Synthesis and Characterization of complexes of ... - Ultrascientist.org
Synthesis and Characterization of complexes of ... - Ultrascientist.org
Synthesis and Characterization of complexes of ... - Ultrascientist.org
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
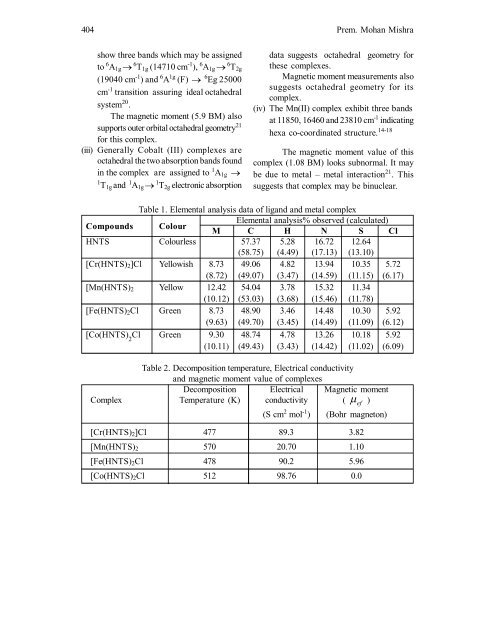
404 Prem. Mohan Mishrashow three b<strong>and</strong>s which may be assignedto 6 A 1g 6 T 1g (14710 cm -1 ), 6 A 1g 6 T 2g(19040 cm -1 ) <strong>and</strong> 6 A 1g (F) 6 Eg 25000cm -1 transition assuring ideal octahedralsystem 20 .The magnetic moment (5.9 BM) alsosupports outer orbital octahedral geometry 21for this complex.(iii) Generally Cobalt (III) <strong>complexes</strong> areoctahedral the two absorption b<strong>and</strong>s foundin the complex are assigned to 1 A 1g 1 T 1g <strong>and</strong> 1 A 1g 1 T 2g electronic absorptiondata suggests octahedral geometry forthese <strong>complexes</strong>.Magnetic moment measurements alsosuggests octahedral geometry for itscomplex.(iv) The Mn(II) complex exhibit three b<strong>and</strong>sat 11850, 16460 <strong>and</strong> 23810 cm -1 indicatinghexa co-coordinated structure. 14-18The magnetic moment value <strong>of</strong> thiscomplex (1.08 BM) looks subnormal. It maybe due to metal – metal interaction 21 . Thissuggests that complex may be binuclear.Table 1. Elemental analysis data <strong>of</strong> lig<strong>and</strong> <strong>and</strong> metal complexCompounds ColourElemental analysis% observed (calculated)M C H N S ClHNTS Colourless 57.37 5.28 16.72 12.64(58.75) (4.49) (17.13) (13.10)[Cr(HNTS) 2 ]Cl Yellowish 8.73 49.06 4.82 13.94 10.35 5.72(8.72) (49.07) (3.47) (14.59) (11.15) (6.17)[Mn(HNTS) 2 Yellow 12.42 54.04 3.78 15.32 11.34(10.12) (53.03) (3.68) (15.46) (11.78)[Fe(HNTS) 2 Cl Green 8.73 48.90 3.46 14.48 10.30 5.92(9.63) (49.70) (3.45) (14.49) (11.09) (6.12)[Co(HNTS) 2Cl Green 9.30 48.74 4.78 13.26 10.18 5.92(10.11) (49.43) (3.43) (14.42) (11.02) (6.09)Table 2. Decomposition temperature, Electrical conductivity<strong>and</strong> magnetic moment value <strong>of</strong> <strong>complexes</strong>Decomposition Electrical Magnetic momentComplex Temperature (K) conductivity ( ef )(S cm 2 mol -1 ) (Bohr magneton)[Cr(HNTS) 2 ]Cl 477 89.3 3.82[Mn(HNTS) 2 570 20.70 1.10[Fe(HNTS) 2 Cl 478 90.2 5.96[Co(HNTS) 2 Cl 512 98.76 0.0