- Page 1: NISTIR 6659Thermodynamic, Transport
- Page 5: Table of ContentsAccomplishments an
- Page 8 and 9: understand that this number is not
- Page 10 and 11: Table 2: A listing of the major com
- Page 12 and 13: 2,6-dimethyloctane1-methyl-3-(2-met
- Page 14 and 15: Figure 2: Representative chromatogr
- Page 16 and 17: Figure 3: A schematic diagram of th
- Page 18 and 19: 200Product Suite Area1501005000 100
- Page 20 and 21: The products of a 40 min decomposit
- Page 22 and 23: ln (propylcyclohexane peak area %)0
- Page 24 and 25: the system. Operation of the densim
- Page 26 and 27: 330.00 2.002 739.17 450.00 1.000 61
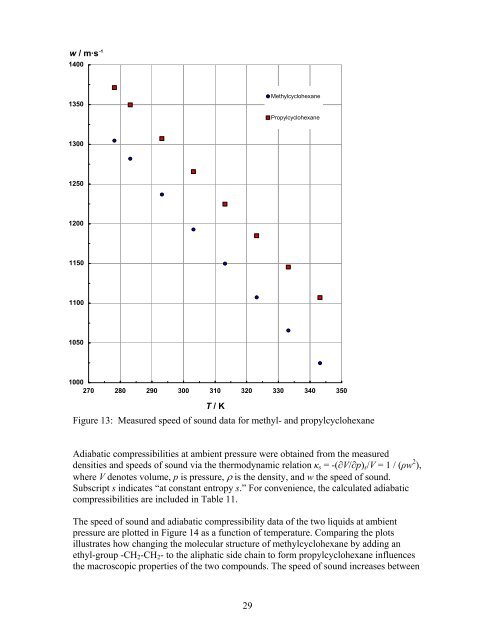
- Page 28 and 29: 290.00 40.005 821.46 410.00 39.998
- Page 30 and 31: Propylcyclohexane840820800Density [
- Page 32 and 33: The viscosity measurement system in
- Page 36 and 37: 5.1 % at 273.15 K and 8.1 % at 343.
- Page 38 and 39: different solution of Fourier's law
- Page 40 and 41: 0.120.10 e / W . m -1 K -10.080.060
- Page 42 and 43: Figure 18: Schematic diagram of the
- Page 44 and 45: During the initial heating of each
- Page 46 and 47: 260250Temperature ( o C)24023022021
- Page 48 and 49: The rather consistent difference in
- Page 50 and 51: Table 14: A summary of the initial
- Page 52 and 53: applied to the distillate fractions
- Page 54 and 55: Table 16: Summary of the results of
- Page 56 and 57: Table 16e: JP-8 3773:Distillate Par
- Page 58 and 59: Figure 26: A plot of the hydrocarbo
- Page 60 and 61: Table 18: Representative distillati
- Page 62 and 63: 45 81.5 16.3 0.0 1.9 0.0 0.250 78.1
- Page 64 and 65: Figure 28: A plot of the hydrocarbo
- Page 66 and 67: 330.00 20.805 805.48 410.00 10.778
- Page 68 and 69: 350.00 4.073 754.74 470.00 25.002 6
- Page 70 and 71: 270.0 1.99 812.6 350.0 19.99 769.52
- Page 72 and 73: 310.0 20.07 754.0 390.0 30.02 711.0
- Page 74 and 75: Jet-A-3638860840820Density [kg/m3]8
- Page 76 and 77: JP-8-3773840820800Density [kg/m3]78
- Page 78 and 79: Table 25: Viscosity Measurements fo
- Page 80 and 81: mPa·s3.02.52.01.51.00.50.0250 260
- Page 82 and 83: mPa·s3.53.02.52.01.51.00.50.0250 2
- Page 84 and 85:
The kinematic viscosity of JP-8-377
- Page 86 and 87:
Thermal Conductivity Measurements o
- Page 88 and 89:
0.1350.1300.1250.120 / W . m -1 K -
- Page 90 and 91:
A correction factor for the heat fl
- Page 92 and 93:
Development of the Thermodynamic an
- Page 94 and 95:
Table 28. Potential constituent flu
- Page 96 and 97:
840820800ρ, kg/m 3780760Jet A-3638
- Page 98 and 99:
35003000Viscosity, uPa.s25002000150
- Page 100 and 101:
The volatility, as indicated by the
- Page 102 and 103:
2.5Deviations in Density for S-8 Su
- Page 104 and 105:
References:1. Bruno, T. J., Svorono
- Page 106 and 107:
28. Smith, B. L., Bruno, T.J., Comp
- Page 108 and 109:
Appendix I: Thermal Conductivity Me
- Page 110 and 111:
2033 322.056 319.876 20.156 0.1136
- Page 112 and 113:
6017 402.656 400.007 5.190 0.0987 0
- Page 114 and 115:
9051 461.127 459.771 40.275 0.1076
- Page 116 and 117:
2029 317.578 320.976 10.218 0.1124
- Page 118 and 119:
6023 397.324 399.294 5.092 0.0999 0
- Page 120 and 121:
9057 460.087 462.193 40.316 0.1089
- Page 122 and 123:
2015 318.519 321.081 5.594 0.1113 0
- Page 124 and 125:
5049 378.062 380.940 30.262 0.1128
- Page 126 and 127:
9033 459.515 461.138 20.446 0.1014
- Page 128 and 129:
1030 300.752 303.416 11.740 0.1187
- Page 130 and 131:
2053 355.304 357.001 40.923 0.1193
- Page 132 and 133:
3087 404.864 406.928 0.783 0.1203 0
- Page 134 and 135:
4095 452.073 453.975 20.454 0.1120
- Page 136 and 137:
6008 547.320 549.917 50.748 0.0753
- Page 138 and 139:
1061 301.816 303.449 20.266 4.330 0
- Page 140 and 141:
3035 338.039 340.147 20.410 4.191 0
- Page 142 and 143:
5029 378.035 381.195 5.181 3.947 0.
- Page 144 and 145:
7025 418.831 421.100 10.554 3.824 0
- Page 146 and 147:
10003 480.867 482.860 0.237 3.433 0