Minerals in the Economy of Alabama, 2007 - Geological Survey of ...
Minerals in the Economy of Alabama, 2007 - Geological Survey of ...
Minerals in the Economy of Alabama, 2007 - Geological Survey of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Cover photograph:View <strong>of</strong> large-scale crushed stone (limestone/dolomite) operation <strong>in</strong> eastern Tuscaloosa County,<strong>Alabama</strong> (courtesy Vulcan Materials Company). Crushed stone accounts for about 28 percent <strong>of</strong><strong>the</strong> total value <strong>of</strong> m<strong>in</strong>eral production <strong>in</strong> <strong>Alabama</strong>.
GEOLOGICAL SURVEY OF ALABAMABerry H. (Nick) Tew, Jr.State GeologistGEOLOGIC INVESTIGATIONS PROGRAMMINERALS IN THE ECONOMY OF ALABAMA, <strong>2007</strong>Information Series 64RbyLewis S. DeanTuscaloosa, <strong>Alabama</strong>2008
GEOLOGICAL SURVEY OF ALABAMABerry H. (Nick) Tew, Jr.State Geologist420 Hackberry LaneP.O. Box 869999Tuscaloosa, <strong>Alabama</strong> 35486-6999Phone (205)349-2852Fax (205)349-2861www.gsa.state.al.usAugust 20, 2008The Honorable Bob RileyGovernor <strong>of</strong> <strong>Alabama</strong>Montgomery, <strong>Alabama</strong>Dear Governor Riley:It is with pleasure that I make available to you this report entitled <strong>M<strong>in</strong>erals</strong> <strong>in</strong> <strong>the</strong><strong>Economy</strong> <strong>of</strong> <strong>Alabama</strong>, <strong>2007</strong>, by Lewis S. Dean, which has been published as InformationSeries 64R by <strong>the</strong> <strong>Geological</strong> <strong>Survey</strong> <strong>of</strong> <strong>Alabama</strong>.M<strong>in</strong>eral resources are <strong>the</strong> basis <strong>of</strong> an important part <strong>of</strong> <strong>Alabama</strong>'s <strong>in</strong>dustrial economy. In<strong>2007</strong> <strong>Alabama</strong> ranked sixteenth among <strong>the</strong> states <strong>in</strong> value <strong>of</strong> nonfuel m<strong>in</strong>erals produced. Theestimated value <strong>of</strong> <strong>in</strong>dustrial m<strong>in</strong>eral production <strong>in</strong> <strong>Alabama</strong> for <strong>2007</strong> was $1.34 billion,about 2 percent <strong>of</strong> <strong>the</strong> value <strong>of</strong> <strong>the</strong> total national m<strong>in</strong>eral production. The <strong>Geological</strong> <strong>Survey</strong><strong>of</strong> <strong>Alabama</strong> has legislative mandate to evaluate <strong>the</strong> state's m<strong>in</strong>eral resources and <strong>the</strong>reforecarries out a comprehensive m<strong>in</strong>erals assessment program for <strong>the</strong> state. Under this program,many reports and maps are published annually, provid<strong>in</strong>g citizens and potential m<strong>in</strong>eral<strong>in</strong>dustry with basic <strong>in</strong>formation useful for <strong>the</strong> development <strong>of</strong> <strong>the</strong>se resources.This summary report provides a brief overview <strong>of</strong> <strong>Alabama</strong>'s m<strong>in</strong>erals and related<strong>in</strong>dustry for <strong>2007</strong>.Respectfully,Berry H. (Nick) Tew, Jr.State GeologistScience and Service for <strong>the</strong> People <strong>of</strong> <strong>Alabama</strong>
CONTENTS—cont<strong>in</strong>uedPageZeolite m<strong>in</strong>erals................................................................................................................ 21Fur<strong>the</strong>r read<strong>in</strong>g ...................................................................................................................... 21References cited .................................................................................................................... 22ILLUSTRATIONSFigure 1. Value <strong>of</strong> <strong>in</strong>dustrial and construction m<strong>in</strong>eral production <strong>in</strong> <strong>Alabama</strong>, 1980-<strong>2007</strong> ........................................................................................................................ 22. Map <strong>of</strong> <strong>Alabama</strong> show<strong>in</strong>g physiographic prov<strong>in</strong>ces and counties ........................... 43. Example <strong>of</strong> a quadrangle series geologic map available for selected areasfrom <strong>the</strong> <strong>Geological</strong> <strong>Survey</strong> <strong>of</strong> <strong>Alabama</strong>.................................................................. 54. Varicolored clays and shales along <strong>the</strong> contact <strong>of</strong> <strong>the</strong> Coastal Pla<strong>in</strong> andAppalachian Plateaus, south <strong>of</strong> Hamilton, Marion County, <strong>Alabama</strong> ...................... 85. Generalized distribution <strong>of</strong> limestone, dolomite, and marble resources <strong>in</strong><strong>Alabama</strong> .................................................................................................................. 116. Quarry operation at Glenco, Etowah County, <strong>Alabama</strong>, for crushed limestone ...... 137. General distribution <strong>of</strong> sand and gravel resources <strong>in</strong> <strong>Alabama</strong>............................... 158. Quartz gravel aggregate.......................................................................................... 16TABLESTable 1. Production and number <strong>of</strong> employees <strong>in</strong> m<strong>in</strong><strong>in</strong>g <strong>of</strong> coal and <strong>in</strong>dustrial m<strong>in</strong>eralresources <strong>in</strong> <strong>Alabama</strong> for fiscal years 2005-<strong>2007</strong> ................................................... 32. Production <strong>of</strong> limestone/dolomite by county <strong>in</strong> <strong>Alabama</strong>, 2000-<strong>2007</strong>...................... 63. Production <strong>of</strong> sand and gravel by county <strong>in</strong> <strong>Alabama</strong>, 2000-<strong>2007</strong> .......................... 74. Production <strong>of</strong> selected clays <strong>in</strong> <strong>Alabama</strong>, 2000-<strong>2007</strong> ............................................. 9
4Figure 2.—Map <strong>of</strong> <strong>Alabama</strong> show<strong>in</strong>g physiographic prov<strong>in</strong>ces and counties.
5Figure 3.—Example <strong>of</strong> a quadrangle series geologic map available forselected areas from <strong>the</strong> <strong>Geological</strong> <strong>Survey</strong> <strong>of</strong> <strong>Alabama</strong>.commodity or a m<strong>in</strong>eral deposit. For example,some types <strong>of</strong> m<strong>in</strong>erals and natural resourceswith<strong>in</strong> <strong>the</strong> regional <strong>in</strong>fluence <strong>of</strong> <strong>the</strong>Tennessee-Tombigbee Waterway may<strong>in</strong>crease <strong>in</strong> value because <strong>of</strong> availability <strong>of</strong>lower cost transportation.Use <strong>of</strong> current and historical nonfuelm<strong>in</strong>eral production data can provide <strong>in</strong>sight<strong>in</strong>to m<strong>in</strong>eral exploration, consumption, andtrade for assess<strong>in</strong>g <strong>the</strong> economic andtechnical development <strong>of</strong> <strong>the</strong> state's m<strong>in</strong>eral<strong>in</strong>dustries. The earliest commercial operation
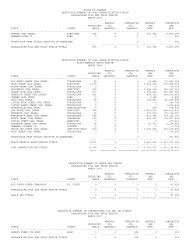
6was <strong>in</strong> 1780 for clay used <strong>in</strong> mak<strong>in</strong>g brick,tile, and pottery at Mobile. O<strong>the</strong>r earlycommercial production occurred <strong>in</strong> 1809 <strong>in</strong>Wash<strong>in</strong>gton County for salt, 1818 <strong>in</strong> Frankl<strong>in</strong>County for iron ore, 1827 <strong>in</strong> TuscaloosaCounty for sandstone (build<strong>in</strong>g stone), 1831<strong>in</strong> Chilton County for gold, 1838 <strong>in</strong> TalladegaCounty for marble, and 1840 for lime andlimestone. M<strong>in</strong>eral production was firstrecorded <strong>in</strong> <strong>Alabama</strong> <strong>in</strong> 1840 for clay (brickand ear<strong>the</strong>nware), stone (limestone andmarble), iron, and gold. S<strong>in</strong>ce <strong>the</strong> 1840s,limestone and dolomite (and associatedmanufacture <strong>of</strong> lime), sand and gravel, ironore (up to 1975), clay, marble, bauxite, andsandstone were <strong>the</strong> pr<strong>in</strong>cipal m<strong>in</strong>eralsproduced <strong>in</strong> <strong>Alabama</strong> (Dean, 2000).From 2000-<strong>2007</strong>, more limestone anddolomite were produced than any o<strong>the</strong>rm<strong>in</strong>erals <strong>in</strong> <strong>Alabama</strong>. The major productionareas are as follows: Shelby County (40percent) and Jefferson County (15 percent)from <strong>the</strong> Cambrian Conasauga Formationand Ketona Dolomite and <strong>the</strong> OrdovicianNewala Limestone; and Madison County (6percent), Colbert County (9 percent), andMorgan County (6 percent) from <strong>the</strong>Mississippian Bangor and TuscumbiaLimestones (table 2).Sand and gravel production from 2000-<strong>2007</strong> has come primarily from Quaternaryalluvium and terrace deposits <strong>in</strong> MontgomeryCounty (14 percent), Mobile County (10percent), Elmore County (8 percent), MaconCounty (5 percent), Tuscaloosa County (2percent), and Russell County (5 percent), andfrom <strong>the</strong> Citronelle Formation <strong>in</strong> MobileCounty (7 percent) (table 3). Clay productionhas consisted <strong>of</strong> brick and common clay (58percent), fuller's earth (20 percent), shale (12percent), kaol<strong>in</strong> (4 percent), bentonite (3percent), and bauxite (3 percent).Currently, o<strong>the</strong>r significant productionconsists <strong>of</strong> lime, cement, salt, build<strong>in</strong>g stone,syn<strong>the</strong>tic gypsum, gemstones, iron oxidepigments, and recovered sulfur (U.S.<strong>Geological</strong> <strong>Survey</strong>, <strong>2007</strong>a). These productiontrends are expected to cont<strong>in</strong>ue <strong>in</strong> 2008. Thefollow<strong>in</strong>g <strong>in</strong>formation provides details <strong>of</strong> <strong>the</strong>occurrence, m<strong>in</strong><strong>in</strong>g history, and generaleconomics <strong>of</strong> specific m<strong>in</strong>eral resources <strong>in</strong><strong>Alabama</strong>.BAUXITEBauxite is a mixture <strong>of</strong> hydrated alum<strong>in</strong>umoxide m<strong>in</strong>erals (pr<strong>in</strong>cipally gibbsite) andm<strong>in</strong>eral impurities formed by <strong>the</strong> wea<strong>the</strong>r<strong>in</strong>g<strong>of</strong> alum<strong>in</strong>um-bear<strong>in</strong>g rocks. Bauxite was firstm<strong>in</strong>ed <strong>in</strong> <strong>Alabama</strong> <strong>in</strong> 1891 from Rock Run <strong>in</strong>Cherokee County. In <strong>the</strong> 1940s, ore fromCalhoun, Cherokee, St. Clair, and ColbertCounties was m<strong>in</strong>ed and shipped to ReynoldsMetals Company at Muscle Shoals forexperimental production <strong>of</strong> alum<strong>in</strong>um. M<strong>in</strong><strong>in</strong>gceased when sufficient tonnage <strong>of</strong>metallurgical-grade bauxite became difficultto obta<strong>in</strong>.Table 2.--Production (short tons) <strong>of</strong> limestone/dolomite by county <strong>in</strong> <strong>Alabama</strong>, 2000-<strong>2007</strong>Data Sources: Modified from <strong>Alabama</strong> Department <strong>of</strong> Industrial Relations,Office M<strong>in</strong>e <strong>of</strong> Safety and Inspection (2000-<strong>2007</strong>).Year Shelby* Jefferson* Madison Colbert Morgan O<strong>the</strong>r*<strong>2007</strong> 21,979,261 7,023,167 1,183,647 5,074,319 2,905,582 15,245,2942006 20,486,051 6,905,091 3,992,785 5,123,983 2,229,948 17,212,9062005 19,377,987 7,289,966 3,522,679 4,915,032 2,234,856 13,828,0442004 19,695,015 7,658,607 3,546,982 4,787,994 2,258,550 13,698,5552003 19,866,357 7,632,369 2,766,845 4,138,349 2,109,151 12,194,9752002 21,378,773 7,390,626 3,020,816 3,153,257 1,888,056 10,474,2472001 15,792,431 6,816,500 3,329,155 3,724,577 1,533,222 10,440,6872000 14,840,589 7,136,408 2,416,303 3,148,602 2,180,702 8,390,506*Includes dolomite.
