NCCLS Guidelines
NCCLS Guidelines
NCCLS Guidelines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
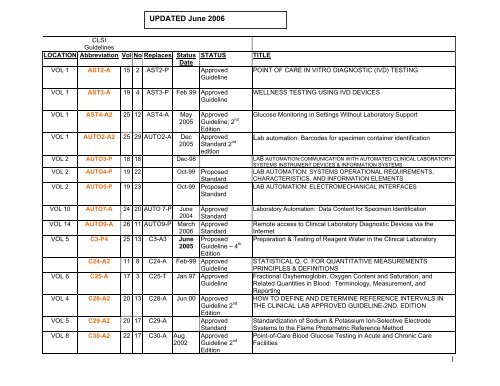
UPDATED June 2006CLSI<strong>Guidelines</strong>LOCATION Abbreviation Vol No Replaces StatusDateSTATUSVOL 1 AST2-A 15 2 AST2-P ApprovedGuidelineVOL 1 AST3-A 19 4 AST3-P Feb 99 ApprovedGuidelineTITLEPOINT OF CARE IN VITRO DIAGNOSTIC (IVD) TESTINGWELLNESS TESTING USING IVD DEVICESVOL 1 AST4-A2 25 12 AST4-A May2005VOL 1 AUTO2-A2 25 29 AUTO2-A Dec2005ApprovedGuideline; 2 ndEditionApprovedStandard 2 ndeditionGlucose Monitoring in Settings Without Laboratory SupportLab automation: Barcodes for specimen container identificationVOL 2 AUTO3-P 18 18 Dec-98 LAB AUTOMATION:COMMUNICATION WITH AUTOMATED CLINICAL LABORATORYSYSTEMS INSTRUMENT DEVICES & INFORMATION SYSTEMSVOL 2 AUTO4-P 19 22 Oct-99 ProposedStandardLAB AUTOMATION: SYSTEMS OPERATIONAL REQUIREMENTS,CHARACTERISTICS, AND INFORMATION ELEMENTSVOL 2 AUTO5-P 19 23 Oct-99 ProposedStandardLAB AUTOMATION: ELECTROMECHANICAL INTERFACESVOL 10 AUTO7-A 24 20 AUTO 7-P June2004ApprovedStandardVOL 14 AUTO9-A 26 11 AUTO9-P March Approved2006 StandardVOL 5 C3-P4 25 13 C3-A3 June Proposed2005 Guideline – 4 thEditionC24-A2 11 8 C24-A Feb-99 ApprovedGuidelineVOL 6 C25-A 17 3 C25-T Jan 97 ApprovedGuidelineVOL 4 C28-A2 20 13 C28-A Jun 00 ApprovedGuideline 2 ndEditionVOL 5 C29-A2 20 17 C29-A ApprovedVOL 8 C30-A2 22 17 C30-A Aug2002StandardApprovedGuideline 2 ndEditionLaboratory Automation: Data Content for Specimen IdentificationRemote access to Clinical Laboratory Diagnostic Devices via theInternetPreparation & Testing of Reagent Water in the Clinical LaboratorySTATISTICAL Q. C. FOR QUANTITATIVE MEASUREMENTSPRINCIPLES & DEFINITIONSFractional Oxyhemoglobin, Oxygen Content and Saturation, andRelated Quantities in Blood: Terminology, Measurement, andReportingHOW TO DEFINE AND DETERMINE REFERENCE INTERVALS INTHE CLINICAL LAB APPROVED GUIDELINE-2ND. EDITIONStandardization of Sodium & Potassium Ion-Selective ElectrodeSystems to the Flame Photometric Reference MethodPoint-of-Care Blood Glucose Testing in Acute and Chronic CareFacilities1
UPDATED June 2006CLSI<strong>Guidelines</strong>LOCATION Abbreviation Vol No Replaces StatusDateVOL 4 C31-A2 15 20 C31-A June2001STATUSApprovedGuideline2 nd EditionVOL 3 C37-A 19 25 C37-P Nov-99 ApprovedGuidelineTITLEIONIZED CALCIUM DETERMINATIONS: PRECOLLECTION VARIABLES,SPECIMEN CHOICE, COLLECTION, AND HANDLING; APPROVEDPREPARATION AMD VALIDATION OF COMMUTABLE FROZEN HUMAN SERUMPOOLS AS SECONDARY REFERENCE MATERIALS FOR CHOLESTEROLMEASUREMENTVOL 2 C39-A 18 23 C39-P Oct-99 ApprovedStandardDESIGNATED COMPARISON METHOD FOR THE MEASUREMENT OFIONIZED CALCIUM IN SERUMVOL 6 C40-A 21 9 C40-P Jun2001ApprovedGuidelineVOL 5 C43-A 22 22 C43-P Nov Approved2002 GuidelineVOL 8 C44-A 22 25 C44-P Dec Approved2002 GuidelineVOL 11 C45-A 24 31 CA45-P Oct 2004 ApprovedGuidelineVOL 4 C46-A 21 14 C46-P Sep Approved2001 GuidelineVOL 11 C48-A 24 22 C48-P Jul 2004 ApprovedGuidelineVOL 7 D12-A2 13 14 1993 ApprovedGuidelineVOL 7 