Foam lecture #1 - Boulder School for Condensed Matter and ...
Foam lecture #1 - Boulder School for Condensed Matter and ...
Foam lecture #1 - Boulder School for Condensed Matter and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
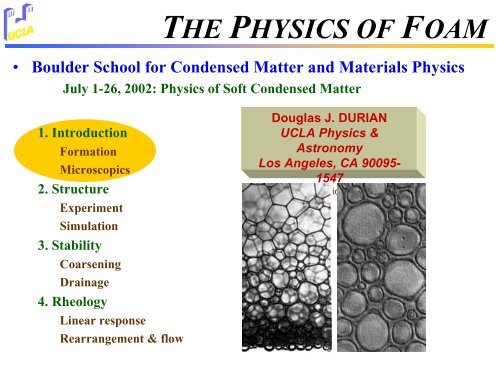
THE PHYSICS OF FOAM<br />
• <strong>Boulder</strong> <strong>School</strong> <strong>for</strong> <strong>Condensed</strong> <strong>Matter</strong> <strong>and</strong> Materials Physics<br />
July 1-26, 2002: Physics of Soft <strong>Condensed</strong> <strong>Matter</strong><br />
1. Introduction<br />
Formation<br />
Microscopics<br />
2. Structure<br />
Experiment<br />
Simulation<br />
3. Stability<br />
Coarsening<br />
Drainage<br />
4. Rheology<br />
Linear response<br />
Rearrangement & flow<br />
Douglas J. DURIAN<br />
UCLA Physics &<br />
Astronomy<br />
Los Angeles, CA 90095-<br />
1547<br />
<strong>Foam</strong> is…<br />
• …a r<strong>and</strong>om packing of bubbles in a relatively small<br />
amount of liquid containing surface-active impurities<br />
– Four levels of structure:<br />
– Three means of time evolution:<br />
• Gravitational drainage<br />
• Film rupture<br />
• Coarsening (gas diffusion from smaller to larger bubbles)
• …a most unusual <strong>for</strong>m of condensed matter<br />
–Like a gas:<br />
• volume ~ temperature / pressure<br />
– Like a liquid:<br />
• Flow without breaking<br />
• Fill any shape vessel<br />
– Under large <strong>for</strong>ce, bubbles rearrange their packing configuration<br />
– Like a solid:<br />
• Support small shear <strong>for</strong>ces elastically<br />
– Under small <strong>for</strong>ce, bubbles distort but don’t rearrange<br />
<strong>Foam</strong> is…
• Everyday life:<br />
– detergents<br />
– foods (ice cream, meringue, beer, cappuccino, ...)<br />
– cosmetics (shampoo, mousse, shaving cream, tooth paste, ...)<br />
• Unique applications:<br />
– firefighting<br />
– isolating toxic materials<br />
– physical <strong>and</strong> chemical separations<br />
– oil recovery<br />
– cellular solids<br />
• Undesirable occurrences:<br />
– mechanical agitation of multicomponent liquid<br />
– pulp <strong>and</strong> paper industry<br />
– paint <strong>and</strong> coating industry<br />
– textile industry<br />
– leather industry<br />
– adhesives industry<br />
– polymer industry<br />
– food processing (sugar, yeast, potatoes)<br />
– metal treatment<br />
– waste water treatment<br />
– polluted natural waters<br />
<strong>Foam</strong> is…<br />
• familiar!<br />
•important!<br />
• need to control stability <strong>and</strong> mechanics<br />
• must first underst<strong>and</strong> microscopic structure <strong>and</strong> dynamics…
<strong>Condensed</strong>-matter challenge<br />
• To underst<strong>and</strong> the stability <strong>and</strong> mechanics of bulk foams<br />
in terms of the behavior at microscopic scales<br />
bubbles are the “particles” from which foams are assembled<br />
– Easy to relate surfactant-film <strong>and</strong> film-bubble behaviors<br />
– Hard to relate bubble-macro behavior<br />
• Opaque: no simple way to image structure<br />
• Disordered: no periodicity<br />
• k BT
• Similar challenge <strong>for</strong> seemingly unrelated systems<br />
Jamming<br />
– Tightly packed collections of bubbles, droplets, grains, cells,<br />
colloids, fuzzy molecules, tectonic plates,.…<br />
• jammed/solid-like: small-<strong>for</strong>ce / low-temperature / high-density<br />
• fluid/liquid-like: large-<strong>for</strong>ce / high-temperature / low-density<br />
<strong>for</strong>ce-chains (S. Franklin) avalanches (S.R. Nagel) universality?