7Table 3.--Production (short tons) <strong>of</strong> sand and gravel by county <strong>in</strong> <strong>Alabama</strong>, 2000-<strong>2007</strong>Data Sources: <strong>Alabama</strong> Department <strong>of</strong> Industrial Relations,Office <strong>of</strong> M<strong>in</strong>e Safety and Inspection (2000-<strong>2007</strong>).Year Montgomery Mobile Elmore Macon Tuscaloosa Russell O<strong>the</strong>r<strong>2007</strong> 2,801,508 3,003,018 1,635,418 1,759,317 399,532 1,032,752 13,541,7502006 3,362,795 3,798,101 1,082,178 1,887,648 261,692 1,236,427 12,289,4632005 2,663,316 979,200 1,087,381 339,216 209,144 930,013 10,009,2372004 3,038,461 1,253,086 1,014,854 302,184 214,052 360,000 8,227,2192003 2,551,345 1,266,537 1,084,240 109,351 198,371 240,000 7,870,5412002 2,202,033 1,474,034 1,500,223 220,000 243,456 935,000 6,519,2882001 406,530 506,000 1,261,148 400,000 261,083 1,000,000 4,453,1822000 653,314 294,696 1,107,166 543,495 235,000 900,000 5,273,506The major economic bauxite deposits <strong>in</strong><strong>Alabama</strong> occur <strong>in</strong> <strong>the</strong> Barbour-HenryCounties (Eufaula) bauxite district with<strong>in</strong>kaol<strong>in</strong>itic clay deposits, which are overla<strong>in</strong> bysands and clays <strong>of</strong> <strong>the</strong> Nanafalia Formation.Production began <strong>in</strong> 1927 and has beenma<strong>in</strong>ta<strong>in</strong>ed on a cont<strong>in</strong>uous basis. Because<strong>of</strong> <strong>the</strong> irregular distribution <strong>of</strong> <strong>the</strong> bauxitedeposits, considerable exploratory test drill<strong>in</strong>gmust be done prior to m<strong>in</strong><strong>in</strong>g (Clarke, 1973).<strong>Alabama</strong> bauxite from <strong>the</strong> Eufaula district isused <strong>in</strong> mak<strong>in</strong>g high-temperature refractoryproducts, abrasives, and chemicals.BUILDING STONEMany stone materials <strong>in</strong> <strong>the</strong> state,<strong>in</strong>clud<strong>in</strong>g sandstone, limestone, granite,quartzite, and marble, have been used forbuild<strong>in</strong>g construction or ornamental stone.Annual production <strong>in</strong> <strong>Alabama</strong> for dimensionstone is about 4,430 metric tons valued atabout $3.63 million (U.S. <strong>Geological</strong> <strong>Survey</strong>,<strong>2007</strong>b).Naturally dimensioned blocks and flags <strong>of</strong>sandstone and limestone have been widelyused <strong>in</strong> north <strong>Alabama</strong>. A unique tan- to buffcoloredsandstone (Pottsville Formation)characterized by small-scale sedimentarystructures is produced from Blount andCherokee Counties for use as exteriorbuild<strong>in</strong>g stone, walkways, and patios(Fousek, 2002). Limestone (BangorLimestone) quarried at Rockwood <strong>in</strong> Frankl<strong>in</strong>County also has been widely used. Whitemarble quarried at Sylacauga has been usedthroughout <strong>the</strong> United States for monumentsand for exterior and <strong>in</strong>terior build<strong>in</strong>g stone(see Marble). A recently developed build<strong>in</strong>gstone operation <strong>in</strong> Lauderdale County was<strong>the</strong> first operation <strong>in</strong> <strong>Alabama</strong> to marketvaricolored "marble" (polished limestone) <strong>of</strong>Silurian age. In south <strong>Alabama</strong>, s<strong>of</strong>tlimestone <strong>of</strong> <strong>the</strong> Clayton Formation, MariannaLimestone, and Salt Mounta<strong>in</strong> Limestone wassaw-quarried <strong>in</strong> <strong>the</strong> 1800s and early 1900sfor build<strong>in</strong>g stone.CEMENTThe cement-manufactur<strong>in</strong>g <strong>in</strong>dustry is amajor consumer <strong>of</strong> raw materials <strong>in</strong> <strong>the</strong> state.This <strong>in</strong>dustry began <strong>in</strong> <strong>Alabama</strong> <strong>in</strong> 1895 andhas s<strong>in</strong>ce played a key role <strong>in</strong> <strong>the</strong>development <strong>of</strong> <strong>the</strong> state. Primary uses arefor ready-mix concrete, concrete products,build<strong>in</strong>g materials, and highway construction.The pr<strong>in</strong>cipal raw materials used <strong>in</strong> mak<strong>in</strong>gcement are limestone or chalk, clay andshale, silica, and iron oxide. Annual cementproduction <strong>in</strong> <strong>Alabama</strong> has <strong>in</strong>creased to 5.1million metric tons <strong>of</strong> portland cement and475,000 metric tons <strong>of</strong> masonry cement.Annual production value <strong>of</strong> cement <strong>in</strong><strong>Alabama</strong> is about $475.8 million (U.S.<strong>Geological</strong> <strong>Survey</strong>, <strong>2007</strong>a).CHERTChert is a siliceous stone <strong>of</strong>tenassociated with wea<strong>the</strong>red limestone or
8dolomite. It is a low value m<strong>in</strong>eral commodity,but where a need exists for fill or basematerial for roads, chert deposits are <strong>of</strong>economic value. Chert quarries have beendeveloped <strong>in</strong> most counties <strong>in</strong> north <strong>Alabama</strong>where <strong>the</strong> Fort Payne Chert and Knox Groupresiduum occur.CLAY AND SHALEClay and shale <strong>of</strong> variable compositionand character occur throughout <strong>the</strong> state (fig.4). Bentonite, kaol<strong>in</strong>, fuller's earth, fireclay,common clays, and shale are m<strong>in</strong>ed <strong>in</strong> <strong>the</strong>state and used for mak<strong>in</strong>g brick, tile,refractory and ceramic products, clay pipe,pottery, and lightweight aggregate (table 4).Brick plants are located <strong>in</strong> Bessemer, PhenixCity, Montgomery, Leeds, Ragland,Huntsville, Selma, and Coosada. Tile plantsare located <strong>in</strong> Fayette and Florence. Theannual value <strong>of</strong> clays produced <strong>in</strong> <strong>the</strong> state isabout $29 million (U.S. <strong>Geological</strong> <strong>Survey</strong>,<strong>2007</strong>a). Potential additional growth can beanticipated <strong>in</strong> <strong>Alabama</strong> because <strong>of</strong> abundantclay and shale resources <strong>in</strong> <strong>the</strong> state.BENTONITEBentonite (smectite clays), a wea<strong>the</strong>r<strong>in</strong>gproduct <strong>of</strong> volcanic ash, occurs near <strong>the</strong> base<strong>of</strong> <strong>the</strong> Ripley Formation at Sandy Ridge <strong>in</strong>sou<strong>the</strong>astern Lowndes County. Nonswell<strong>in</strong>gcalcium bentonite is m<strong>in</strong>ed from Sandy Ridgeand processed for use <strong>in</strong> agricultural<strong>in</strong>dustries and as a b<strong>in</strong>der <strong>in</strong> foundry sand.S<strong>in</strong>ce m<strong>in</strong><strong>in</strong>g began <strong>in</strong> 1964, about 5.3 millionshort tons <strong>of</strong> bentonite has been produced.The Lowndes County bentonite has highplasticity and a very high level<strong>in</strong>g strength.S<strong>in</strong>ce 1980, an average <strong>of</strong> 183,000 short tons<strong>of</strong> bentonite has been produced annually,represent<strong>in</strong>g <strong>the</strong> only bentonite productionfrom <strong>the</strong> Selma Group <strong>in</strong> <strong>the</strong> sou<strong>the</strong>astCoastal Pla<strong>in</strong> Prov<strong>in</strong>ce (Hosterman, 1984;Dean, 2000). Annual production value <strong>of</strong>bentonite is about $3.48 million (U.S.<strong>Geological</strong> <strong>Survey</strong>, <strong>2007</strong>a). Bentonite alsohas been reported <strong>in</strong> Cretaceous deposits <strong>of</strong>Montgomery County and from <strong>the</strong> Tertiary <strong>in</strong>Clarke County.Figure 4.— Varicolored clays and shales along <strong>the</strong> contact <strong>of</strong> <strong>the</strong> Coastal Pla<strong>in</strong>and Appalachian Plateaus, south <strong>of</strong> Hamilton, Marion County, <strong>Alabama</strong>.