D13-A 13 15 1993 ApprovedGuidelineVOL 1 EP5-A2 24 25 EP5-A Aug2004VOL 6 EP6-A 23 16 EP6-P2 April2003ApprovedGuideline – 2 ndEditionApprovedGuidelineAnalytical Procedures for the Determination of Lead in Blood and UrineGas Chromatography/Mass (GC/MS) Spectometry Confirmation of DrugsHarmonization of Glycohemoglobin MeasurementsMeasurement of Free Thyroid HormonesBLOOD GAS & pH ANALYSIS & RELATED MEASUREMENTS;PROPOSED GUIDELINEApplication of Biochemical Markers of Bone Turnover in the Assessmentand Monitoring of Bone DiseasesImmunoprecipitin Analyses: procedures for Evaluating the performance ofmaterialsAgglutination Analyses: Antibody Characteristics, Methodology,Limitation, and Clinical ValidationEvaluation of Precision Performance of Quantitative MeasurementMethodsEvaluation of the Linearity of Quantitative Measurement Procedures; AStatistical Approach2
UPDATED June 2006CLSI<strong>Guidelines</strong>LOCATION Abbreviation Vol No Replaces StatusDateVOL 8 EP7-A2 25 27 EP7-A Nov2005VOL 6 EP9-A2 22 19 EP9-A Sept2002VOL 6 EP10-A2 22 29 EP10-A Dec2002VOL 7 EP12-A 22 14 EP12-P Aug2002VOL 13 EP13-R 15 8 Aug1995VOL 5 EP14-A2 25 4 EP14A Jan2005VOL 7 EP15-A2 25 17 EP15-A April2006VOL 11 EP17-A 24 34 EP17-P Oct2004VOL 4 EP18-A 22 28 EP18-P Dec2002VOL 7 EP19-R 22 10 EP19-P JuN2002VOL 9 EP21-A 23 20 EP21-P APRIL2003STATUSApprovedGuideline 2 ndeditionApprovedGuideline 2 ndEditionApprovedGuideline- 2 ndEditionApprovedGuidelineReportApprovedGuideline -2 ndEditionApprovedGuideline –2 nd PrintingApprovedGuidelineApprovedGuidelineReportApprovedGuidelineTITLEInterference Testing in Clinical ChemistryMethod Comparison and Bias Estimation Using Patient SamplesPreliminary Evaluation of Quantitative Clinical Laboratory MethodsUser Protocol for Evaluation of Qualitative Test PerformanceLaboratory Statistics – Standard DeviationEvaluation of Matrix EffectsUser Verification of Performance for Precision and TruenessProtocols for Determination of Limits of Detection and Limits ofQuantitationQuality Management for Unit-Use TestingA Framework for <strong>NCCLS</strong> Evaluation Protocols; A ReportEstimation of Total Analytical Error for Clinical MethodsVOL 4 GP2-A5 26 12 GP2-A43 Mar2006ApprovedGuideline 5thEditionLaboratory Documents: Development and ControlVOL 7 GP5-A2 22 3 GP5-A Mar2002ApprovedGuidelineClinical Laboratory Waste Management3
UPDATED June 2006` CLSI<strong>Guidelines</strong>LOCATION Abbreviation Vol No Replaces StatusSTATUSDateVOL 3 GP9-A 18 15 GP9-T Nov-98 ApprovedGuidelineVOL 5 GP10-A 15 19 GP10-T DEC1995ApprovedGuidelineTITLESELECTING AND EVALUATING A REFERRAL LABORATORYAssessment of the Clinical Accuracy of Laboratory Tests UsingReceiver Operating Characteristic (ROC)PlotsVOL 3 GP11-A 18 14 GP11-T Nov-98 ApprovedGuidelineVOL 7 GP15-A2 21 17 GP15-A Nov2001VOL 5 GP16-A2 21 19 GP16-A Nov2001VOL 5 GP17-A2 24 13 GP17-A APR2004VOL 8 GP18-A 18 3 GP18-P Apr1998VOL 5 GP20-A2 23 27 GP20-A Oct2003VOL 5 GP21-A2 24 14 GP21-A APR2004VOL 4 GP22-A2 24 35 GP22-A Nov2004BASIC COST ACCOUNTING FOR CLINICAL SERVICESApproved-2 