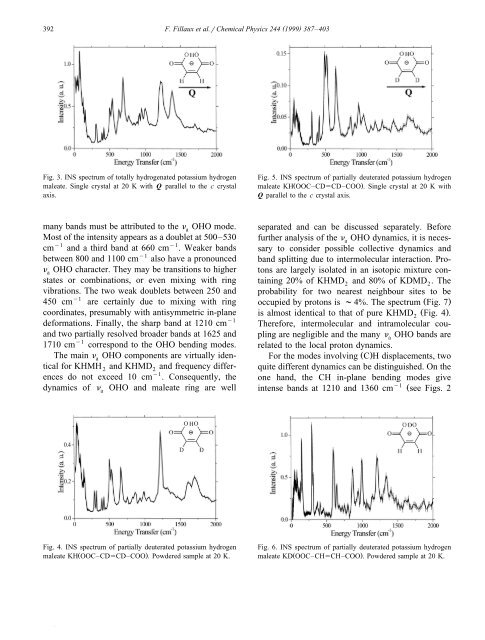
392( )F. Fillaux et al.rChemical Physics 244 1999 387–403Fig. 3. INS spectrum <strong>of</strong> totally <strong>hydrogen</strong>ated potassium <strong>hydrogen</strong>maleate. Single crystal at 20 K with Q parallel to <strong>the</strong> c crystalaxis.Fig. 5. INS spectrum <strong>of</strong> partially deuterated potassium <strong>hydrogen</strong>maleate KHŽ OOC–CD5CD–COO .. Single crystal at 20 K withQ parallel to <strong>the</strong> c crystal axis.many b<strong>and</strong>s must be attributed to <strong>the</strong> n OHO mode.aMost <strong>of</strong> <strong>the</strong> intensity appears as a doublet at 500–530cm y1 <strong>and</strong> a third b<strong>and</strong> at 660 cm y1 . Weaker b<strong>and</strong>sbetween 800 <strong>and</strong> 1100 cm y1 also have a pronouncedn OHO character. They may be transitions to higherastates or combinations, or even mixing with ringvibrations. The two weak doublets between 250 <strong>and</strong>450 cm y1 are certainly due to mixing with ringcoordinates, presumably with anti<strong>symmetric</strong> in-planedeformations. Finally, <strong>the</strong> sharp b<strong>and</strong> at 1210 cm y1<strong>and</strong> two partially resolved broader b<strong>and</strong>s at 1625 <strong>and</strong>1710 cm y1 correspond to <strong>the</strong> OHO bending modes.The main n OHO components are virtually idenatical for KHMH 2 <strong>and</strong> KHMD2<strong>and</strong> frequency differencesdo not exceed 10 cm y1 . Consequently, <strong>the</strong><strong>dynamics</strong> <strong>of</strong> naOHO <strong>and</strong> maleate ring are wellseparated <strong>and</strong> can be discussed separately. Beforefur<strong>the</strong>r analysis <strong>of</strong> <strong>the</strong> naOHO <strong>dynamics</strong>, it is neces-sary to consider possible collective <strong>dynamics</strong> <strong>and</strong>b<strong>and</strong> splitting due to intermolecular interaction. Protonsare largely isolated in an isotopic mixture containing20% <strong>of</strong> KHMD2 <strong>and</strong> 80% <strong>of</strong> KDMD 2. Theprobability for two nearest neighbour sites to beoccupied by protons is ;4%. The spectrum Ž Fig. 7.is almost identical to that <strong>of</strong> pure KHMD Ž Fig. 4 .2 .Therefore, intermolecular <strong>and</strong> intramolecular couplingare negligible <strong>and</strong> <strong>the</strong> many naOHO b<strong>and</strong>s arerelated to <strong>the</strong> local proton <strong>dynamics</strong>.For <strong>the</strong> modes involving Ž C.H displacements, twoquite different <strong>dynamics</strong> can be distinguished. On <strong>the</strong>one h<strong>and</strong>, <strong>the</strong> CH in-plane bending modes givey1intense b<strong>and</strong>s at 1210 <strong>and</strong> 1360 cm Žsee Figs. 2Fig. 4. INS spectrum <strong>of</strong> partially deuterated potassium <strong>hydrogen</strong>Ž .maleate KH OOC–CD5CD–COO . Powdered sample at 20 K.Fig. 6. INS spectrum <strong>of</strong> partially deuterated potassium <strong>hydrogen</strong>Ž .maleate KD OOC–CH5CH–COO . Powdered sample at 20 K.
