OWLS: Electrophilic Aromatic Substitution Solutions - UCLA
OWLS: Electrophilic Aromatic Substitution Solutions - UCLA
OWLS: Electrophilic Aromatic Substitution Solutions - UCLA
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>OWLS</strong>: <strong>Electrophilic</strong> <strong>Aromatic</strong> <strong>Substitution</strong> <strong>Solutions</strong><br />
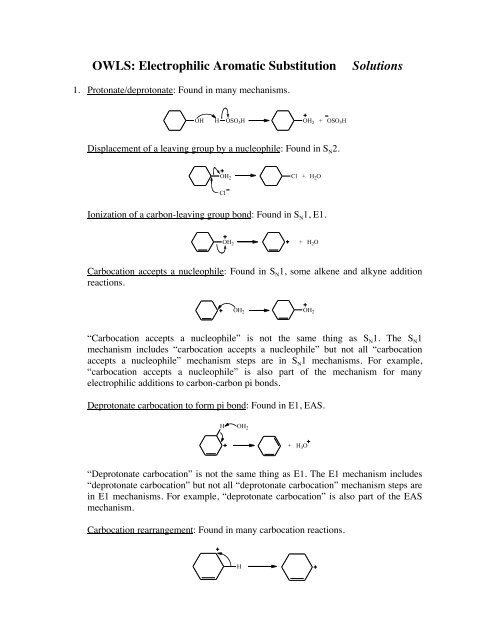
1. Protonate/deprotonate: Found in many mechanisms.<br />
OH H OSO 3H OH 2 + OSO 3H<br />
Displacement of a leaving group by a nucleophile: Found in S N2.<br />
OH 2<br />
Cl<br />
Cl + H 2O<br />
Ionization of a carbon-leaving group bond: Found in S N1, E1.<br />
OH 2<br />
+ H 2O<br />
Carbocation accepts a nucleophile: Found in S N1, some alkene and alkyne addition<br />
reactions.<br />
OH 2<br />
“Carbocation accepts a nucleophile” is not the same thing as S N1. The S N1<br />
mechanism includes “carbocation accepts a nucleophile” but not all “carbocation<br />
accepts a nucleophile” mechanism steps are in S N1 mechanisms. For example,<br />
“carbocation accepts a nucleophile” is also part of the mechanism for many<br />
electrophilic additions to carbon-carbon pi bonds.<br />
Deprotonate carbocation to form pi bond: Found in E1, EAS.<br />
H OH 2<br />
+ H 3O<br />
“Deprotonate carbocation” is not the same thing as E1. The E1 mechanism includes<br />
“deprotonate carbocation” but not all “deprotonate carbocation” mechanism steps are<br />
in E1 mechanisms. For example, “deprotonate carbocation” is also part of the EAS<br />
mechanism.<br />
Carbocation rearrangement: Found in many carbocation reactions.<br />
H<br />
OH 2
β-Elimination: Found in E2.<br />
H OCH 3<br />
I<br />
+ HOCH 3 + I<br />
<strong>Electrophilic</strong> addition to pi bond: Found in some carbon-carbon pi bond addition<br />
reactions and EAS.<br />
H OH 2<br />
+ H 2O<br />
Resonance: Not really a mechanism step because the resonance contributors have no<br />
lifetime (only the resonance hybrid really exists). Included because it is common in<br />
reaction mechanisms.<br />
2. (a) Cl Cl AlCl 3 Cl Cl AlCl 3<br />
O<br />
O CH 3<br />
Cl Cl AlCl 3<br />
O<br />
O CH 3<br />
Cl H Cl AlCl 3<br />
O<br />
O CH 3<br />
Cl H<br />
O CH 3<br />
Cl<br />
O<br />
O CH 3<br />
Cl H<br />
<strong>OWLS</strong>: <strong>Electrophilic</strong> <strong>Aromatic</strong> <strong>Substitution</strong> <strong>Solutions</strong> 2<br />
O<br />
O<br />
O CH 3<br />
Cl H<br />
O<br />
O CH 3<br />
Cl H<br />
The ester group is large enough to cause the para isomer to be formed in greater<br />
amount than the ortho isomer.<br />
(b) To determine which reaction is faster, compare the rate-determining steps of the<br />
two reactions. For any EAS mechanism, the rds is the step in which the aromatic
3. (a)<br />
ring is attacked by the electrophile and aromaticity is lost. In this case, the only<br />
difference between the reactants is the presence of a hydroxyl (OH, which makes<br />
this molecule a phenol) or an ester group. How does this effect the rds? Recall<br />
that a more stable carbocation is formed more quickly. How do the hydroxyl and<br />
ester groups affect the carbocation stability? Both arenium ions have four<br />
significant resonance contributors, but the ester oxygen lone pairs are not as<br />
readily available to stabilize the carbocation as the hydroxyl oxygen lone pairs<br />
because the ester oxygen lone pairs are delocalized by resonance with the ester<br />
carbonyl.<br />
Lone pair delocalized<br />
by C=O resonance<br />
O<br />
O CH 3<br />
Cl H<br />
O<br />
O CH 3<br />
Cl H<br />
Lone pair not delocalized<br />
by C=O resonance<br />
<strong>OWLS</strong>: <strong>Electrophilic</strong> <strong>Aromatic</strong> <strong>Substitution</strong> <strong>Solutions</strong> 3<br />
O<br />
Cl H<br />
Arenium ion from ester Arenium ion from phenol<br />
