Monitoring Adap-ve Clinical Trials a Case Study
Monitoring Adappve Clinical Trials; a Case Study - Cytel
Monitoring Adappve Clinical Trials; a Case Study - Cytel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
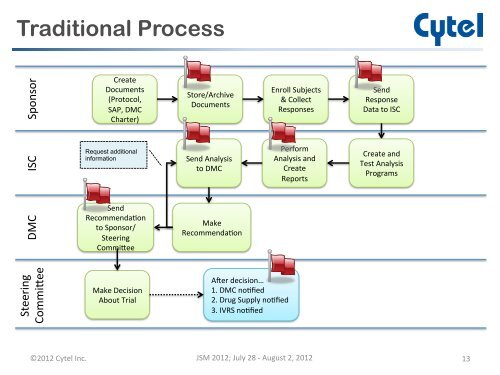
Traditional ProcessSponsor Create Documents (Protocol, SAP, DMC Charter) Store/Archi<strong>ve</strong> Documents Enroll Subjects & Collect Responses Send Response Data to ISC ISC Request additionalinformationSend Analysis to DMC Perform Analysis and Create Reports Create and Test Analysis Programs DMC Send Recommenda-on to Sponsor/ Steering Commitee Make Recommenda-on Steering Commitee Make Decision About Trial Ager decision… 1. DMC no-fied 2. Drug Supply no-fied 3. IVRS no-fied ©2012 Cytel Inc. JSM 2012; July 28 -‐ August 2, 2012 13