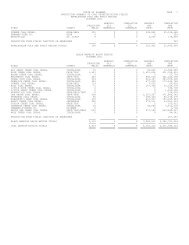
9Table 4.--Production (short tons) <strong>of</strong> selected clays <strong>in</strong> <strong>Alabama</strong>, 2000-<strong>2007</strong>Data Sources: Modified from <strong>Alabama</strong> Department <strong>of</strong> Industrial Relations,Office <strong>of</strong> Safety and Inspection (2000-<strong>2007</strong>).Year Brick clay Fuller’s earth Common clay Bauxite Kaol<strong>in</strong>* Bentonite<strong>2007</strong> 1,159,832 782,952 1,095,810 141,914 167,585 110,6252006 1,003,520 835,419 735,476 397,986 185,679 113,4462005 746,925 851,817 302,507 133,586 185,427 124,3282004 766,735 946,696 179,263 286,458 189,943 208,5802003 896,801 775,157 151,082 150,442 210,673 243,4612002 773,669 702,246 30,144 277,378 197,969 143,5622001 850,327 883,044 24,900 273,002 195,388 254,5062000 930,423 880,400 63,070 281,852 195,388 290,801*St. Clair County.FIRECLAYFireclay is a siliceous clay rich <strong>in</strong> hydrousalum<strong>in</strong>um silicates, useful for <strong>the</strong>manufacture <strong>of</strong> refractory ceramic productssuch as crucibles and firebrick. Many <strong>of</strong> <strong>the</strong>clays that occur below coal seams(underclays) <strong>of</strong> <strong>the</strong> Pottsville Formation <strong>in</strong><strong>Alabama</strong> commonly have refractorycharacteristics. These underclays aredistributed throughout <strong>the</strong> coal-produc<strong>in</strong>gareas <strong>in</strong> north-central <strong>Alabama</strong>—Blount,Fayette, Jefferson, Marion, Tuscaloosa, andWalker Counties—and can be m<strong>in</strong>ed <strong>in</strong>conjunction with coal. The clay that occursbelow <strong>the</strong> Mary Lee coal group <strong>in</strong> <strong>the</strong>Cordova district <strong>of</strong> Walker County is used <strong>in</strong>mak<strong>in</strong>g structural products, such as brick andtile. S<strong>in</strong>ce 1925, an estimated 6 million shorttons <strong>of</strong> fireclay has been m<strong>in</strong>ed from WalkerCounty with an average annual productions<strong>in</strong>ce 1980 <strong>of</strong> 90,980 short tons, represent<strong>in</strong>gabout 38 percent <strong>of</strong> <strong>the</strong> state's refractory clayproduction (Dean, 2000). Siliceous fireclayhas also been m<strong>in</strong>ed <strong>in</strong> Calhoun and ShelbyCounties.KAOLINKaol<strong>in</strong>, popularly known as ch<strong>in</strong>a clay, is<strong>the</strong> best known <strong>of</strong> <strong>the</strong> pottery clays. It iscomposed pr<strong>in</strong>cipally <strong>of</strong> <strong>the</strong> m<strong>in</strong>eral kaol<strong>in</strong>ite(hydrous alum<strong>in</strong>um silicate) and has beenproduced from deposits <strong>in</strong> <strong>the</strong> Coastal Pla<strong>in</strong>Prov<strong>in</strong>ce for more than a century. Economicdeposits are associated with bauxite <strong>in</strong> <strong>the</strong>Eufaula district <strong>in</strong> Barbour and HenryCounties (Clarke, 1973). Kaol<strong>in</strong> productionfrom <strong>the</strong> Eufaula district began <strong>in</strong> 1958. S<strong>in</strong>ce1961, about 1.8 million short tons <strong>of</strong> kaol<strong>in</strong>has been m<strong>in</strong>ed from <strong>the</strong> Eufaula district foran average annual production s<strong>in</strong>ce 1980 <strong>of</strong>about 55,500 short tons. Kaol<strong>in</strong> was alsom<strong>in</strong>ed <strong>in</strong> Marion County from 1939 to 1977,with an estimated 666,000 short tons m<strong>in</strong>edfrom 1942 to 1977 (Dean, 2000). This kaol<strong>in</strong>was used <strong>in</strong> floor and wall tiles, firebrick, andas a filler <strong>in</strong> paper and fertilizer. Kaol<strong>in</strong> is alsom<strong>in</strong>ed <strong>in</strong> St. Clair County for use <strong>in</strong>manufactur<strong>in</strong>g cement.FULLER'S EARTHClay suitable for <strong>the</strong> manufacture <strong>of</strong>lightweight aggregate occurs <strong>in</strong> a zone thatextends from western Wilcox County throughMarengo County and across sou<strong>the</strong>rn SumterCounty (Clarke and Tyrrell, 1976). Fuller'searth <strong>in</strong> <strong>the</strong> Porters Creek Formation is m<strong>in</strong>ednear Liv<strong>in</strong>gston <strong>in</strong> Sumter County forproduction <strong>of</strong> lightweight aggregate.Lightweight aggregate is made from clay orshale conta<strong>in</strong><strong>in</strong>g carbonaceous or calcareousmaterial. Heat<strong>in</strong>g it to fusion temperatureproduces carbon dioxide gas, which shapesvesicles. Upon cool<strong>in</strong>g, <strong>the</strong> expanded productis full <strong>of</strong> cavities. This material can be usedfor low density, or lightweight, aggregate <strong>in</strong>mak<strong>in</strong>g concrete blocks. It is also used to
10make nonskid pavement and <strong>in</strong> horticulturalapplications (Hosterman and Patterson,1992).CRUSHED STONERock types <strong>in</strong> <strong>Alabama</strong> with goodphysical characteristics (loose unit mass, bulkspecific gravity, absorption and abrasionvalues) for crushed stone <strong>in</strong>clude limestone,dolomite, marble, granite, sandstone, andquartzite. The pr<strong>in</strong>cipal uses are <strong>in</strong>construction for aggregate, agricultural uses,chemical and metallurgical uses (cement andlime manufacture, flux stone), and o<strong>the</strong>rspecial uses such as water treatment, m<strong>in</strong>edust<strong>in</strong>g, and fillers and extenders. The annualproduction <strong>of</strong> f<strong>in</strong>e to coarse constructionaggregate sold or used by <strong>Alabama</strong>producers amounts to about 26.76 millionmetric tons with a value <strong>of</strong> $175 million (unitvalue <strong>of</strong> $6.54 per metric ton). The totalamount <strong>of</strong> all crushed stone produced <strong>in</strong> <strong>the</strong>state <strong>in</strong> <strong>2007</strong> was 51.7 million metric tonsvalued at $375 million, account<strong>in</strong>g for about28 percent <strong>of</strong> <strong>the</strong> total value <strong>of</strong> m<strong>in</strong>eralproduction (U.S. <strong>Geological</strong> <strong>Survey</strong>, <strong>2007</strong>a,2008b).DOLOMITEDolomite, a carbonate rock that consistspr<strong>in</strong>cipally <strong>of</strong> calcium magnesium carbonate,occurs extensively <strong>in</strong> nor<strong>the</strong>astern <strong>Alabama</strong>(fig. 5). Dolomite is used to manufacture rockwool (used for <strong>in</strong>sulation), <strong>in</strong> <strong>the</strong> chemical<strong>in</strong>dustry to extract metallic magnesium, <strong>in</strong>agricultural applications to condition soil, asflux, and as an alternative to limestone <strong>in</strong> <strong>the</strong>production <strong>of</strong> aggregate. The unit value <strong>of</strong>crushed dolomite is about $6.37 per metricton (U.S. <strong>Geological</strong> <strong>Survey</strong>, <strong>2007</strong>a). Most <strong>of</strong><strong>the</strong> dolomite rock units <strong>in</strong> <strong>Alabama</strong> (forexample, <strong>the</strong> Knox Group dolomites) have ahigh content <strong>of</strong> silica and o<strong>the</strong>r impurities,which generally preclude commercialdevelopment for most applications. TheCambrian Ketona Dolomite, however, isknown for its purity and is quarried <strong>in</strong>Jefferson and Shelby Counties for a variety <strong>of</strong>applications. The Ketona Dolomite was m<strong>in</strong>edfrom <strong>the</strong> Dolonah quarry at Bessemer from1924 to 1981 for flux<strong>in</strong>g material and was <strong>the</strong>first large-scale <strong>in</strong>dustrial quarry, produc<strong>in</strong>g1.1 million short tons <strong>of</strong> dolomite annually asearly as 1942. The Ketona Dolomite ispresently m<strong>in</strong>ed <strong>in</strong> <strong>the</strong> Dolcito quarry <strong>in</strong>Birm<strong>in</strong>gham, which has been operat<strong>in</strong>g s<strong>in</strong>ce<strong>the</strong> early 1900s. Production <strong>of</strong> dolomite from<strong>the</strong> Dolcito quarry exceeds 1 million shorttons annually (<strong>Alabama</strong> Department <strong>of</strong>Industrial Relations, <strong>2007</strong>). Beg (1984)reported on <strong>the</strong> distribution andcharacteristics <strong>of</strong> dolomites <strong>in</strong> <strong>Alabama</strong>.GEMSTONES AND OTHER MINERALSA variety <strong>of</strong> gemstones and o<strong>the</strong>rm<strong>in</strong>erals which are <strong>of</strong> value to collectors <strong>of</strong>m<strong>in</strong>eral specimens occur <strong>in</strong> <strong>Alabama</strong>. Thesem<strong>in</strong>erals <strong>in</strong>clude amethyst, beryl (emerald),calcite, fluorite, gypsum, magnetite, quartz(agate), rutile, tourmal<strong>in</strong>e, turquoise, andwavellite (Cook and Smith, 1982). Also,freshwater mussel shells are exported for use<strong>in</strong> <strong>the</strong> production <strong>of</strong> cultured pearls. Sectionsfrom a mussel shell are cut out, partitioned,rounded, polished, and <strong>in</strong>serted <strong>in</strong>to oystersas <strong>the</strong> pearl nuclei. After a period <strong>of</strong> time, <strong>the</strong>cultured pearl is removed from <strong>the</strong> oyster andused <strong>in</strong> jewelry production. The Tennesseeand <strong>Alabama</strong> River systems are currently <strong>the</strong>most important sources <strong>of</strong> commercialmussels (Outdoor <strong>Alabama</strong>, 2008). Theyearly value <strong>of</strong> exported shell is about$370,000 (U.S. <strong>Geological</strong> <strong>Survey</strong>, <strong>2007</strong>a).In 1990 “star blue quartz” was designatedas <strong>the</strong> <strong>of</strong>ficial gemstone for <strong>Alabama</strong>. Thisgemstone is asteriated (rutilated) light-bluegrayquartz from Chambers County <strong>in</strong> <strong>the</strong>sou<strong>the</strong>rn Piedmont. It exhibits a bluish castwhen viewed <strong>in</strong> direct sunlight. Facetedmaterial exhibits a strong, central, six-rayedstar. The raw material consists <strong>of</strong> residualve<strong>in</strong> quartz found <strong>in</strong> a red clay soil pr<strong>of</strong>ile(Rohrbach, 1989).GRANITEGranite is a plutonic rock composedprimarily <strong>of</strong> <strong>the</strong> m<strong>in</strong>erals feldspar, quartz, andmica. Rocks with granitic m<strong>in</strong>eral compositionoccur throughout <strong>the</strong> <strong>Alabama</strong> Piedmont.These granitic rocks commonly have a
Figure 5.— Generalized distribution <strong>of</strong> limestone, dolomite, and marble resources <strong>in</strong> <strong>Alabama</strong>.11
12banded texture and are termed "granitegneiss." Such stone has been quarried foruse as dimension stone and crushed stone.Granite has been quarried for crushed stoneon an <strong>in</strong>termittent basis s<strong>in</strong>ce 1936. Granitegneiss <strong>of</strong> <strong>the</strong> Elkahatchee Quartz DioriteGneiss is presently quarried <strong>in</strong> Coosa Countyfor use as crushed stone, riprap, andaggregate. Granite is also quarried <strong>in</strong>Randolph County and was used extensively<strong>in</strong> construction <strong>of</strong> <strong>the</strong> R.L. Harris hydroelectricdam on <strong>the</strong> Tallapoosa River. O<strong>the</strong>r granitequarries operate <strong>in</strong> Lee and Elmore Counties.Granite production currently exceeds 1 millionshort tons annually (table 1). The unit value <strong>of</strong>crushed granite is about $6.88 per metric ton(U.S. <strong>Geological</strong> <strong>Survey</strong>, <strong>2007</strong>a).LIMELime is pr<strong>in</strong>cipally calcium oxide (CaO) ora mixture <strong>of</strong> CaO and magnesium oxide(MgO) produced by <strong>the</strong> calc<strong>in</strong><strong>in</strong>g <strong>of</strong> limestoneor dolomite. Large-scale lime production by<strong>the</strong> Shelby Lime Company began <strong>in</strong> <strong>the</strong>1850s near Montevallo. The major uses <strong>of</strong>lime <strong>in</strong>clude steel mak<strong>in</strong>g, papermanufacture, water purification, andagricultural soil condition<strong>in</strong>g. In <strong>Alabama</strong>, <strong>the</strong>Ordovician Newala Limestone is <strong>the</strong> primarysource <strong>of</strong> raw material for <strong>the</strong> production <strong>of</strong>lime (Puckett and o<strong>the</strong>rs, 1990). Annualproduction <strong>in</strong> <strong>Alabama</strong> is about 2.24 millionmetric tons <strong>of</strong> lime valued at about $181million (U.S. <strong>Geological</strong> <strong>Survey</strong>, <strong>2007</strong>a).LIMESTONELimestone suitable for crushed stone andconsist<strong>in</strong>g pr<strong>in</strong>cipally <strong>of</strong> calcium carbonate iswidely distributed <strong>in</strong> <strong>Alabama</strong> (fig. 5). Mostlimestone quarries <strong>in</strong> <strong>the</strong> state are open pits(fig. 6), but underground m<strong>in</strong><strong>in</strong>g has beenused <strong>in</strong> Frankl<strong>in</strong>, Jefferson, Marshall, andShelby Counties. The limestone units <strong>of</strong><strong>Alabama</strong> have a wide variety <strong>of</strong> chemical andphysical properties and are suitable forvarious applications (Beg, 1984). Limestone<strong>of</strong> vary<strong>in</strong>g grade is used for flux, aggregate,dimension stone, and soil conditioner, and <strong>in</strong>many <strong>in</strong>dustrial processes <strong>in</strong>clud<strong>in</strong>g <strong>the</strong>manufacture <strong>of</strong> glass, refractories, fillers,abrasives, cement, lime, and chemicals. TheMississippian Bangor and TuscumbiaLimestones are quarried throughout north<strong>Alabama</strong>. In Jefferson and Shelby Counties,<strong>the</strong> Cambrian Conasauga Formation andOrdovician Newala Limestone are extensivelyquarried (Rheams, 1992). Limestone is <strong>the</strong>lead<strong>in</strong>g crushed stone produced <strong>in</strong> <strong>Alabama</strong>with an annual value <strong>of</strong> about $269 millionand a unit value <strong>of</strong> $6.56 per metric ton (U.S.