The arenium ion of reaction (i) is more stable, and thus reaction (i) is faster.<br />
(c) A wide variety of changes can be made. Extending the idea from part (b), any<br />
structural change that further reduces the availability of the ester oxygen lone<br />
pairs will decrease the stability of the arenium ion and hence decrease the reaction<br />
rate. Replacement of the ester methyl group (weak electron donating group) with<br />
a trifluoromethyl group (powerful electron withdrawing group) decreases the<br />
availability of the ester oxygen lone pairs due to an inductive effect.<br />
(b)<br />
Br 2, Fe<br />
or Br 2, FeBr 3<br />
CH 3<br />
Cl 2<br />
AlCl 3<br />
O<br />
O CF 3<br />
Br<br />
Cl 2<br />
AlCl 3<br />
CH 3<br />
Cl<br />
O<br />
O CF 3<br />
Cl<br />
H
4.<br />
(c)<br />
(d)<br />
(e)<br />
(f)<br />
(g)<br />
H 3C<br />
OCH 3<br />
H OSO 3H<br />
or<br />
O<br />
NH 2<br />
Cl<br />
CH 3<br />
SO 3<br />
H 2SO 4<br />
Cl<br />
AlCl 3<br />
NO 2<br />
benzene<br />
aq. HONO 2<br />
aq. H 2SO 4<br />
HO 3S<br />
AlCl 3<br />
1. NaNO 2, aq. HCl<br />
2. PhOH<br />
H 3C<br />
H 3C<br />
<strong>OWLS</strong>: <strong>Electrophilic</strong> <strong>Aromatic</strong> <strong>Substitution</strong> <strong>Solutions</strong> 4<br />
CH 3O<br />
O 2N<br />
O<br />
N<br />
NO 2<br />
Consider arenium ion<br />
stability plus steric effects.<br />
N<br />
OH<br />
H OSO 3H<br />
5. In each case, electrophilic attack on the aromatic ring gives the more stable arenium<br />
ion. Draw all of the resonance contributors if necessary to convince yourself.<br />
(a)<br />
aq. HONO 2<br />
aq. H 2SO 4<br />
NO 2
(b)<br />
(c)<br />
OCH 3<br />
SO 3<br />
H 2SO 4<br />
<strong>OWLS</strong>: <strong>Electrophilic</strong> <strong>Aromatic</strong> <strong>Substitution</strong> <strong>Solutions</strong> 5<br />
OCH 3<br />
The major product choice is based on the assumption that a benzene ring<br />
offers more steric hindrance than a methoxy group.<br />
O<br />
+<br />
CH 3<br />
O<br />
Cl<br />
AlCl 3<br />
6. Remember that Ph = phenyl = C 6H 5 = monosubstituted benzene ring.<br />
7.<br />
O N O H OH 2 O N OH<br />
H OH 2<br />
Ph NH2 N O Ph N N O<br />
Ph N<br />
Ph N N OH<br />
Ph N N OH<br />
H OH 2<br />
NH 2<br />
CH 3<br />
excess Br 2<br />
H<br />
NH 2<br />
Br Br<br />
CH 3<br />
O<br />
O<br />
CH 3<br />
SO 3H<br />
H OH 2 O N OH2 O N + OH 2<br />
H OH 2<br />
H<br />
N O<br />
H OH 2<br />
Ph<br />
N<br />
H<br />
N OH<br />
Ph N N OH2 Ph N N<br />
Ph N N<br />
excess CH 3I<br />
K 2CO 3<br />
N(CH 3) 3 Br<br />
Br Br<br />
The NH 2 and CH 3 (electron-donating) substituents of 4-methylaniline give its benzene<br />
ring enough nucleophilicity so that it can react with Br 2 in the absence of a Lewis acid<br />
catalyst such as FeBr 3. The reactivity is also enhanced by the increased stability<br />
imparted to the arenium ion by the NH 2 and CH 3 groups.<br />
Potassium carbonate is a weak base that is necessary to convert all of the amine to the<br />
ammonium salt. (Work out the mechanism to see how this happens.) Other weak<br />
bases could also be used.<br />
Bromination must be conducted before alkylation because the R 3N + substituent is a<br />
meta directing group. When there are multiple directing groups on the benzene ring,<br />
the strongest electron donor dominates. (In this case a methyl group is a stronger<br />
electron donor than an ammonium group, which is not an electron donor at all! For<br />
more on this concept ask Dr H for the EAS Supplemental Reading.)<br />
CH 3
NH 2<br />
CH 3<br />
excess CH 3I<br />
K 2CO 3<br />
N(CH 3) 3 Br<br />
CH 3<br />
excess Br 2<br />
FeBr 3<br />
N(CH 3) 3 Br<br />
Br Br<br />
<strong>OWLS</strong>: <strong>Electrophilic</strong> <strong>Aromatic</strong> <strong>Substitution</strong> <strong>Solutions</strong> 6<br />
CH 3