WHO Drug Information Vol. 20, No. 1, 2006 - World Health ...
WHO Drug Information Vol. 20, No. 1, 2006 - World Health ...
WHO Drug Information Vol. 20, No. 1, 2006 - World Health ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
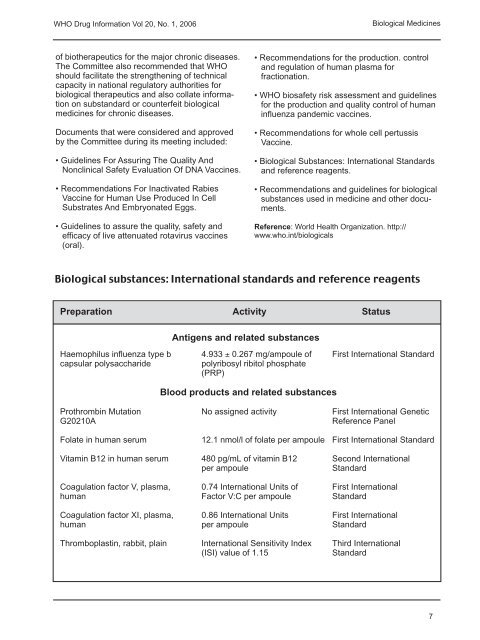
<strong>WHO</strong> <strong>Drug</strong> <strong>Information</strong> <strong>Vol</strong> <strong>20</strong>, <strong>No</strong>. 1, <strong>20</strong>06Biological Medicinesof biotherapeutics for the major chronic diseases.The Committee also recommended that <strong>WHO</strong>should facilitate the strengthening of technicalcapacity in national regulatory authorities forbiological therapeutics and also collate informationon substandard or counterfeit biologicalmedicines for chronic diseases.Documents that were considered and approvedby the Committee during its meeting included:• Guidelines For Assuring The Quality And<strong>No</strong>nclinical Safety Evaluation Of DNA Vaccines.• Recommendations For Inactivated RabiesVaccine for Human Use Produced In CellSubstrates And Embryonated Eggs.• Guidelines to assure the quality, safety andefficacy of live attenuated rotavirus vaccines(oral).• Recommendations for the production. controland regulation of human plasma forfractionation.• <strong>WHO</strong> biosafety risk assessment and guidelinesfor the production and quality control of humaninfluenza pandemic vaccines.• Recommendations for whole cell pertussisVaccine.• Biological Substances: International Standardsand reference reagents.• Recommendations and guidelines for biologicalsubstances used in medicine and other documents.Reference: <strong>World</strong> <strong>Health</strong> Organization. http://www.who.int/biologicalsBiological substances: International standards and reference reagentsPreparation Activity StatusAntigens and related substancesHaemophilus influenza type b 4.933 ± 0.267 mg/ampoule of First International Standardcapsular polysaccharidepolyribosyl ribitol phosphate(PRP)Blood products and related substancesProthrombin Mutation <strong>No</strong> assigned activity First International GeneticG<strong>20</strong>210AReference PanelFolate in human serum 12.1 nmol/l of folate per ampoule First International StandardVitamin B12 in human serum 480 pg/mL of vitamin B12 Second Internationalper ampouleStandardCoagulation factor V, plasma, 0.74 International Units of First Internationalhuman Factor V:C per ampoule StandardCoagulation factor XI, plasma, 0.86 International Units First Internationalhuman per ampoule StandardThromboplastin, rabbit, plain International Sensitivity Index Third International(ISI) value of 1.15Standard7