<strong>Geological</strong> <strong>Survey</strong>, <strong>2007</strong>a). Shelby County is<strong>the</strong> state's lead<strong>in</strong>g limestone-produc<strong>in</strong>g areawith an average annual production s<strong>in</strong>ce2000 <strong>of</strong> 19.2 million short tons, represent<strong>in</strong>groughly 40 percent <strong>of</strong> <strong>Alabama</strong>'s entireproduction (table 2).Chalk is a s<strong>of</strong>t f<strong>in</strong>e-textured variety <strong>of</strong>limestone that occurs <strong>in</strong> <strong>the</strong> Coastal Pla<strong>in</strong>Prov<strong>in</strong>ce <strong>of</strong> west-central <strong>Alabama</strong> (figs. 2, 5).The Demopolis Chalk <strong>of</strong> <strong>the</strong> Selma Grouphas been m<strong>in</strong>ed for use <strong>in</strong> <strong>the</strong> manufacture <strong>of</strong>portland cement and agricultural soilconditioner s<strong>in</strong>ce 1900 from <strong>the</strong> Spocariquarry near Demopolis. Approximately 29million short tons <strong>of</strong> chalk has been m<strong>in</strong>edfrom <strong>the</strong> Spocari quarry s<strong>in</strong>ce 1942. S<strong>in</strong>ce1980, this quarry has produced 1.2 millionshort tons <strong>of</strong> chalk annually. At St. Stephens<strong>in</strong> Wash<strong>in</strong>gton County, <strong>the</strong> Tertiary MariannaLimestone was m<strong>in</strong>ed from 1927 to 1980 foruse <strong>in</strong> cement. About 26 million short tons <strong>of</strong>limestone was m<strong>in</strong>ed from 1942 to 1980(Dean, 2000).MARBLEIn <strong>Alabama</strong> <strong>the</strong> major source <strong>of</strong> marble—<strong>the</strong> metamorphosed equivalent <strong>of</strong> limestoneand dolomite—is <strong>in</strong> Talladega County (fig. 5),where it occurs <strong>in</strong> a narrow outcrop belt from<strong>the</strong> Coosa River to sou<strong>the</strong>ast <strong>of</strong> Talladega(Guthrie, 1989). This area is known as <strong>the</strong>Sylacauga Marble belt, named for exposuresnear Sylacauga, where marble has beenquarried for more than 160 years. In <strong>the</strong>Sylacauga area, marble known for its highgradecrystall<strong>in</strong>e texture, whiteness, andbeauty has been extensively quarried with anestimated total production <strong>of</strong> 42 million shorttons (Dean, 2000). In 1969, SylacaugaMarble was designated as <strong>the</strong> <strong>of</strong>ficial rock <strong>of</strong>
13Figure 6.—Quarry operation at Glenco, Etowah County, <strong>Alabama</strong>, for crushed limestone(photo courtesy <strong>of</strong> Vulcan Materials Company).<strong>Alabama</strong>. Although marble quarried <strong>in</strong> earlyoperations was used primarily for monumentsand for decorative purposes, today'soperations produce predom<strong>in</strong>antly crushedmarble. Crushed marble is used <strong>in</strong> a number<strong>of</strong> diversified applications <strong>in</strong>clud<strong>in</strong>g <strong>in</strong>dustrialfillers, fertilizer, aggregate, and micronizedmarble, which is shipped as a slurry for use <strong>in</strong>paper pigment and coat<strong>in</strong>g. In <strong>2007</strong> marbleproduction was 4.4 million short tons (table1). Marble production has a unit value <strong>of</strong>$6.62 per metric ton (U.S. <strong>Geological</strong> <strong>Survey</strong>,<strong>2007</strong>a).In addition to <strong>the</strong> Sylacauga Marble, <strong>the</strong>dolomitic Chewacla Marble crops out <strong>in</strong> anumber <strong>of</strong> large isolated areas <strong>in</strong> Lee Countyand has been quarried for aggregate nearAuburn (Fousek, <strong>2007</strong>). Dolomitic marble wasquarried as early as 1849 south <strong>of</strong> Auburnand used <strong>in</strong> lime production. Nearly 35 millionshort tons <strong>of</strong> Chewacla Marble has beenquarried s<strong>in</strong>ce 1958, when large-scale m<strong>in</strong><strong>in</strong>gfor crushed stone began (Dean, 2000).MICAMuscovite mica (hydrous potassiumalum<strong>in</strong>um silicate) is found as sheets or"books" associated with granitic pegmatitesoccurr<strong>in</strong>g as dikes and sills with<strong>in</strong> <strong>the</strong>metamorphic and igneous rocks <strong>of</strong> <strong>the</strong><strong>Alabama</strong> Piedmont. Mica-bear<strong>in</strong>g pegmatiteshave been m<strong>in</strong>ed extensively <strong>in</strong> five majorareas: <strong>the</strong> P<strong>in</strong>etucky area, Randolph andCleburne Counties; <strong>the</strong> Pyriton area, westernClay County; <strong>the</strong> L<strong>in</strong>eville area, eastern ClayCounty; <strong>the</strong> Rockford area, Coosa County;and <strong>the</strong> Dadeville area, Tallapoosa County.Large concentrations <strong>of</strong> flake mica also occuras f<strong>in</strong>ely dissem<strong>in</strong>ated flakes <strong>in</strong> mica schists<strong>of</strong> <strong>the</strong> <strong>Alabama</strong> Piedmont (Epperson andRheams, 1984).Sheet mica is used primarily <strong>in</strong> <strong>the</strong>electrical <strong>in</strong>dustry as <strong>in</strong>sulation <strong>in</strong> a variety <strong>of</strong>electrical equipment. Scrap or flake mica isused by <strong>in</strong>dustry <strong>in</strong> dry-, wet-, or micronizedgroundform. Major uses <strong>of</strong> dry-ground micaare <strong>in</strong> drill<strong>in</strong>g muds for <strong>the</strong> petroleum <strong>in</strong>dustry,
14<strong>in</strong> <strong>the</strong> manufacture <strong>of</strong> asphalt ro<strong>of</strong><strong>in</strong>gmaterial, and as a filler-extender <strong>in</strong> gypsumplasterboard. Wet-ground mica is usedpr<strong>in</strong>cipally by <strong>the</strong> pa<strong>in</strong>t <strong>in</strong>dustry as aspecialized pigment extender. Micronizedgroundmica is used similarly as a fillerextender<strong>in</strong> pa<strong>in</strong>ts and plastics, and as adust<strong>in</strong>g agent <strong>in</strong> <strong>the</strong> manufacture <strong>of</strong> rubberproducts. A new mica recovery operationcont<strong>in</strong>ues to be active at Micaville <strong>in</strong> CleburneCounty. Scrap or flake mica produced atMicaville is used by <strong>in</strong>dustry <strong>in</strong> dry-, wet-, ormicronized-ground form.OCHEROcher is <strong>the</strong> clayey and unconsolidatedform <strong>of</strong> <strong>the</strong> m<strong>in</strong>erals limonite and hematite,with various impurities. Ocher <strong>in</strong> <strong>Alabama</strong>occurs <strong>in</strong> sedimentary and residual deposits.The sedimentary deposits are found <strong>in</strong> <strong>the</strong>Coastal Pla<strong>in</strong> Prov<strong>in</strong>ce <strong>in</strong> Autauga, Barbour,Clarke, Monroe, and Tuscaloosa Counties.Residual ocher occurs <strong>in</strong> <strong>the</strong> Valley andRidge Prov<strong>in</strong>ce and Piedmont Prov<strong>in</strong>ce <strong>in</strong>Calhoun, Cherokee, Elmore, Shelby, St.Clair, and Talladega Counties. Hematite usedas iron oxide pigment is m<strong>in</strong>ed <strong>in</strong> easternTuscaloosa County.QUARTZITEQuartzite (recrystallized or silicacementedsandstone) has been quarried atseveral sites <strong>in</strong> east <strong>Alabama</strong> and used forbuild<strong>in</strong>g stone, riprap, road metal, and <strong>in</strong> <strong>the</strong>manufacture <strong>of</strong> silica brick. The CheahaQuartzite Member along <strong>the</strong> crest <strong>of</strong>Talladega Mounta<strong>in</strong> <strong>in</strong> <strong>the</strong> <strong>Alabama</strong> Piedmonthas been quarried <strong>in</strong> Clay County. Quartzite<strong>in</strong> <strong>the</strong> Weisner Formation has been quarried<strong>in</strong> Cherokee and Calhoun Counties. TheHollis Quartzite <strong>in</strong> Lee County is presentlyused as road metal and as a filler <strong>in</strong>manufactur<strong>in</strong>g brick. Production <strong>in</strong> <strong>2007</strong> was59,000 short tons (<strong>Alabama</strong> Department <strong>of</strong>Industrial Relations, <strong>2007</strong>).SALTSalt deposits underlie much <strong>of</strong> southwest<strong>Alabama</strong> at depths from 400 feet to greaterthan 18,000 feet. Salt seeps <strong>in</strong> Wash<strong>in</strong>gtonand Clarke Counties were used to producesalt <strong>in</strong>termittently dur<strong>in</strong>g <strong>the</strong> 1800s. Largescaleproduction <strong>of</strong> salt br<strong>in</strong>e began <strong>in</strong> 1952to supply a chlor<strong>in</strong>e and caustic soda plant atMcIntosh <strong>in</strong> Wash<strong>in</strong>gton County. Salt isproduced by solution m<strong>in</strong><strong>in</strong>g for <strong>the</strong>manufacture <strong>of</strong> <strong>in</strong>dustrial chemicals.Numerous o<strong>the</strong>r salt structures occur deeper<strong>in</strong> <strong>the</strong> subsurface <strong>of</strong> southwest <strong>Alabama</strong>.SAND AND GRAVEL<strong>Alabama</strong> has an abundance <strong>of</strong> sand andgravel suitable for most construction needsand many <strong>in</strong>dustrial uses (fig. 7). The mostextensive <strong>of</strong> many sand and gravel depositsdistributed throughout <strong>the</strong> state occur <strong>in</strong> <strong>the</strong>Coastal Pla<strong>in</strong> Prov<strong>in</strong>ce <strong>in</strong> an area extend<strong>in</strong>gfrom Dallas County through MontgomeryCounty and <strong>in</strong>to Russell County. Thesedeposits have <strong>the</strong> potential for production <strong>of</strong>high-silica construction sand and <strong>in</strong>dustrialgravel (fig. 8). Sand and gravel deposits <strong>in</strong><strong>the</strong> Valley and Ridge Prov<strong>in</strong>ce and <strong>the</strong>Interior Low Plateaus Prov<strong>in</strong>ce (figs. 2, 7)conta<strong>in</strong> a high percentage <strong>of</strong> chert gravel,which is unsuited for most <strong>in</strong>dustrialapplications or as aggregate <strong>in</strong> concrete.Chert gravel can be used for road-basematerial and fill.Industrial and construction sand andgravel comb<strong>in</strong>ed was <strong>the</strong> second lead<strong>in</strong>g<strong>in</strong>dustrial m<strong>in</strong>eral commodity produced <strong>in</strong><strong>Alabama</strong> dur<strong>in</strong>g <strong>2007</strong>, valued at $87.8 million(U.S. <strong>Geological</strong> <strong>Survey</strong>, 2008b). TheMontgomery district, located <strong>in</strong> <strong>the</strong> CoastalPla<strong>in</strong> Prov<strong>in</strong>ce <strong>of</strong> south-central <strong>Alabama</strong>, was<strong>the</strong> largest sand and gravel produc<strong>in</strong>g area <strong>in</strong><strong>the</strong> state dur<strong>in</strong>g <strong>the</strong> period 2000-<strong>2007</strong> (table3). Sand and gravel resources occurpr<strong>in</strong>cipally <strong>in</strong> Quaternary alluvium and terracedeposits <strong>of</strong> <strong>the</strong> <strong>Alabama</strong>, Coosa, andTallapoosa Rivers border<strong>in</strong>g Montgomery,Autauga, Lowndes, Elmore, and MaconCounties. Large-scale production <strong>of</strong> sand andgravel <strong>in</strong> <strong>the</strong> Montgomery district began <strong>in</strong> <strong>the</strong>early 1920s. S<strong>in</strong>ce 2000, <strong>the</strong> Montgomerydistrict has produced about 2.2 million shorttons <strong>of</strong> sand and gravel annually, about 14percent <strong>of</strong> <strong>the</strong> entire production <strong>in</strong> <strong>Alabama</strong>(table 3).