nd Papanicolaou TechniqueEditionApproved –2 nd Urinalysis and Collection, Transportation, and Preservation of UrineEdition SpecimensClinical Laboratory SafetyApprovedGuideline- 2 ndEditionApprovedGuidelineApprovedGuideline –2 nd EditionApprovedGuideline – 2 ndEditionApprovedGuideline – 2 ndEditionVOL 3 GP23-A 19 14 GP23-P Aug-99 ApprovedGuidelineVOL 8 G26-A3 24 36 GP26-A Nov2004VOL 10 GP28-A 25 7 GP28-P Feb2005VOL 7 GP29-A 22 26 GP29-P Dec2002ApprovedGuideline-3rdEditionApprovedEditionApprovedGuidelineLaboratory DesignFine Needle Aspiration Biopsy (FNAB) TechniquesTraining and Competence AssessmentContinuous Quality Improvement: Integrating Five Key Quality SystemComponentsNON GYNECOLOGIC CYTOLOGIC. SPECMINS COLLECTION & CYTOPREPATORYTECHNIQUESApplication of a Quality Management System Model for LaboratoryServicesMicrowave Device Use in the Clinical LaboratoryAssessment of Laboratory Tests When Proficiency Testing is NotAvailableVOL 10 H1-A5 23 33 H1-A4 Dec2003ApprovedStandard – 5 thEditionTubes and Additives for Venous Blood Specimen Collection4
UPDATED June 2006CLSIGUIDELINESLOCATION Abbreviation Vol No Replaces StatusDateSTATUSVOL 4 H2-A4 20 27 H2-A3 Dec00 ApprovedStandard – 4 thEditionVOL 7 * H3-A5 23 32 H3-A4 Dec Approved2003 Standard – 5 thEditionVOL 3 * H4-A5 24 21 H4-A4 June Approved2004 Standard – 5 thEditionVOL 5 H7-A3 20 18 H7-A2 ApprovedStandardVOL 3 H11-A4 24 28 H11-A3 Sept2004ApprovedStandard-4 thEditionVOL 4 H15-A3 20 28 H15-A2 Dec00 ApprovedStandard – 3 rdEditionVOL 8 H17-A 18 19 H17-P DEC1998VOL 2 H18-A3 24 38 H18-A2 Nov2004ApprovedStandardApprovedGuideline –3 rd EditionTITLEREFERENCE & SELECTED PROCEDURE FOR THEERYTHROCYTE SEDIMENTATION RATE (ESR) TESTPROCEDURE FOR THE COLLECTION OF DIAGNOSTIC BLOOD SPECIMENS BYVENIPUNCTUREPROCEDURES AND DEVICES FOR COLLECTION OF DIAGNOSTIC CAPILLARYBLOOD SPECIMENSProcedure for Determining Packed Cell Volume by the MicrohematocritMethodPROCEDURES FOR THE COLLECTION OF ARTERIAL BLOOD SPECIMENSREFERENCE & SELECTED PROCEDURES FOR THEQUANTITATIVE DETERMINATION OF HEMOGLOBIN IN BLOODDetermination of Serum Iron,Total Iron-Binding Capacity and PercentTransferrin SaturationProcedures for the Handling and Processing of Blood SpecimensVOL 7 H21-A4 23 35 H21-A3 Dec2003ApprovedGuideline –4th EditionVOL 5 H26-A 16 12 H26-P DEC 96 ApprovedStandardCollection, transport, and Processing of Blood specimens forCoagulation Testing and Based Coagulation AssaysPerformance Goals for the Internal Quality Control of MultichannelHematology AnalyzersVOL 7 H30-A2 21 18 H30-A Nov2001ApprovedGuideline-2ndEditionPROCEDURE FOR THE DETERMINATION OF FIBRINOGEN INPLASMAVOL 3 H38-P 19 7 Apr-99 ProposedStandardCALIBRATION AND QUALITY CONTROL OF AUTOMATEDHEMATOLOGYANALYZERS5