( )F. Fillaux et al.rChemical Physics 244 1999 387–403 393Fig. 7. INS spectrum <strong>of</strong> an isotopic mixture <strong>of</strong> partially deuteratedpotassium <strong>hydrogen</strong> maleate KHŽ OOC–CD5CD–COO ., 20%,<strong>and</strong> KDŽ OOC–CD5CD–COO ., 80%. Powdered sample at 20 K.<strong>and</strong> 6 ., still observed in <strong>the</strong> single-crystal spectrumŽ Fig. 3 .. The CH out-<strong>of</strong>-plane bending at 875 <strong>and</strong>1010 cm y1 is totally extinguished for <strong>the</strong> singlecrystal Ž compare Figs. 2 <strong>and</strong> 3 .. On <strong>the</strong> o<strong>the</strong>r h<strong>and</strong>,<strong>the</strong> sharp b<strong>and</strong>s at 155, 305 <strong>and</strong> 605 cm y1 for <strong>the</strong>powdered KHMH Ž Fig. 2.2 which disappear for <strong>the</strong>single crystal Ž Fig. 3. <strong>and</strong> for KHMD Ž2 Figs. 4 <strong>and</strong>5.correspond to out-<strong>of</strong>-plane deformations <strong>of</strong> <strong>the</strong>maleate ring. The observed intensities are due tocontributions <strong>of</strong> <strong>the</strong> Ž C.H protons.The lattice density <strong>of</strong> states below 150 cm y1comprises many sharp components compatible withra<strong>the</strong>r weak intermolecular coupling. The ra<strong>the</strong>rmodest intensities confirm that <strong>the</strong> proton <strong>dynamics</strong>are largely decoupled from <strong>the</strong> lattice. However, <strong>the</strong>sharp b<strong>and</strong> at 80 cm y1 observed for <strong>the</strong> singlecrystal <strong>of</strong> KHMH Ž Fig. 3. suggests that <strong>the</strong> Ž C.2Hprotons may ride some lattice modes. The very weaklattice mode intensity for <strong>the</strong> KHMD2single crystalshows that <strong>the</strong> naOHO mode is largely decoupledfrom <strong>the</strong> lattice, in agreement with <strong>the</strong> spectrum <strong>of</strong><strong>the</strong> isotopic mixture Ž Fig. 7 .. The anomalous largelattice mode intensity for <strong>the</strong> KHMD2powder islikely due to <strong>the</strong> presence <strong>of</strong> some N2ice in <strong>the</strong>cryostat.5. Proton <strong>dynamics</strong> in <strong>the</strong> <strong>hydrogen</strong> bondAccording to <strong>the</strong> spectra presented above, intra<strong>and</strong> intermolecular coupling are <strong>of</strong> minor importancefor <strong>the</strong> n OHO mode <strong>and</strong> we assume that <strong>the</strong>amultiple components arise from a very anharmonicpotential function. According to <strong>the</strong> crystal structure,<strong>the</strong> potential function is <strong>symmetric</strong>al with a well-definedcentral minimum. Any double minimum potentialfunction similar to those proposed for strong<strong>symmetric</strong> <strong>hydrogen</strong> bonds w1–10,31,38,39xcan beeliminated. For <strong>the</strong> KHMD single crystal Ž Fig. 5 .2,<strong>the</strong> energy level scheme <strong>of</strong> naOHO should accountfor <strong>the</strong> main components at 500, 530 <strong>and</strong> 655 cm y1y1<strong>and</strong> higher transitions above 800 cm Ž Table 5 ..The three components can be regarded as an almosttriply degenerate level <strong>and</strong> <strong>the</strong> rule <strong>of</strong> thumb is tosuppose a <strong>symmetric</strong> potential function with threeminima. To be consistent with <strong>the</strong> structure, <strong>the</strong>central minimum should be at a lower energy than<strong>the</strong> o<strong>the</strong>r two. The <strong>dynamics</strong> are ra<strong>the</strong>r well representedwith <strong>the</strong> potential presented in Fig. 8 <strong>and</strong>Table 6. However, this potential is not totally free <strong>of</strong>some arbitrariness because, unfortunately, it is notpossible to precisely identify <strong>the</strong> naOHO b<strong>and</strong>samongst <strong>the</strong> features observed between 800 <strong>and</strong> 1500cm y1 , in <strong>the</strong> range <strong>of</strong> <strong>the</strong> ring deformations. Theseundesignated naOHO levels leave some flexibility to<strong>the</strong> shape <strong>of</strong> <strong>the</strong> upper part <strong>of</strong> <strong>the</strong> potential. Therefore,potential functions with different power termsŽ2namely x or x 4. give broadly <strong>the</strong> same reasonableagreement with observations Ž see Table 6 .. Never<strong>the</strong>less,<strong>the</strong>se potentials are very similar in shape <strong>and</strong>correspond to <strong>the</strong> same <strong>dynamics</strong>.INS intensities provide fur<strong>the</strong>r support for <strong>the</strong>sepotential functions. In <strong>the</strong> harmonic case, intensitiesdecrease rapidly Ž exponentially.as <strong>the</strong> quantumnumber <strong>of</strong> <strong>the</strong> final state increases w36 x. In contrast tothis, calculated intensities remain quite high in <strong>the</strong>triple minimum potential Žat least for n-8, see. Žy1Table 6 <strong>and</strong> <strong>the</strong> 03 transition 665 cm . is <strong>the</strong>most intense, as is observed. Intensities calculatedy1between 700 <strong>and</strong> 1100 cm Ž ns4–7.confirm thatb<strong>and</strong>s in this region can be attributed to naOHO.Some support to <strong>the</strong> x 2 potential function, over thatwith x 4 , comes from a consideration <strong>of</strong> <strong>the</strong> relativeintensities for <strong>the</strong> doublet at 500–530 cm y1 in Table6. Beyond 1100 cm y1 , <strong>the</strong> spectra provide littlediscriminatory information by which <strong>the</strong> potentialscould be fur<strong>the</strong>r restricted.Supposing <strong>the</strong> potential <strong>of</strong> Fig. 8 also applies to<strong>the</strong> deuterated analogue, <strong>the</strong> calculated frequencyratios naOHOrnaODO, range from 0.9 to 1.1