15The lead<strong>in</strong>g use <strong>of</strong> construction sand andgravel <strong>in</strong> <strong>Alabama</strong> was <strong>in</strong> concrete aggregatewith a value <strong>of</strong> $36.7 million (unit value <strong>of</strong>$4.19 per metric ton). O<strong>the</strong>r major uses<strong>in</strong>clude asphaltic concrete aggregate with avalue <strong>of</strong> $10.1 million (unit value <strong>of</strong> $7.34 permetric ton), road base and cover<strong>in</strong>gs with avalue <strong>of</strong> $1.9 million (unit value <strong>of</strong> $2.89 permetric ton), and fill sand and gravel with avalue <strong>of</strong> $2.29 million (unit value <strong>of</strong> $2.50 permetric ton). O<strong>the</strong>r uses totaled $19.5 millionand <strong>in</strong>clude plaster and gunite sands (unitvalue <strong>of</strong> $6.27 per metric ton), concreteproducts (unit value <strong>of</strong> $7.10 per metric ton),Figure 7.— General distribution <strong>of</strong> sand and gravel resources <strong>in</strong> <strong>Alabama</strong> (Dean, 2000).
16Figure 8.—Quartz gravel aggregate.road stabilization, and water filtration (U.S.<strong>Geological</strong> <strong>Survey</strong>, <strong>2007</strong>a). A develop<strong>in</strong>g<strong>in</strong>dustry <strong>in</strong> <strong>Alabama</strong> is <strong>the</strong> use <strong>of</strong> <strong>of</strong>fshoresand resources to renourish and restoreerod<strong>in</strong>g and hurricane-damaged beaches(see <strong>M<strong>in</strong>erals</strong> Related Activity section). In <strong>the</strong>past, small plants alone produced sand andgravel for a specific job or area. Recenttrends have been to develop large, highcapacityplants that use long-distancetransportation.SANDSTONESandstone is a medium-gra<strong>in</strong>edsedimentary rock typically composed <strong>of</strong>quartz sand gra<strong>in</strong>s cemented toge<strong>the</strong>r bysilica or iron oxide and frequently conta<strong>in</strong><strong>in</strong>gsmall-scale sedimentary structures. ThePennsylvanian Pottsville Formation is <strong>the</strong>most widespread unit conta<strong>in</strong><strong>in</strong>g sandstoneand has been quarried throughout north<strong>Alabama</strong> for build<strong>in</strong>g stone and crushedaggregate for more than 160 years. Pottsvillesandstone was quarried as early as 1827 atTuscaloosa for build<strong>in</strong>g stone used <strong>in</strong> <strong>the</strong>construction <strong>of</strong> <strong>the</strong> State Capitol build<strong>in</strong>g.Pottsville sandstone has recently beenquarried <strong>in</strong> Blount, Cherokee, Marshall, andWalker Counties for build<strong>in</strong>g stone, sand andgravel, and crushed aggregate. TheMississippian Hartselle Sandstone <strong>of</strong> north<strong>Alabama</strong> has been used as a source <strong>of</strong>build<strong>in</strong>g stone and silica sand. Production <strong>in</strong><strong>2007</strong> was 1.7 million short tons (table 1).Recent annual sandstone production wasvalued at $7.69 million and a unit value <strong>of</strong>$5.96 per metric ton (U.S. <strong>Geological</strong> <strong>Survey</strong>,<strong>2007</strong>a).SILICONHigh-grade silica sand and gravel (greaterthan 99 percent SiO2) from Quaternaryalluvial deposits are used <strong>in</strong> <strong>the</strong> ferroalloyfurnace production <strong>of</strong> ferrosilicon and siliconmetal at plants <strong>in</strong> Selma, Montgomery, andBridgeport. Ferrosilicon is used as an additiveto steel to achieve complete deoxidization,and silicon is used as an alloy<strong>in</strong>g elementand as a component <strong>in</strong> silicone production.
17SULFURSulfur is produced as a by-product <strong>of</strong> gascleans<strong>in</strong>gprocesses <strong>in</strong> plants operated <strong>in</strong>Escambia, Mobile, and Wash<strong>in</strong>gton Counties.Sulfur recovery started <strong>in</strong> 1972 from south<strong>Alabama</strong>, and it is also currently recoveredfrom petroleum ref<strong>in</strong>eries <strong>in</strong> TuscaloosaCounty. Recent annual production <strong>of</strong> sulfur <strong>in</strong><strong>Alabama</strong> was 236,000 metric tons valued atabout $9.42 million (U.S. <strong>Geological</strong> <strong>Survey</strong>,2006).POTENTIAL MINERAL RESOURCESA number <strong>of</strong> <strong>the</strong> state's m<strong>in</strong>eral depositsare now classified as potential resourcesei<strong>the</strong>r because <strong>the</strong>y are not presently m<strong>in</strong>edcommercially or because <strong>the</strong>y occur <strong>in</strong>quantity and grade unacceptable for presentcommercial operations. As knowledge <strong>of</strong> <strong>the</strong>state's geology <strong>in</strong>creases, or as economiccircumstances change, m<strong>in</strong><strong>in</strong>g <strong>of</strong> <strong>the</strong>sem<strong>in</strong>erals may become economically feasible.ASPHALTIC ROCKAsphalt-impregnated sandstone andlimestone <strong>of</strong> <strong>the</strong> Mississippian HartselleSandstone, Pride Mounta<strong>in</strong> Formation, andTuscumbia Limestone underlie some areas <strong>of</strong>Colbert, Frankl<strong>in</strong>, Lawrence, and MorganCounties <strong>in</strong> northwest <strong>Alabama</strong>. Crushedasphaltic stone was first used for pav<strong>in</strong>gstreets <strong>in</strong> Florence, <strong>Alabama</strong>. Later, <strong>Alabama</strong>was one <strong>of</strong> only three states produc<strong>in</strong>g nativeasphalt. For many years crushed asphalticlimestone from <strong>the</strong> Tuscumbia Limestonewas produced at <strong>the</strong> Margerum quarry <strong>in</strong>Colbert County. From 1923 to 1982 about 14million short tons <strong>of</strong> asphaltic limestone wasm<strong>in</strong>ed from Colbert County. Renewed <strong>in</strong>terest<strong>in</strong> <strong>the</strong> development <strong>of</strong> new energy resourceshas prompted <strong>in</strong>vestigations <strong>of</strong> <strong>the</strong>se depositsas potential sources <strong>of</strong> petroleum (Wilson,1987).BARITEBarite (barium sulfate) is a colorless towhite, chemically <strong>in</strong>ert nonmetallic m<strong>in</strong>eralwith a relatively high specific gravity. Themajority <strong>of</strong> barite consumed <strong>in</strong> <strong>the</strong> UnitedStates is used as an additive to drill<strong>in</strong>g mudfor rotary drill<strong>in</strong>g <strong>of</strong> oil and gas wells. O<strong>the</strong>ruses <strong>in</strong>clude filler <strong>in</strong> paper, rubber products,pa<strong>in</strong>t, textiles, and as heavy constructionaggregate. Barite is also used as a source <strong>of</strong>barium <strong>in</strong> <strong>the</strong> chemical <strong>in</strong>dustry.In <strong>Alabama</strong>, barite occurs as epigeneticve<strong>in</strong> and breccia deposits <strong>in</strong> <strong>the</strong> OrdovicianChepultepec Dolomite, Longview Limestone,Newala Limestone, and possibly <strong>the</strong>Mosheim Limestone Member <strong>of</strong> <strong>the</strong> LenoirLimestone (Hughes and Lynch, 1973). It alsooccurs <strong>in</strong> secondary residual deposits thatare wea<strong>the</strong>r<strong>in</strong>g products <strong>of</strong> dolomites andlimestones. These deposits are situated <strong>in</strong> a13-county area that extends from BibbCounty <strong>in</strong> central <strong>Alabama</strong> to CherokeeCounty <strong>in</strong> nor<strong>the</strong>ast <strong>Alabama</strong>. In addition,barite occurs as a ve<strong>in</strong> m<strong>in</strong>eral with<strong>in</strong> <strong>the</strong>Piedmont Prov<strong>in</strong>ce <strong>of</strong> eastern <strong>Alabama</strong>. Itwas m<strong>in</strong>ed <strong>in</strong> <strong>Alabama</strong> as early as <strong>the</strong> 1840s.M<strong>in</strong><strong>in</strong>g cont<strong>in</strong>ued sporadically <strong>in</strong> <strong>Alabama</strong>until World War II, with most <strong>of</strong> <strong>the</strong> productionfrom Bibb, Calhoun, and Shelby Counties.From 1915 to 1924, 28,600 tons <strong>of</strong> barite wasm<strong>in</strong>ed, 80 percent <strong>of</strong> which came fromCalhoun County.COLUMBITE-TANTALITEThe columbite-tantalite (iron manganeseniobium-tantalum oxides) series <strong>of</strong> m<strong>in</strong>eralswas only <strong>of</strong> m<strong>in</strong>eralogical <strong>in</strong>terest whendiscovered <strong>in</strong> <strong>the</strong> 1870s near Rockford <strong>in</strong>Coosa County. Detailed exploration led to <strong>the</strong>discovery <strong>of</strong> m<strong>in</strong>eralization hosted by <strong>the</strong>Rockford Granite. M<strong>in</strong><strong>in</strong>g and production <strong>of</strong>tantalum concentrate <strong>in</strong> Coosa Countyoccurred dur<strong>in</strong>g 1989-91 for <strong>the</strong> nationaldefense stockpile. There has been recent<strong>in</strong>terest <strong>in</strong> assess<strong>in</strong>g <strong>the</strong> m<strong>in</strong><strong>in</strong>g possibilities<strong>of</strong> <strong>the</strong> deposits <strong>in</strong> Coosa County. Thecolumbite-tantalite m<strong>in</strong>erals are <strong>the</strong> pr<strong>in</strong>cipalsources <strong>of</strong> tantalum, which is a refractorymetal with several strategic <strong>in</strong>dustrialapplications.GARNETThe garnet group <strong>of</strong> m<strong>in</strong>erals occurs <strong>in</strong>schists and gneisses <strong>of</strong> <strong>the</strong> PiedmontProv<strong>in</strong>ce <strong>in</strong> east-central <strong>Alabama</strong>. Rocks <strong>of</strong>