UPDATED June 2006CLSIGUIDELINESLOCATION Abbreviation Vol No Replaces StatusDateVOL 9 H42-A 17 18 H42-P Dec1998VOL 9 H43-A 17 18 H43-P June1998VOL 8 H44-A2 24 8 H44-A FEB2004Vol 12 H45-A2 25 15 H45-A June2005VOL 7 H47-A 16 3 H28T &H29TJUN1996VOL 11 H49-A 24 23 H49-P JULY2004VOL 8 H51-A 22 20 H51-P SEPT2002VOL 5 H52-A 21 26 H52-P DEC2001VOL 13 H54-A 25 23 H54-P AUG2005VOL 13 H56-A 25 20 Aug2005VOL 2 HS1-A2 24 37 HS1-A Nov2004VOL 6 HS2-A 23 5 HS2-P Feb2003VOL 12 HS3-A 25 5 HS3-P Jan2005VOL 5 HS4-A2 26 15 HS4-A May2006VOL 8 HS5-A2 26 16 HS5-AP May2006STATUSApprovedGuidelineApprovedGuidelineApprovedGuideline –2 nd EditionApprovedGuideline –2 nd EditionApprovedGuidelineApprovedGuidelineApprovedGuidelineApprovedGuidelineApprovedGuidelineProposedGuidelineApprovedGuideline –2 nd EditionApprovedGuidelineApprovedGuidelineApprovedGuideline –2 nd EditionApprovedGuideline –2 nd EditionTITLEClinical Applications of Flow Cytometry: Quality Assurance andImmunophenotyping of LymphocytesClinical Applications of Flow Cytometry: Immunophenotyping ofLeukemic CellsMethods for Reticulocyte Counting (Automated Blood Cell Counters,Flow Cytometry, and Supravital Dyes)Performance of the Bleeding Time TestOne-Stage Prothrombin Time (PT) Test and Activated PartialThromboplastin Time (APTT) TestPoint-Of-Care Monitoring of Anticoagulation TherapyAssays of von Willebrand Factor Antigen and Ristocetic Cofactor ActivityFetal Red Cell DetectionProcedures for Validation of INR and Local Calibration of PT/INRSystemsBody Fluid Analysis for Cellular CompositionA Quality Management System Model for Health CareProvider-Performed Microscopy TestingPulse OximetryApplication of a Quality Management System Model for RespiratoryServicesApplication of a Quality Management System Model for Medical ImagingServices6
UPDATED June 2006Abbreviation Vol No Replaces StatusLOCATIONDateVOL 11 HS6-A 24 32 HS6-P Oct2004VOL 11 HS10-A2 264 174 HS10-A May2006VOL 13 HS11-A 25 30 HS11-P Dec2005VOL 4 I/LA2-A2 26 13 I/LA2-A March2006VOL 6 I/LA18-A2 21 15 I/LA18-A Sep2001VOL 2 I/LA 21-A 22 9 I/LA21-P Jun2002VOL 10 I/LA23-A 24 16 I/L23 May2004VOL 11 I/LA24-A 24 26 I/LA24-P Aug2004VOL 12 I/LA25-A 24 30 I/LA25-P Dec2004VOL 11 I/LA26-A 24 29 I/LA26-A Oct2004VOL 14 I/LA27-A 26 18 I/LA27-P May2006VOL 10 ISO 15189 Jul2003VOL 8 LA1-A2 14 17 LA1-A Dec1994VOL 9 LA4-A4 23 21 LA4-A3 Jul2003VOL 11 LIS2-A2 24 33 LIS2-A Oct2004STATUSApprovedGuidelineApprovedGuideline –2 nd EditionApprovedGuidelineApprovedGuideline –2 nd editionApprovedGuidelineApprovedGuidelineApprovedGuidelineApprovedGuidelineApprovedStandardApprovedGuidelineApprovedGuidelineInternationalStandardApprovedGuidelineApprovedStandard -4thEditionApprovedStandard – 2 ndEditionTITLEStudies to Evaluate Patient OutcomesApplication of a Quality Management System Model for InpatientMedication UseA Model for Managing Medical Device Alerts (Hazards and Recalls) forHealthcare OrganizationsQuality Assurance of Laboratory Tests for Autoantibodies to NuclearAntigens: (1)Indirecto Fluorescence Assay for Microscopy and (2)Microtiter Enzyme Immunoassay MethodsSpecifications for Immunological Testing for Infectious DiseasesClinical Evaluation of ImmunoassaysAssessing the Quality of Immunoassay Systems: Radioimmunoassaysand Enzyme, Fluorescence, and Luminescence Immunoassays;Approved GuidelineFluorescence Calibration and Quantitative Measurement ofFluorescence IntensityMaternal Serum ScreeningPerformance of Single Cell Immune Response AssaysNewborn Screening Follow-upMedical Laboratories Particular Requirements for Quality andCompetenceAssessing the Quality of Radioimmunoassay Systems-Second EditionBlood Collection on Filter Paper for Newborn Screening ProgramsSpecification for Transferring Information Between Clinical LaboratoryInstruments and Information Systems7
