Dressings and Topical Agents for Chronic Wounds - NIHR Health ...
Dressings and Topical Agents for Chronic Wounds - NIHR Health ...
Dressings and Topical Agents for Chronic Wounds - NIHR Health ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
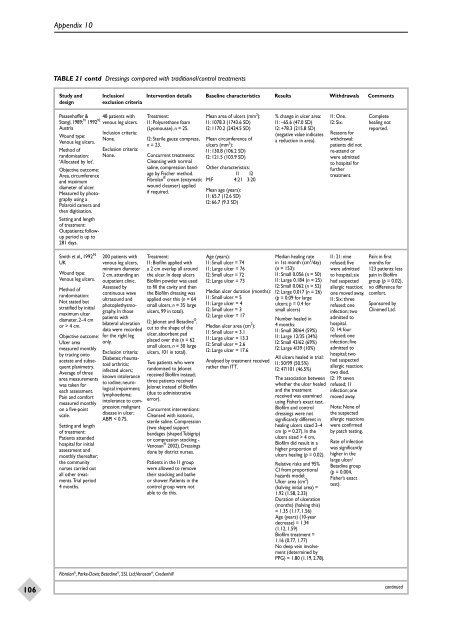
Appendix 10TABLE 21 contd <strong>Dressings</strong> compared with traditional/control treatmentsStudy <strong>and</strong> Inclusion/ Intervention details Baseline characteristics Results Withdrawals Commentsdesignexclusion criteriaPessenhoffer &Stangl, 1989; 91 1992 92AustriaWound type:Venous leg ulcers.Method ofr<strong>and</strong>omisation:‘Allocated by lot’.Objective outcome:Area, circumference<strong>and</strong> maximumdiameter of ulcer.Measured by photographyusing aPolaroid camera <strong>and</strong>then digitisation.Setting <strong>and</strong> lengthof treatment:Outpatients; followupperiod is up to281 days.48 patients withvenous leg ulcers.Inclusion criteria:None.Exclusion criteria:None.Treatment:I1: Polyurethane foam(Lyomousse), n = 25.I2: Sterile gauze compress,n = 23.Concurrent treatments:Cleansing with normalsaline, compression b<strong>and</strong>ageby Fischer method.Fibrolan ® cream (enzymaticwound cleanser) appliedif required.Mean area of ulcers (mm 2 ):I1: 1078.3 (1743.6 SD)I2: 1170.2 (2424.5 SD)Mean circumference ofulcers (mm 2 ):I1: 130.8 (106.2 SD)I2: 121.5 (103.9 SD)Other characteristics:I1 I2M:F 4:21 3:20Mean age (years):I1: 65.7 (12.6 SD)I2: 66.7 (9.3 SD)% change in ulcer area:I1: –65.6 (47.0 SD)I2: +78.3 (215.8 SD)(negative value indicatesa reduction in area).I1: One.I2: Six.Reasons <strong>for</strong>withdrawal:patients did notre-attend orwere admittedto hospital <strong>for</strong>furthertreatment.Completehealing notreported.Smith et al., 1992 95UKWound type:Venous leg ulcers.Method ofr<strong>and</strong>omisation:Not stated butstratified by initialmaximum ulcerdiameter, 2–4 cmor > 4 cm.Objective outcome:Ulcer areameasured monthlyby tracing ontoacetate <strong>and</strong> subsequentplanimetry.Average of threearea measurementswas taken <strong>for</strong>each assessment.Pain <strong>and</strong> com<strong>for</strong>tmeasured monthlyon a five-pointscale.Setting <strong>and</strong> lengthof treatment:Patients attendedhospital <strong>for</strong> initialassessment <strong>and</strong>monthly thereafter;the communitynurses carried outall other treatments.Trialperiod4 months.200 patients withvenous leg ulcers,minimum diameter2 cm, attending anoutpatient clinic.Assessed bycontinuous waveultrasound <strong>and</strong>photoplethysmography.In thosepatients withbilateral ulcerationdata were recorded<strong>for</strong> the right legonly.Exclusion criteria:Diabetes; rheumatoidarthritis;infected ulcers;known intoleranceto iodine; neurologicalimpairment;lymphoedema;intolerance to compression;malignantdisease in ulcer;ABPI < 0.75.Treatment:I1: Biofilm applied witha 2 cm overlap all aroundthe ulcer. In deep ulcersBiofilm powder was usedto fill the cavity <strong>and</strong> thenthe Biofilm dressing wasapplied over this (n = 64small ulcers, n = 35 largeulcers, 99 in total).I2: Jelonet <strong>and</strong> Betadine ® ,cut to the shape of theulcer, absorbent padplaced over this (n = 62small ulcers, n = 30 largeulcers, 101 in total).Two patients who werer<strong>and</strong>omised to Jelonetreceived Biofilm instead;three patients receivedJelonet instead of Biofilm(due to administrativeerror).Fibrolan ® , Parke-Davis; Betadine ® , SSL Ltd;Venosan ® , CredenhillConcurrent interventions:Cleansed with isotonic,sterile saline. Compression(two shaped supportb<strong>and</strong>ages (shaped Tubigrip)or compression stocking -Venosan ® 2002). <strong>Dressings</strong>done by district nurses.Patients in the I1 groupwere allowed to removetheir stocking <strong>and</strong> batheor shower. Patients in thecontrol group were notable to do this.Age (years):I1: Small ulcer = 74I1: Large ulcer = 76I2: Small ulcer = 72I2: Large ulcer = 73Median ulcer duration (months):I1: Small ulcer = 5I1: Large ulcer = 4I2: Small ulcer = 3I2: Large ulcer = 17Median ulcer area (cm 2 ):I1: Small ulcer = 3.1I1: Large ulcer = 13.3I2: Small ulcer = 2.6I2: Large ulcer = 17.6Analysed by treatment receivedrather than ITT.Median healing ratein 1st month (cm 2 /day)(n = 153):I1: Small 0.056 (n = 50)I1: Large 0.184 (n = 25)I2: Small 0.062 (n = 52)I2: Large 0.017 (n = 26)(p = 0.09 <strong>for</strong> largeulcers; p = 0.4 <strong>for</strong>small ulcers)Number healed in4 months:I1: Small 38/64 (59%)I1: Large 12/35 (34%)I2: Small 43/62 (69%)I2: Large 4/39 (10%)All ulcers healed in trial:I1: 50/99 (50.5%)I2: 47/101 (46.5%)The association betweenwhether the ulcer healed<strong>and</strong> the treatmentreceived was examinedusing Fisher’s exact test.Biofilm <strong>and</strong> controldressings were notsignificantly different inhealing ulcers sized 2–4cm (p = 0.27). In theulcers sized > 4 cm,Biofilm did result in ahigher proportion ofulcers healing (p = 0.02).Relative risks <strong>and</strong> 95%CI from proportionalhazards model:Ulcer area (cm 2 )(halving initial area) =1.92 (1.58, 2.33)Duration of ulceration(months) (halving this)= 1.35 (1.17, 1.56)Age (years) (10-yeardecrease) = 1.34(1.12, 1.59)Biofilm treatment =1.16 (0.77, 1.77)No deep vein involvement(determined byPPG) = 1.80 (1.19, 2.78).I1: 21: ninerefused; fivewere admittedto hospital; sixhad suspectedallergic reaction;one moved away.I1: Six: threerefused; oneinfection; twoadmitted tohospital.I2: 14: fourrefused; oneinfection; fiveadmitted tohospital; twohad suspectedallergic reaction;two died.I2: 19: sevenrefused; 11infection; onemoved away.Note: None ofthe suspectedallergic reactionswere confirmedby patch testing.Rate of infectionwas significantlyhigher in thelarge ulcer/Betadine group(p = 0.004,Fisher’s exacttest).Pain: in firstmonths <strong>for</strong>123 patients: lesspain in Biofilmgroup (p = 0.02),no difference <strong>for</strong>com<strong>for</strong>t.Sponsored byClinimed Ltd.106continued