Monitoring Adap-ve Clinical Trials a Case Study
Monitoring Adappve Clinical Trials; a Case Study - Cytel
Monitoring Adappve Clinical Trials; a Case Study - Cytel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
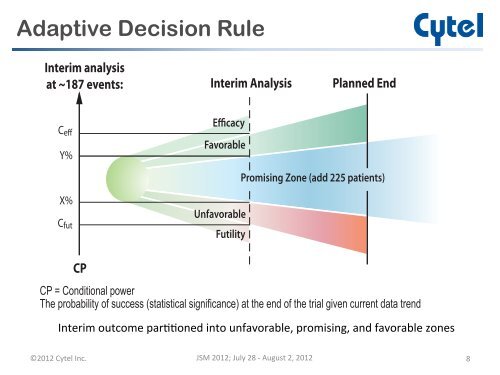
<strong>Adap</strong>ti<strong>ve</strong> Decision RuleInterim analysisat ~187 e<strong>ve</strong>nts:Interim AnalysisPlanned EndC effY%EfficacyFavorablePromising Zone (add 225 patients)X%C futUnfavorableFutilityCPCP = Conditional powerThe probability of success (statistical significance) at the end of the trial gi<strong>ve</strong>n current data trendInterim outcome par--oned into unfavorable, promising, and favorable zones ©2012 Cytel Inc. JSM 2012; July 28 -‐ August 2, 2012 8