OWLS: Electrophilic Aromatic Substitution Solutions - UCLA
OWLS: Electrophilic Aromatic Substitution Solutions - UCLA
OWLS: Electrophilic Aromatic Substitution Solutions - UCLA
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
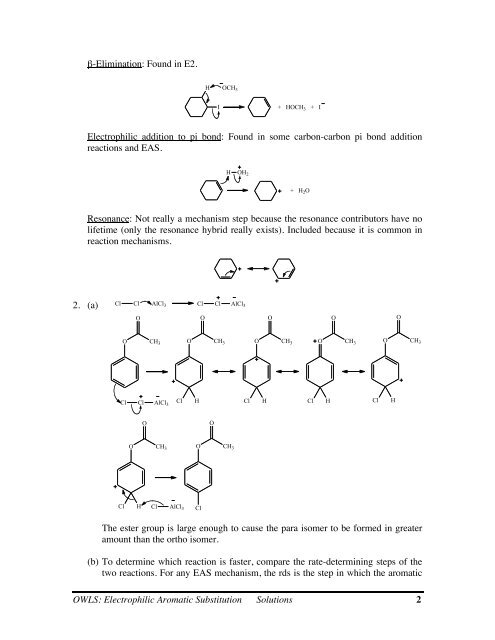
β-Elimination: Found in E2.<br />
H OCH 3<br />
I<br />
+ HOCH 3 + I<br />
<strong>Electrophilic</strong> addition to pi bond: Found in some carbon-carbon pi bond addition<br />
reactions and EAS.<br />
H OH 2<br />
+ H 2O<br />
Resonance: Not really a mechanism step because the resonance contributors have no<br />
lifetime (only the resonance hybrid really exists). Included because it is common in<br />
reaction mechanisms.<br />
2. (a) Cl Cl AlCl 3 Cl Cl AlCl 3<br />
O<br />
O CH 3<br />
Cl Cl AlCl 3<br />
O<br />
O CH 3<br />
Cl H Cl AlCl 3<br />
O<br />
O CH 3<br />
Cl H<br />
O CH 3<br />
Cl<br />
O<br />
O CH 3<br />
Cl H<br />
<strong>OWLS</strong>: <strong>Electrophilic</strong> <strong>Aromatic</strong> <strong>Substitution</strong> <strong>Solutions</strong> 2<br />
O<br />
O<br />
O CH 3<br />
Cl H<br />
O<br />
O CH 3<br />
Cl H<br />
The ester group is large enough to cause the para isomer to be formed in greater<br />
amount than the ortho isomer.<br />
(b) To determine which reaction is faster, compare the rate-determining steps of the<br />
two reactions. For any EAS mechanism, the rds is the step in which the aromatic