18<strong>the</strong> Wedowee, Mad Indian, Poe BridgeMounta<strong>in</strong>, and Higg<strong>in</strong>s Ferry Groups conta<strong>in</strong>garnets as ubiquitous m<strong>in</strong>erals <strong>in</strong> Randolph,Clay, Tallapoosa, Coosa, Elmore, and ChiltonCounties. Garnet crystals are usually wellformeddodecahedrons or trapezohedrons,rang<strong>in</strong>g <strong>in</strong> size from microscopic to 0.5 <strong>in</strong>ch <strong>in</strong>diameter. The larger crystals usually conta<strong>in</strong><strong>in</strong>clusions <strong>of</strong> mica, quartz, and feldspar. Thesmaller crystals tend to be purer and free <strong>of</strong><strong>in</strong>clusions.Garnet's resistance to wea<strong>the</strong>r<strong>in</strong>g results<strong>in</strong> garnet-bear<strong>in</strong>g zones <strong>of</strong> heavy m<strong>in</strong>erals <strong>in</strong><strong>the</strong> alluvial sediments associated with <strong>the</strong>Tallapoosa and Coosa River systems as wellas <strong>in</strong> <strong>the</strong> coastal pla<strong>in</strong> sedimentary apron on<strong>the</strong> crystall<strong>in</strong>e rocks <strong>of</strong> <strong>the</strong> PiedmontProv<strong>in</strong>ce. Garnets are used primarily asabrasives. The garnets occurr<strong>in</strong>g <strong>in</strong> <strong>the</strong>metamorphic rocks <strong>of</strong> <strong>the</strong> Piedmont Prov<strong>in</strong>cepossibly can be m<strong>in</strong>ed <strong>in</strong> areas where <strong>the</strong>schists conta<strong>in</strong> up to 25 percent garnets.GOLD AND SILVERGold was discovered <strong>in</strong> <strong>Alabama</strong> <strong>in</strong> <strong>the</strong>early 1830s. Recorded production to 1939was about 49,000 troy ounces <strong>of</strong> gold;however, probably an equal amount hasbeen m<strong>in</strong>ed but not reported. Gold has beenproduced from seven districts <strong>in</strong> <strong>the</strong> <strong>Alabama</strong>Piedmont Prov<strong>in</strong>ce:1. <strong>the</strong> Chulaf<strong>in</strong>nee-Arbacoochee district <strong>in</strong>sou<strong>the</strong>rn Cleburne and nor<strong>the</strong>rnRandolph Counties;2. <strong>the</strong> Idaho district southwest <strong>of</strong> Pyriton <strong>in</strong>west-central Clay County;3. <strong>the</strong> Cragford district <strong>in</strong> west-centralRandolph and east-central Clay Counties;4. <strong>the</strong> Riddles Mill district <strong>in</strong> sou<strong>the</strong>asternTalladega County;5. <strong>the</strong> Goldville-Hog Mounta<strong>in</strong> district <strong>in</strong>north-central Tallapoosa County;6. <strong>the</strong> Devil's Backbone-Eagle Creek district<strong>in</strong> Tallapoosa County; and7. <strong>the</strong> western Coosa and eastern ChiltonCounties district.More than 100 prospects and m<strong>in</strong>es areknown <strong>in</strong> <strong>the</strong>se seven districts (Lesher ando<strong>the</strong>rs, 1989).The towns <strong>of</strong> Arbacoochee, Chulaf<strong>in</strong>nee,and Goldville, which developed from <strong>the</strong> goldm<strong>in</strong><strong>in</strong>g rush <strong>of</strong> <strong>the</strong> 1830s, have now beenabandoned. The Hog Mounta<strong>in</strong> m<strong>in</strong>e <strong>in</strong>Tallapoosa County was <strong>the</strong> most extensivelydeveloped gold m<strong>in</strong>e <strong>in</strong> <strong>Alabama</strong> and iscredited with a total production <strong>of</strong> about24,000 troy ounces <strong>of</strong> gold.Gold m<strong>in</strong><strong>in</strong>g ceased <strong>in</strong> <strong>Alabama</strong>, excepton an <strong>in</strong>dividual basis, dur<strong>in</strong>g <strong>the</strong> late 1930s.Exploration <strong>of</strong> gold deposits <strong>in</strong> <strong>Alabama</strong> hasbeen carried out <strong>in</strong>termittently dur<strong>in</strong>g <strong>the</strong> pastseveral years <strong>in</strong> <strong>the</strong> historic gold districts. In2008, gold prices exceeded $1,000 per troyounce, <strong>in</strong>creas<strong>in</strong>g <strong>in</strong>terest <strong>in</strong> <strong>the</strong> historic golddistricts <strong>of</strong> <strong>the</strong> state.Silver deposits have not beenau<strong>the</strong>nticated <strong>in</strong> <strong>Alabama</strong>, although rumors <strong>of</strong>"silver m<strong>in</strong>es" have been prevalentthroughout <strong>the</strong> state. These rumors may bebased on <strong>the</strong> occasional discovery <strong>of</strong> galenacrystals (lead sulfide). Approximately 2,600troy ounces <strong>of</strong> silver has been produced as aby-product <strong>of</strong> gold m<strong>in</strong><strong>in</strong>g operations <strong>in</strong><strong>Alabama</strong> (Cook and Smith, 1982).GRAPHITEGraphite is <strong>the</strong> most common m<strong>in</strong>eralform <strong>of</strong> native, or naturally occurr<strong>in</strong>g, purecarbon. In <strong>Alabama</strong>, graphite generallyoccurs as dissem<strong>in</strong>ated flakes <strong>in</strong> <strong>the</strong>metamorphic rocks <strong>of</strong> <strong>the</strong> Piedmont Prov<strong>in</strong>ce<strong>in</strong> <strong>the</strong> east-central part <strong>of</strong> <strong>the</strong> state. Them<strong>in</strong>eral is particularly abundant <strong>in</strong> Clay,Coosa, and sou<strong>the</strong>astern Chilton Counties,and <strong>the</strong> graphite-rich rock units with<strong>in</strong> <strong>the</strong>secounties form one <strong>of</strong> <strong>the</strong> largest graphitedeposits <strong>in</strong> <strong>the</strong> United States. The richestgraphite occurrences are associated withmicaceous quartzites and mica schists <strong>of</strong> <strong>the</strong>Poe Bridge Mounta<strong>in</strong> and Higg<strong>in</strong>s FerryGroups, which may conta<strong>in</strong> up to 5 percentdissem<strong>in</strong>ated graphite. The graphite-richzones occur as elongate lenses or layerswith<strong>in</strong> <strong>the</strong> rock, and <strong>the</strong>ir thicknesses rangefrom a few feet to more than 100 feet.Vanadium-bear<strong>in</strong>g mica is commonlyassociated with graphite-rich zones.Graphite is an important commodity used<strong>in</strong> <strong>the</strong> manufacture <strong>of</strong> electrical products and
19high-temperature refractory crucibles for <strong>the</strong>metals <strong>in</strong>dustry. Graphite is commonly usedas a dry lubricant or is mixed with clay to form<strong>the</strong> "lead" <strong>of</strong> pencils.Flake graphite was m<strong>in</strong>ed extensively <strong>in</strong><strong>Alabama</strong> from 1890 to 1931. Dur<strong>in</strong>g WorldWar I, graphite m<strong>in</strong><strong>in</strong>g reached its peak with7.8 million pounds <strong>of</strong> graphite produced <strong>in</strong>Clay and Coosa Counties <strong>in</strong> 1918 alone.Forty-three major m<strong>in</strong>es and 30 process<strong>in</strong>gplants were <strong>in</strong> operation dur<strong>in</strong>g this period.From 1910 to 1929 roughly 50.8 millionpounds <strong>of</strong> graphite was produced, and from1942 to 1953 nearly 30 million pounds wasproduced. Technological advances led to <strong>the</strong>development and production <strong>of</strong> artificialgraphite, and foreign production rendered<strong>Alabama</strong>'s graphite deposits subeconomic.Graphite m<strong>in</strong>es <strong>in</strong> <strong>the</strong> state ceased activityafter 1953.HEAVY MINERALS"Heavy m<strong>in</strong>erals" is a general term for agroup <strong>of</strong> m<strong>in</strong>erals which, because <strong>of</strong> <strong>the</strong>irdurability and relatively high specific gravity,are concentrated by natural stream or mar<strong>in</strong>eprocesses. Small concentrations <strong>of</strong> heavym<strong>in</strong>erals, sometimes called "black sands,"occur <strong>in</strong> <strong>the</strong> sediment <strong>of</strong> most streams <strong>in</strong> <strong>the</strong>state. Large, concentrated deposits that mayoccur <strong>in</strong> beach and <strong>of</strong>fshore sand <strong>in</strong> coastalareas are important because heavy m<strong>in</strong>eralsconta<strong>in</strong> significant quantities <strong>of</strong> economicallyimportant and potentially critical rareelements. Some <strong>of</strong> <strong>the</strong> most commonm<strong>in</strong>erals <strong>in</strong>cluded <strong>in</strong> this group aretourmal<strong>in</strong>e, which conta<strong>in</strong>s lithium and boron;rutile, which conta<strong>in</strong>s titanium; zircon, whichconta<strong>in</strong>s zirconium and hafnium; ilmenite,which conta<strong>in</strong>s titanium; monazite, whichconta<strong>in</strong>s several <strong>of</strong> <strong>the</strong> rare earth elements;and magnetite, an oxide <strong>of</strong> iron.IRON OREThe development <strong>of</strong> <strong>Alabama</strong>'s steel<strong>in</strong>dustry marked <strong>the</strong> beg<strong>in</strong>n<strong>in</strong>g <strong>of</strong> <strong>the</strong><strong>in</strong>dustrial era <strong>in</strong> <strong>Alabama</strong> <strong>in</strong> <strong>the</strong> late 1800s.First efforts at iron mak<strong>in</strong>g employed massivestone furnaces and used local "brown ironore" (limonite) and charcoal. Such furnaceswere located near Russellville (1818),Tannehill (1830), Polkville (1843), Shelby(1848), Round Mounta<strong>in</strong> (1853), and atseveral o<strong>the</strong>r localities <strong>in</strong> <strong>Alabama</strong>. The iron<strong>in</strong>dustry began to expand with <strong>the</strong> discovery<strong>of</strong> red ore (hematite) <strong>in</strong> <strong>the</strong> Birm<strong>in</strong>gham area,and <strong>the</strong> mill camps toge<strong>the</strong>r with <strong>the</strong> m<strong>in</strong><strong>in</strong>gcamps formed <strong>the</strong> basis for <strong>the</strong> present city <strong>of</strong>Birm<strong>in</strong>gham. In 1967 hematite wasdesignated as <strong>the</strong> <strong>of</strong>ficial state m<strong>in</strong>eral.Iron ore m<strong>in</strong><strong>in</strong>g was once <strong>the</strong> state's mostimportant m<strong>in</strong>eral <strong>in</strong>dustry, but now allunderground m<strong>in</strong>es have ceased operationbecause higher grade imported ores areavailable. Red iron ore from <strong>the</strong> Silurian RedMounta<strong>in</strong> Formation has been m<strong>in</strong>ed <strong>in</strong> Bibb,Blount, Cherokee, DeKalb, Etowah,Jefferson, and Tuscaloosa Counties. Browniron ore has been m<strong>in</strong>ed <strong>in</strong> Barbour, Butler,Calhoun, Cherokee, Chilton, Colbert,Conecuh, Crenshaw, Frankl<strong>in</strong>, Jefferson,Pike, Shelby, and Tuscaloosa Counties.Brown iron ore wash<strong>in</strong>g and screen<strong>in</strong>g plantswere generally near <strong>the</strong> open-pit m<strong>in</strong>e andwere moved as m<strong>in</strong><strong>in</strong>g operations shiftedfrom one m<strong>in</strong>e to ano<strong>the</strong>r. In TalladegaCounty specular hematite (gray iron ore)occurs <strong>in</strong> quartzite <strong>of</strong> <strong>the</strong> Cambrian WeisnerFormation. This iron ore conta<strong>in</strong>s a low-grademetamorphic m<strong>in</strong>eral assemblage, whichconta<strong>in</strong>s pyrite and chalcopyrite. The totaliron ore production for <strong>Alabama</strong> from 1840 to1975 was about 376 million long tons (Dean,2000).<strong>Alabama</strong> has significant resources <strong>of</strong> ironore. Although most outcrop areas <strong>of</strong> <strong>the</strong>major red ore beds have been strip m<strong>in</strong>ed, anestimated 4.2 billion tons <strong>of</strong> red ore underlieareas <strong>in</strong> DeKalb, Etowah, Jefferson, and St.Clair Counties. These iron ore resourcescould be m<strong>in</strong>ed underground if economicconditions became favorable or if it becamestrategically necessary. In addition, resources<strong>of</strong> brown iron ore <strong>in</strong> <strong>the</strong> sou<strong>the</strong>astern,northwestern, and central counties areestimated at 925 million tons (Simpson ando<strong>the</strong>rs, 1978).