UPDATED June 2006CLSIGUIDELINESLOCATION Abbreviation Vol No Replaces StatusDateVOL 5 M2-A9 26 1 M2-A8 Jan2006VOL 13 M6-A2 26 6 M6-A Jan2006VOL 3 M7-A7 26 2 M7-A6 Jan2006VOL 6 M11-A6 24 2 M11-A5 Jan2004STATUSApprovedStandard9 th EditionApprovedStandard -2 ndEditionApprovedStandard 7 thEditionApprovedStandard 6thEditionVOL 4 M15-A 20 12 M15-T Jun 00 ApprovedGuidelineTITLEPerformance Standards of Antimicrobial Disk Susceptibility TestsProtocols for Evaluating Dehydrated Mueller-Hinton AgarMethods for Antimicrobial Susceptibility Tests for Bacteria That GrowAerobicallyMethods for Antimicrobial Susceptibility Testing of Anaerobic BacteriaLAB DX. OF BLOOD BORNE PARASITIC DISEASES; ApprovedVOL 3 M21-A 19 17 M21-T Sep-99 ApprovedGuidelineVOL 10 M22-A3 24 19 M22-A2 June2004VOL 9 M24-A 23 18 M24-T2 APRIL2003ApprovedStandard – 3 rdEditionApprovedStandardVOL 3 M26-A 19 18 M26-T Sep-99 ApprovedGuidelineVOL 8 M27-A2 22 15 M27-A Aug2002ApprovedStandard 2ndEditionMETHODOLOGY FOR THE SERUM BACTERIAL TESTQuality Control for Commercially Prepared Microbiological CultureMediaSusceptibility Testing of Mycobacteria, Nocardiae, and Other AerobicActinomycetesMETHODS FOR DETERMINING BACTERICIDAL ACTIVITY OFANTIMICROBIAL AGENTSReference Method for Broth Dilution Antifungal Susceptibility Testing ofYeasts8
UPDATED June 2006CLSIGUIDELINESLOCATION Abbreviation Vol No Replaces Status STATUSDateVOL 12 M28-A2 25 16 M28-A June 25 ApprovedGuideline-2 ndVOL 6 M29-A3 25 10 M29-A2 March2005VOL 3 M31-A2 22 6 M31-A May2002EditionApprovedGuideline- 3rd EditionApprovedStandard2nd EditionApprovedStandardVOL 10 M33-A 24 7 M33-P FEB2004VOL 5 M34-A 20 20 M34-P ApprovedGuidelineVOL 4 M35-A 22 18 M35-P Sept Approved2002 GuidelineVOL 10 M36-A 24 6 M36-P FEB Approved2004 GuidelineVOL 1 M37-A2 22 7 M37-A May2002VOL 8 M38-A 22 16 M38-P Aug2002ApprovedGuideline2nd EditionApprovedStandardTITLEProcedures for the Recovery and Identification of Parasites from theIntestinal TractProtection of Laboratory Workers from Occupationally AcquiredInfectionsPERFORMANCE STANDARDS FOR ANTIMICROBIAL DISK AND DILUTIONSUSCEPTIBILITY TESTS FOR BACTERIA ISOLATED FROM ANIMALSAntiviral Susceptibility Testing:Herpes Simplex Virus by PlaqueReduction AssayWestern Blot Assay for Antibodies to Borrelia burgdorferiABBREVIATED IDENTIFICATION OF BACTERIA & YEASTClinical Use and Interpretation of Serologic Tests for ToxoplasmagondiiDEVELOPMENT OF IN VITRO SUSCEPTIBILITY TESTINGCRITERIA & QC PARAMETERS FOR VET ANTIMICROBIAL AGENTSReference Method for Broth Dilution Antifungal Susceptibility Testing ofFilamentous FungiVOL 7 M39-A2 25 28 M39-A Nov2005VOL 10 M40-A 23 34 M40-P Dec2003ApprovedGuideline2 nd EditionApprovedStandard – 5 thEditionAnalysis and Presentation of Cumulative Antimicrobial SusceptibilityTest DataQuality Control of Microbiological Transport Systems9
