Hydrogen Peroxide Decomposition by Pyrite in the Presence of Fe ...
Hydrogen Peroxide Decomposition by Pyrite in the Presence of Fe ...
Hydrogen Peroxide Decomposition by Pyrite in the Presence of Fe ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
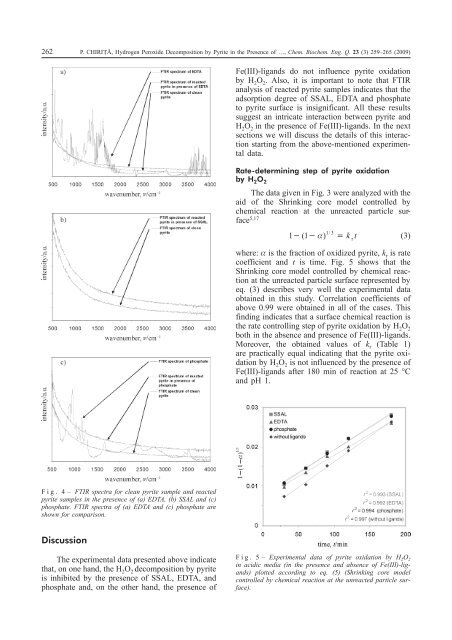
262 P. CHIRIȚÃ, <strong>Hydrogen</strong> <strong>Peroxide</strong> <strong>Decomposition</strong> <strong>by</strong> <strong>Pyrite</strong> <strong>in</strong> <strong>the</strong> <strong>Presence</strong> <strong>of</strong> …, Chem. Biochem. Eng. Q. 23 (3) 259–265 (2009)<strong>Fe</strong>(III)-ligands do not <strong>in</strong>fluence pyrite oxidation<strong>by</strong> H 2 O 2 . Also, it is important to note that FTIRanalysis <strong>of</strong> reacted pyrite samples <strong>in</strong>dicates that <strong>the</strong>adsorption degree <strong>of</strong> SSAL, EDTA and phosphateto pyrite surface is <strong>in</strong>significant. All <strong>the</strong>se resultssuggest an <strong>in</strong>tricate <strong>in</strong>teraction between pyrite andH 2 O 2 <strong>in</strong> <strong>the</strong> presence <strong>of</strong> <strong>Fe</strong>(III)-ligands. In <strong>the</strong> nextsections we will discuss <strong>the</strong> details <strong>of</strong> this <strong>in</strong>teractionstart<strong>in</strong>g from <strong>the</strong> above-mentioned experimentaldata.Rate-determ<strong>in</strong><strong>in</strong>g step <strong>of</strong> pyrite oxidation<strong>by</strong> H 2 O 2The data given <strong>in</strong> Fig. 3 were analyzed with <strong>the</strong>aid <strong>of</strong> <strong>the</strong> Shr<strong>in</strong>k<strong>in</strong>g core model controlled <strong>by</strong>chemical reaction at <strong>the</strong> unreacted particle surface5,17 131( 1 ) / k t r (3)where: is <strong>the</strong> fraction <strong>of</strong> oxidized pyrite, k r is ratecoefficient and t is time. Fig. 5 shows that <strong>the</strong>Shr<strong>in</strong>k<strong>in</strong>g core model controlled <strong>by</strong> chemical reactionat <strong>the</strong> unreacted particle surface represented <strong>by</strong>eq. (3) describes very well <strong>the</strong> experimental dataobta<strong>in</strong>ed <strong>in</strong> this study. Correlation coefficients <strong>of</strong>above 0.99 were obta<strong>in</strong>ed <strong>in</strong> all <strong>of</strong> <strong>the</strong> cases. Thisf<strong>in</strong>d<strong>in</strong>g <strong>in</strong>dicates that a surface chemical reaction is<strong>the</strong> rate controll<strong>in</strong>g step <strong>of</strong> pyrite oxidation <strong>by</strong> H 2 O 2both <strong>in</strong> <strong>the</strong> absence and presence <strong>of</strong> <strong>Fe</strong>(III)-ligands.Moreover, <strong>the</strong> obta<strong>in</strong>ed values <strong>of</strong> k r (Table 1)are practically equal <strong>in</strong>dicat<strong>in</strong>g that <strong>the</strong> pyrite oxidation<strong>by</strong> H 2 O 2 is not <strong>in</strong>fluenced <strong>by</strong> <strong>the</strong> presence <strong>of</strong><strong>Fe</strong>(III)-ligands after 180 m<strong>in</strong> <strong>of</strong> reaction at 25 °Cand pH 1.Fig. 4– FTIR spectra for clean pyrite sample and reactedpyrite samples <strong>in</strong> <strong>the</strong> presence <strong>of</strong> (a) EDTA, (b) SSAL and (c)phosphate. FTIR spectra <strong>of</strong> (a) EDTA and (c) phosphate areshown for comparison.DiscussionThe experimental data presented above <strong>in</strong>dicatethat, on one hand, <strong>the</strong> H 2 O 2 decomposition <strong>by</strong> pyriteis <strong>in</strong>hibited <strong>by</strong> <strong>the</strong> presence <strong>of</strong> SSAL, EDTA, andphosphate and, on <strong>the</strong> o<strong>the</strong>r hand, <strong>the</strong> presence <strong>of</strong>Fig. 5– Experimental data <strong>of</strong> pyrite oxidation <strong>by</strong> H 2 O 2<strong>in</strong> acidic media (<strong>in</strong> <strong>the</strong> presence and absence <strong>of</strong> <strong>Fe</strong>(III)-ligands)plotted accord<strong>in</strong>g to eq. (5) (Shr<strong>in</strong>k<strong>in</strong>g core modelcontrolled <strong>by</strong> chemical reaction at <strong>the</strong> unreacted particle surface).