Monitoring Adap-ve Clinical Trials a Case Study
Monitoring Adappve Clinical Trials; a Case Study - Cytel
Monitoring Adappve Clinical Trials; a Case Study - Cytel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
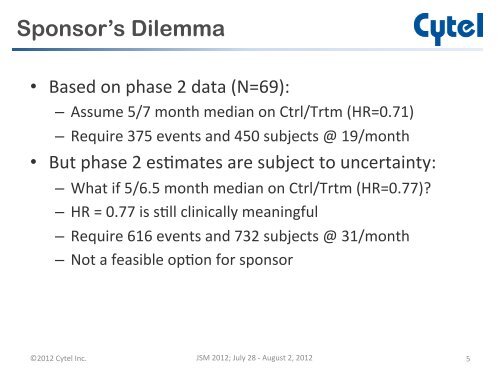
Sponsor’s Dilemma• Based on phase 2 data (N=69): – Assume 5/7 month median on Ctrl/Trtm (HR=0.71) – Require 375 e<strong>ve</strong>nts and 450 subjects @ 19/month • But phase 2 es-mates are subject to uncertainty: – What if 5/6.5 month median on Ctrl/Trtm (HR=0.77)? – HR = 0.77 is s-ll clinically meaningful – Require 616 e<strong>ve</strong>nts and 732 subjects @ 31/month – Not a feasible op-on for sponsor ©2012 Cytel Inc. JSM 2012; July 28 -‐ August 2, 2012 5