UPDATED June 2006CLSIGUIDELINESLOCATION Abbreviation Vol No Replaces StatusDateVOL 9 M42-R 23 19 April2003VOL 10 M44-A 24 15 M44-A May2004VOL 14 M45-A 26 19 M45-P May2006M100-S13VOL 10 M100-S14VOL 10 M100-S15 25 1 M100-S14 Jan2005VOL 13 M100-S16 26 3 M100-S15 Jan2006VOL 9 MM2-A2 22 12 MM2-A Aug2002VOL 8 MM3-A2 26 8 MM3-A Feb2006STATUSReportPublishedApprovedGuidelineApprovedGuideline15 thInformationalSupplement16 thInformationalSupplementApprovedGuideline -2nd EdApprovedGuideline –2 nd EditionVOL 3 MM4-A 19 26 MM4-P 1999 ApprovedGuidelineVOL 9 MM5-A 23 17 MM5-P April Approved2003 GuidelineVOL 10 MM6-A 23 28 MM6-P Oct Approved2003 GuidelineMM7-A 24 5 MM7-P JAN ApprovedVOL 92004 GuidelineVOL 12 MM9-A 24 40 MM9-P Dec Approved2004 GuidelineVol 14 MM10-A 26 9 MM10-P Feb2006ApprovedGuideline-2 ndEditionTITLEMethods for Antimicrobial Disk Susceptibility Testing of BacteriaIsolated from Aquatic AnimalsMethod for Antifungal Disk Diffusion Susceptibility Testing of Yeast;Approved GuidelineMethods for Antimicrobial Dilution and Disk Susceptibility Testing ofInfrequently Isolated or Fastidious BacteriaWallchart – Glossary ofAntimicrobial Terms and AbbreviatioonsPerformance Standards for Antimicrobial Susceptibility Testing; 15 thInformational SupplementPerformance Standards for Antimicrobial Susceptibility Testing –16 thInformational SupplementImmunoglobulin and T-Cell Receptor Gene Rearrangement AssaysMolecular Diagnostic Methods for Infectious DiseasesQuality Insurance for ImmunocytochemistryNucleic Acid Amplification Assays for Molecular HematopathologyQuantitative Molecular Methods for Infectious DiseasesFluorescence In Situ Hybridization (FISH) Methods for MedicalGeneticsNucleic Acid Sequencing Methods in Diagnostic Laboratory MedicineGenotyping for Infectious Diseases: Identification and Characterization10
UPDATED June 2006CLSIGUIDELINESLOCATION Abbreviation Vol No Replaces StatusDateVOL 14 MM12-A 26 20 MM12-P May2006VOL 12 MM13-A 25 31 MM13-P Dec2005VOL 11 MM14-A 25 24 MM14-P AugVOL 5 NRSCL13-A 20 21 NRSCL13-P2005STATUSApprovedGuidelineApprovedGuidelineApprovedGuidelineApprovedGuidelineCD Case I POCT1-A Dec2001ApprovedStandardVOL 13 T/DM6-A 17 14 T/DM6-P Sep Approved1997 GuidelineVOL 1 T/DM8-A 19 6 T/DM8-P Feb 99 ApprovedGuidelineVOL 14 X6-R 25 14 June Approved2005TITLEDiagnostic Nucleic Acid MicroarraysCollection, Transport, Preparation, and Storage of Specimens forMolecular MethodsProficiency Testing (External Quality Assessment) for MolecularMethodsThe Reference System for the Clinical Laboratory: Criteria forDevelopment and Credentialing of Methods and Materials forHarmonization of ResultsPoint of Care ConnectivityBlood Alcohol Testing in the Clinical LaboratoryURING DRUG TESTING IN THE CLINICAL LABProceedings from the QC for the Future Workshop* Video is also available through CQI Office11