20PHOSPHATE ROCKPhosphatic material occurs <strong>in</strong> <strong>the</strong> CoastalPla<strong>in</strong> Prov<strong>in</strong>ce at <strong>the</strong> contact between <strong>the</strong>Cretaceous Eutaw Formation and MoorevilleChalk <strong>in</strong> Perry and Dallas Counties.Phosphate occurs <strong>in</strong> nodules, gra<strong>in</strong>s, bonefragments, and shark teeth with<strong>in</strong> a narrowsand and clay zone. This phosphatic zonerepresents only a potential reserve <strong>of</strong> lowgradephosphate.Phosphate rock has been m<strong>in</strong>ed fromLimestone County, but operations werediscont<strong>in</strong>ued <strong>in</strong> 1983. In this area phosphaticmaterial occurs <strong>in</strong> Ordovician limestone, but itwas m<strong>in</strong>ed only from <strong>the</strong> residuum andwea<strong>the</strong>red portions <strong>of</strong> <strong>the</strong> phosphaticlimestone. From 1978 to 1983 roughly721,000 short tons <strong>of</strong> collophane, orphosphate-bear<strong>in</strong>g residuum, was m<strong>in</strong>ed.The residual deposits conta<strong>in</strong>ed appreciableamounts <strong>of</strong> phosphate, but no attempts havebeen made to recover phosphate economicallyfrom <strong>the</strong> unwea<strong>the</strong>red limestone.PYRITEPyrite (iron disulfide) is a common sulfidem<strong>in</strong>eral <strong>in</strong> <strong>the</strong> <strong>Alabama</strong> Piedmont Prov<strong>in</strong>ce,occurr<strong>in</strong>g as "fool's gold" <strong>in</strong> metamorphic,igneous, and sedimentary rocks. Pyrite has avariety <strong>of</strong> uses <strong>in</strong>clud<strong>in</strong>g manufacture <strong>of</strong> irons<strong>in</strong>ter, sulfuric acid, and sulfur. Pyritedeposits <strong>in</strong> <strong>the</strong> Hillabee Greenstone <strong>in</strong> <strong>the</strong>vic<strong>in</strong>ity <strong>of</strong> Pyriton, Clay County, were firstm<strong>in</strong>ed <strong>in</strong> <strong>the</strong> 1850s, and production <strong>of</strong> pyritefor sulfur cont<strong>in</strong>ued <strong>in</strong>termittently until WorldWar I.SLATESlate is a compact, f<strong>in</strong>e-gra<strong>in</strong>edmetamorphic rock formed by <strong>the</strong> alterationand recrystallization <strong>of</strong> clay m<strong>in</strong>erals <strong>in</strong>sedimentary rock. Its most dist<strong>in</strong>guish<strong>in</strong>gfeature is a pronounced cleavage alongplanes that are <strong>in</strong>dependent <strong>of</strong> orig<strong>in</strong>albedd<strong>in</strong>g. In <strong>Alabama</strong>, slate and phyllite with aslaty cleavage (will split <strong>in</strong>to slabs and th<strong>in</strong>plates) crop out <strong>in</strong> parts <strong>of</strong> Shelby, Chilton,Talladega, and Calhoun Counties. Althoughslate was orig<strong>in</strong>ally used for build<strong>in</strong>g stone,modern uses <strong>in</strong>clude <strong>in</strong>dustrial extenders,<strong>in</strong>ert fillers, lightweight aggregate, and roadaggregate. Slate with <strong>the</strong> physical propertiesnecessary for use as ro<strong>of</strong><strong>in</strong>g material hasbeen reported <strong>in</strong> Talladega County. Also,slate for build<strong>in</strong>g stone and o<strong>the</strong>r applicationshas been m<strong>in</strong>ed <strong>in</strong> nor<strong>the</strong>rn Chilton andsou<strong>the</strong>rn Shelby Counties.TALCTalc (hydrated magnesium silicate)occurs <strong>in</strong> association with hydro<strong>the</strong>rmallyaltered dolomite <strong>of</strong> <strong>the</strong> Shady Dolomite nearW<strong>in</strong>terboro, Talladega County (Blount andHelbig, 1987). In <strong>the</strong> W<strong>in</strong>terboro area,prospect<strong>in</strong>g for talc began as early as 1918,but <strong>the</strong> majority <strong>of</strong> production occurredbetween 1953 and 1976. From 1955 to 1976,<strong>the</strong> American Talc Co. m<strong>in</strong>ed nearly 130,000short tons <strong>of</strong> talc from this area. In 1963, aflotation mill was constructed at nearbyAlp<strong>in</strong>e, where local and imported high-qualitytalc is processed for use as a filler <strong>in</strong> goodssuch as pharmaceuticals and cosmetics. Talcproduction from <strong>the</strong> W<strong>in</strong>terboro area resumedon a small scale from 1988 to 1992.TRIPOLITripoli (silicon dioxide) is a light-colored,porous, friable, sedimentary rock that resultsfrom <strong>the</strong> wea<strong>the</strong>r<strong>in</strong>g <strong>of</strong> chert or siliceouslimestone. Commercial tripoli averages 98 to99 percent silicon dioxide with m<strong>in</strong>or amounts<strong>of</strong> alum<strong>in</strong>a (clay m<strong>in</strong>erals), titanium, and irondioxide. Large, undeveloped deposits <strong>of</strong>commercial-quality tripoli occur <strong>in</strong> <strong>the</strong> upperpart <strong>of</strong> <strong>the</strong> Fort Payne Chert <strong>in</strong> westernLauderdale and Colbert Counties. The tripoli<strong>in</strong> northwest <strong>Alabama</strong> is composed <strong>of</strong>crystall<strong>in</strong>e aggregates <strong>of</strong> quartz with generallyless than 1.5 percent impurities. Smaller,scattered deposits <strong>of</strong> tripoli occur <strong>in</strong> chertsand dolomites <strong>in</strong> Calhoun and TalladegaCounties. These deposits are composedpr<strong>in</strong>cipally <strong>of</strong> <strong>in</strong>dividual, frosted, subangular torounded quartz gra<strong>in</strong>s.Tripoli has excellent abrasive qualitiesand is used ma<strong>in</strong>ly as a component <strong>of</strong> buff<strong>in</strong>gand polish<strong>in</strong>g compounds. Because it ischemically <strong>in</strong>ert and resists abrasion, tripoli isused as a filler and extender <strong>in</strong> pa<strong>in</strong>t, plastics,
21and enamels. Dur<strong>in</strong>g <strong>the</strong> mid-1960s, a smallamount <strong>of</strong> tripoli was m<strong>in</strong>ed nor<strong>the</strong>ast <strong>of</strong>Waterloo <strong>in</strong> Lauderdale County, for use asfoundry fac<strong>in</strong>g or silica brick.ZEOLITE MINERALSZeolites are hydrated alum<strong>in</strong>osilicates <strong>of</strong>alkali and alkal<strong>in</strong>e earth metals and areunique <strong>in</strong> that <strong>the</strong>ir crystal structure conta<strong>in</strong>smicropores. Zeolite m<strong>in</strong>erals occur <strong>in</strong> <strong>the</strong> P<strong>in</strong>eBarren Member <strong>of</strong> <strong>the</strong> Clayton Formation, <strong>the</strong>lower clays <strong>of</strong> <strong>the</strong> Porters Creek Formation,<strong>the</strong> Grampian Hills Member <strong>of</strong> <strong>the</strong> NanafaliaFormation, and <strong>the</strong> Tallahatta Formation—all<strong>in</strong> <strong>the</strong> Paleocene and Eocene deposits <strong>in</strong>south <strong>Alabama</strong> (Beg, 1992). The zeolitem<strong>in</strong>erals identified <strong>in</strong> <strong>the</strong>se formations areheulandite, cl<strong>in</strong>optilolite, and phillipsite. Themajor occurrence <strong>of</strong> zeolites is <strong>in</strong> <strong>the</strong>Tallahatta Formation; zeolites have yet to beexploited commercially.The discovery <strong>of</strong> unusual properties <strong>of</strong>zeolite m<strong>in</strong>erals, particularly <strong>the</strong>ir ability to actas molecular sieves, <strong>the</strong>ir ion exchangecapabilities, and hydration and rehydrationproperties, has created a great demand forzeolites by <strong>in</strong>dustry. Zeolites are used topurify natural and <strong>in</strong>dustrial gases, and forwater filtration systems, soil condition<strong>in</strong>g,aquaculture systems, carriers <strong>of</strong> pesticides,desiccants, and many o<strong>the</strong>r uses requir<strong>in</strong>gmolecular capillaries. At Chickasaw <strong>in</strong> MobileCounty, artificial zeolites are produced for useas molecular sieve absorbents.FURTHER READINGPublished reports on <strong>the</strong> state's geologyand m<strong>in</strong>eral resources are too numerous to<strong>in</strong>clude a complete citation <strong>in</strong> this report. Acomplete list <strong>of</strong> <strong>Geological</strong> <strong>Survey</strong> <strong>of</strong><strong>Alabama</strong> publications can be accessed on<strong>the</strong> <strong>Survey</strong>’s web site athttp://www.gsa.state.al.us. Also, <strong>in</strong>formationon <strong>Survey</strong> publications is available from <strong>the</strong>Publications Sales Office, <strong>Geological</strong> <strong>Survey</strong><strong>of</strong> <strong>Alabama</strong>, P.O. Box 869999, Tuscaloosa,<strong>Alabama</strong> 35486-6999 (telephone 205-247-3636). The <strong>Geological</strong> <strong>Survey</strong> PublicationsSales Office is also a repository for U.S.<strong>Geological</strong> <strong>Survey</strong> topographic maps, whichmay be purchased. An <strong>in</strong>dex to topographicmaps may be obta<strong>in</strong>ed free <strong>of</strong> charge.The <strong>Geological</strong> <strong>Survey</strong> <strong>of</strong> <strong>Alabama</strong>reports listed below summarize <strong>in</strong>formationon <strong>the</strong> state's m<strong>in</strong>eral resources and may beobta<strong>in</strong>ed from <strong>the</strong> Publications Sales Office.Summary Report on <strong>the</strong> M<strong>in</strong>eralResources <strong>of</strong> Southwest <strong>Alabama</strong>, 1986, byMirza A. Beg, Bennett L. Bearden, Otis M.Clarke, Jr., Robert L. Barnett, and ThomasW. Daniel, Jr., <strong>Geological</strong> <strong>Survey</strong> <strong>of</strong> <strong>Alabama</strong>Information Series 65, 33 p.M<strong>in</strong>eral Filler and Extender Resources <strong>in</strong><strong>Alabama</strong>, 1990, by Karen F. Rheams,<strong>Geological</strong> <strong>Survey</strong> <strong>of</strong> <strong>Alabama</strong> Circular 145,77 p.Geologic Map <strong>of</strong> <strong>Alabama</strong>, 1988,compiled by Michael W. Szabo, W. EdwardOsborne, Charles W. Copeland, Jr., andThornton L. Nea<strong>the</strong>ry, <strong>Geological</strong> <strong>Survey</strong> <strong>of</strong><strong>Alabama</strong> Special Map 220 (scale 1:250,000).Geologic Map <strong>of</strong> <strong>Alabama</strong>, 1989,compiled by W. Edward Osborne, Michael W.Szabo, Charles W. Copeland, Jr., andThornton L. Nea<strong>the</strong>ry, <strong>Geological</strong> <strong>Survey</strong> <strong>of</strong><strong>Alabama</strong> Special Map 221 (scale 1:500,000).Assessment <strong>of</strong> Nonhydrocarbon M<strong>in</strong>eralResources <strong>in</strong> <strong>the</strong> Exclusive Economic Zone <strong>in</strong>Offshore <strong>Alabama</strong>, 1990, by Steven J.Parker, <strong>Geological</strong> <strong>Survey</strong> <strong>of</strong> <strong>Alabama</strong>Circular 147, 73 p.Industrial <strong>M<strong>in</strong>erals</strong> <strong>of</strong> <strong>the</strong> Sou<strong>the</strong>asternUnited States—Economic M<strong>in</strong>eral Resources<strong>in</strong> Central <strong>Alabama</strong>, 1990, by Karen F.Rheams and Guerry H. McClellan, <strong>Geological</strong><strong>Survey</strong> <strong>of</strong> <strong>Alabama</strong> Guidebook 3-3, 60 p.Selected Industrial M<strong>in</strong>eral ResourceSites and Process<strong>in</strong>g Facilities <strong>in</strong> West<strong>Alabama</strong>, by Karen F. Rheams, 1991,<strong>Geological</strong> <strong>Survey</strong> <strong>of</strong> <strong>Alabama</strong> Guidebook 4,56 p.Industrial <strong>M<strong>in</strong>erals</strong> <strong>of</strong> <strong>the</strong> Sou<strong>the</strong>asternUnited States—Proceed<strong>in</strong>gs <strong>of</strong> <strong>the</strong> 2ndAnnual Symposium on Industrial <strong>M<strong>in</strong>erals</strong>,1992, edited by Karen F. Rheams and GuerryH. McClellan, <strong>Geological</strong> <strong>Survey</strong> <strong>of</strong> <strong>Alabama</strong>Circular 161, 111 p.In addition to <strong>the</strong>se resources, onl<strong>in</strong>esources <strong>of</strong> m<strong>in</strong>erals-related data, <strong>in</strong>clud<strong>in</strong>g
22current production statistics and companiesnow operat<strong>in</strong>g <strong>in</strong> <strong>the</strong> state, are listed <strong>in</strong> <strong>the</strong>“References Cited” section below.REFERENCES CITED<strong>Alabama</strong> Department <strong>of</strong> Industrial Relations,Office <strong>of</strong> M<strong>in</strong>e Safety and Inspection,<strong>2007</strong>, Annual statistical report, accessedMay 30, 2008, at http://dir.alabama.gov/mr/.___2000-2006, Annual statistical reports:Montgomery, <strong>Alabama</strong>.<strong>Alabama</strong> Department <strong>of</strong> Transportation,2008, List I-1, Aggregates—Sources <strong>of</strong>coarse and f<strong>in</strong>e aggregates, accessedMay 30, 2008, at http://www.dot.state.al.us/Docs/Bureaus/Materials+and+Tests/Test<strong>in</strong>g/MSDSAR/MSDSAR_Lists.htm.<strong>Alabama</strong> Development Office, <strong>2007</strong>, <strong>Alabama</strong>2006 new and expand<strong>in</strong>g <strong>in</strong>dustry report:Montgomery, <strong>Alabama</strong>, 24 p.___2008, <strong>Alabama</strong> new and expand<strong>in</strong>g<strong>in</strong>dustry announcements, <strong>2007</strong>:Montgomery, <strong>Alabama</strong>, 23 p.Beg, M. A., 1984, Limestone resources <strong>of</strong><strong>Alabama</strong>: <strong>Alabama</strong> <strong>Geological</strong> <strong>Survey</strong>Special Map 172 with text, 24 p.___1992, Zeolite m<strong>in</strong>erals <strong>in</strong> <strong>the</strong> TallahattaFormation, <strong>Alabama</strong>: <strong>Alabama</strong> <strong>Geological</strong><strong>Survey</strong> Circular 160, 27 p.Blount, A. M., and Helbig, S. R., 1987, Talcand chlorite <strong>in</strong> <strong>the</strong> W<strong>in</strong>terboro area,Talladega County, <strong>Alabama</strong>: <strong>Alabama</strong><strong>Geological</strong> <strong>Survey</strong> Circular 129, 43 p.Clarke, O. M., Jr., 1973, Bauxite and kaol<strong>in</strong> <strong>in</strong><strong>the</strong> Eufaula bauxite district, <strong>Alabama</strong>:<strong>Alabama</strong> <strong>Geological</strong> <strong>Survey</strong> Bullet<strong>in</strong> 100,90 p.Clarke, O. M., Jr., and Tyrrell, M. E., 1976,Porters Creek lightweight aggregate:<strong>Alabama</strong> <strong>Geological</strong> <strong>Survey</strong> Circular 100,35 p.Cook, R. B., and Smith, W. E., 1982,M<strong>in</strong>eralogy <strong>of</strong> <strong>Alabama</strong>: <strong>Alabama</strong><strong>Geological</strong> <strong>Survey</strong> Bullet<strong>in</strong> 120, 285 p.Dean, L. S., 2000, <strong>M<strong>in</strong>erals</strong> <strong>in</strong> <strong>Alabama</strong>,1998-99: <strong>Alabama</strong> <strong>Geological</strong> <strong>Survey</strong>Information Series 64Q, 44 p.Epperson, R. S., and Rheams, K. F., 1984,Mica <strong>in</strong> <strong>Alabama</strong>: <strong>Alabama</strong> <strong>Geological</strong><strong>Survey</strong> Atlas 18, 63 p.Fousek, R. S., editor, 2002, The geology,m<strong>in</strong><strong>in</strong>g methods, and process<strong>in</strong>g <strong>of</strong>selected <strong>in</strong>dustrial m<strong>in</strong>erals <strong>in</strong>nor<strong>the</strong>astern <strong>Alabama</strong>: <strong>Alabama</strong><strong>Geological</strong> Society 39 th Annual Field TripGuidebook, 123 p.___<strong>2007</strong>, Locat<strong>in</strong>g, permitt<strong>in</strong>g, and operation<strong>of</strong> construction aggregate m<strong>in</strong><strong>in</strong>goperations <strong>in</strong> <strong>Alabama</strong>: <strong>Alabama</strong><strong>Geological</strong> Society 44 th Annual Field TripGuidebook, 105 p.<strong>Geological</strong> <strong>Survey</strong> <strong>of</strong> <strong>Alabama</strong>, 2008,STATEMAP—<strong>Alabama</strong> Metadata Portal,accessed May 30, 2008, at http://portal.gsa.state.al.us/Portal/ptk?command=openchannel&channel=8.Guthrie, G. M., 1989, Geology and marbleresources <strong>of</strong> <strong>the</strong> Sylacauga marbledistrict: <strong>Alabama</strong> <strong>Geological</strong> <strong>Survey</strong>Bullet<strong>in</strong> 131, 81 p.Hosterman, J. W., 1984, Ball clay andbentonite deposits <strong>of</strong> <strong>the</strong> central andwestern Gulf <strong>of</strong> Mexico Coastal Pla<strong>in</strong>,United States: U.S. <strong>Geological</strong> <strong>Survey</strong>Bullet<strong>in</strong> 1558-C, 22 p.Hosterman, J. W., and Patterson, S. H.,1992, Bentonite and fuller's earthresources <strong>of</strong> <strong>the</strong> United States: U.S.<strong>Geological</strong> <strong>Survey</strong> Pr<strong>of</strong>essional Paper1522, 45 p.Hughes, T. H., and Lynch, R. E., 1973, Barite<strong>in</strong> <strong>Alabama</strong>: <strong>Alabama</strong> <strong>Geological</strong> <strong>Survey</strong>Circular 85, 43 p.Lesher, C. M., Cook, R. B., and Dean, L. S.,eds., 1989 [repr<strong>in</strong>t 1999], Gold deposits <strong>of</strong><strong>Alabama</strong>: <strong>Alabama</strong> <strong>Geological</strong> <strong>Survey</strong>Bullet<strong>in</strong> 136, 229 p.National Cooperative Geologic Mapp<strong>in</strong>gProgram, <strong>2007</strong>, accessed May 30, 2008,at http://www.gsa.state.al.us/documents/misc_gsa/<strong>Alabama</strong>.pdf.Outdoor <strong>Alabama</strong>, 2008, Freshwater mussels<strong>in</strong> <strong>Alabama</strong>, accessed June 27, 2008, athttp://www.outdooralabama.com/education/general<strong>in</strong>fo/mussels.
23Puckett, T. M., Roberson, K. E., and Tew, B.H., 1990, High calcium deposits <strong>in</strong> <strong>the</strong>Newala Limestone: <strong>Alabama</strong> <strong>Geological</strong><strong>Survey</strong> Circular 149, 29 p.Rheams, K. F., 1992, M<strong>in</strong>eral resources <strong>of</strong><strong>the</strong> Valley and Ridge Prov<strong>in</strong>ce, <strong>Alabama</strong>:<strong>Alabama</strong> <strong>Geological</strong> <strong>Survey</strong> Bullet<strong>in</strong> 147,263 p.Rohrbach, R. P., 1989, The stars fell <strong>in</strong><strong>Alabama</strong>: Lapidary Journal, v. 42, no. 12,p.20-22, 24-26, 28.Simpson, T. A., McCarl, H. N., Hilleke, A. F.,and Hanna, H. S., 1978, Assessment <strong>of</strong>iron ore availability <strong>in</strong> <strong>Alabama</strong> and <strong>the</strong>sou<strong>the</strong>astern Appalachian region:Tuscaloosa, University <strong>of</strong> <strong>Alabama</strong>,M<strong>in</strong>eral Resources Institute, State M<strong>in</strong>eExperiment Station, 102 p.U.S. <strong>Geological</strong> <strong>Survey</strong>, 2006, Sulfur—2005<strong>M<strong>in</strong>erals</strong> Yearbook, accessed May 30,2008, at http://m<strong>in</strong>erals.usgs.gov/m<strong>in</strong>erals/pubs/commodity/.___<strong>2007</strong>a, The m<strong>in</strong>eral <strong>in</strong>dustry <strong>of</strong><strong>Alabama</strong>—2005 <strong>M<strong>in</strong>erals</strong> Yearbook, p.3.1-3.6, accessed May 30, 2008, athttp://m<strong>in</strong>erals.usgs.gov/m<strong>in</strong>erals/pubs/state/al.html.___<strong>2007</strong>b, Stone, Dimension—2006 <strong>M<strong>in</strong>erals</strong>Yearbook, accessed May 30, 2008, athttp://m<strong>in</strong>erals.usgs.gov/m<strong>in</strong>erals/pubs/commodity/.___2008a, <strong>Alabama</strong> <strong>2007</strong> prelim<strong>in</strong>ary nonfuelm<strong>in</strong>eral production data: Arnold Tanner,e-mail communication.___2008b, M<strong>in</strong>eral Industry <strong>Survey</strong>s—Crushed stone and sand and gravel <strong>in</strong> <strong>the</strong>fourth quarter <strong>2007</strong>, accessed May 30,2008, at http://m<strong>in</strong>erals.usgs.gov/m<strong>in</strong>erals/pubs/commodity/.Wilson, G. V., 1987, Characteristics andresource evaluation <strong>of</strong> <strong>the</strong> asphalt andbitumen deposits <strong>of</strong> nor<strong>the</strong>rn <strong>Alabama</strong>:<strong>Alabama</strong> <strong>Geological</strong> <strong>Survey</strong> Bullet<strong>in</strong> 111,110 p., accessed May 30, 2008, athttp://www.gsa.state.al.us/onl<strong>in</strong>e_pubs.aspx.
Published byGEOLOGICAL SURVEY OF ALABAMAServ<strong>in</strong>g <strong>Alabama</strong> s<strong>in</strong>ce 1848Berry H. (Nick) Tew, Jr., State GeologistFor additional copies write:Publications Sales OfficeP.O. Box 869999Tuscaloosa, <strong>Alabama</strong> 35486-6999205/247-3636e-mail: PubSales@gsa.state.al.usA complete list <strong>of</strong> <strong>Geological</strong> <strong>Survey</strong> <strong>of</strong> <strong>Alabama</strong> and Oil and Gas Board publicationscan be obta<strong>in</strong>ed at <strong>the</strong> Publications Sales Office or through our web site athttp://www.gsa.state.al.us/.As a recipient <strong>of</strong> Federal f<strong>in</strong>ancial assistance from <strong>the</strong> U.S. Department <strong>of</strong> <strong>the</strong>Interior, <strong>the</strong> GSA prohibits discrim<strong>in</strong>ation on <strong>the</strong> basis <strong>of</strong> race, color, national orig<strong>in</strong>,age, or disability <strong>in</strong> its programs or activities. Discrim<strong>in</strong>ation on <strong>the</strong> basis <strong>of</strong> sex isprohibited <strong>in</strong> federally assisted GSA education programs. If anyone believes that heor she has been discrim<strong>in</strong>ated aga<strong>in</strong>st <strong>in</strong> any <strong>of</strong> <strong>the</strong> GSA’s programs or activities,<strong>in</strong>clud<strong>in</strong>g its employment practices, <strong>the</strong> <strong>in</strong>dividual may contact <strong>the</strong> U.S. <strong>Geological</strong><strong>Survey</strong>, U.S. Department <strong>of</strong> <strong>the</strong> Interior, Wash<strong>in</strong>gton, D.C. 20240.AN EQUAL OPPORTUNITY EMPLOYER