<strong>Foam</strong> Physics Today<br />
• visit the websites of these Summer 2002 conferences to<br />
see examples of current research on aqueous foams<br />
– Gordon Research Conference on Complex Fluids<br />
• Ox<strong>for</strong>d, UK<br />
– Euro<strong>Foam</strong> 2002<br />
• Manchester, UK<br />
– <strong>Foam</strong>s <strong>and</strong> Minimal Surfaces<br />
• Isaac Newton Institute <strong>for</strong> Mathematical Sciences<br />
– Geometry <strong>and</strong> Mechanics of Structured Materials<br />
• Max Planck Institute <strong>for</strong> the Physics of Complex Systems<br />
• after these <strong>lecture</strong>s, you should be in a good position to underst<strong>and</strong><br />
the issues being addressed & progress being made!
General references<br />
1. D. Weaire <strong>and</strong> N. Rivier, “Soap, cells <strong>and</strong> statistics - r<strong>and</strong>om patterns in two dimensions,” Contemp. Phys. 25, 55 (1984).<br />
2. J. P. Heller <strong>and</strong> M. S. Kuntamukkula, “Critical review of the foam rheology literature,” Ind. Eng. Chem. Res. 26, 318-325 (1987).<br />
3. A. M. Kraynik, “<strong>Foam</strong> flows,” Ann. Rev. Fluid Mech. 20, 325-357 (1988).<br />
4. J. H. Aubert, A. M. Kraynik, <strong>and</strong> P. B. R<strong>and</strong>, “Aqueous foams,” Sci. Am. 254, 74-82 (1989).<br />
5. A. J. Wilson, ed., <strong>Foam</strong>s: Physics, Chemistry <strong>and</strong> Structure (Springer-Verlag, New York, 1989).<br />
6. J. A. Glazier <strong>and</strong> D. Weaire, “The kinetics of cellular patterns,” J. Phys.: Condens. <strong>Matter</strong> 4, 1867-1894 (1992).<br />
7. C. Isenberg, The Science of Soap Films <strong>and</strong> Soap Bubbles (Dover Publications, New York, 1992).<br />
8. J. Stavans, “The evolution of cellular structures,” Rep. Prog. Phys. 56, 733-789 (1993).<br />
9. D. J. Durian <strong>and</strong> D. A. Weitz, “<strong>Foam</strong>s,” in Kirk-Othmer Encyclopedia of Chemical Technology, 4 ed., edited by J.I. Kroschwitz<br />
(Wiley, New York, 1994), Vol. 11, pp. 783-805.<br />
10. D. M. A. Buzza, C. Y. D. Lu, <strong>and</strong> M. E. Cates, “Linear shear rheology of incompressible foams,” J. de Phys. II 5, 37-52 (1995).<br />
11. R. K. Prud'homme <strong>and</strong> S. A. Khan, ed., <strong>Foam</strong>s: Theory, Measurement, <strong>and</strong> Application. Surfactant Science Series 57, (Marcel<br />
Dekker, NY, 1996).<br />
12. J.F. Sadoc <strong>and</strong> N. Rivier, Eds. <strong>Foam</strong>s <strong>and</strong> Emulsions (Kluwer Academic: Dordrecht, The Netherl<strong>and</strong>s, 1997).<br />
13. D. Weaire, S. Hutzler, G. Verbist, <strong>and</strong> E. Peters, “A review of foam drainage,” Adv. Chem. Phys. 102, 315-374 (1997).<br />
14. D. J. Durian, “Fast, nonevolutionary dynamics in foams,” Current Opinion in Colloid <strong>and</strong> Interface Science 2, 615-621 (1997).<br />
15. L.J. Gibson <strong>and</strong> M.F. Ashby, Cellular Solids: Structure <strong>and</strong> Properties (Cambridge University Press, Cambridge, 1997).<br />
16. M. Tabor, J. J. Chae, G. D. Burnett, <strong>and</strong> D. J. Durian, “The structure <strong>and</strong> dynamics of foams,” Nonlinear Science Today (1998).<br />
17. D. Weaire <strong>and</strong> S. Hutzler, The Physics of <strong>Foam</strong>s (Clarendon Press, Ox<strong>for</strong>d, 1999).<br />
18. S.A. Koehler, S. Hilgenfeldt, <strong>and</strong> H.A. Stone, "A generalized view of foam drainage,” Langmuir 16, 6327-6341 (2000).<br />
19. A.J. Liu <strong>and</strong> S.R. Nagel, eds., Jamming <strong>and</strong> Rheology (Taylor <strong>and</strong> Francis, New York, 2001).<br />
20. J. Banhart <strong>and</strong> D. Weaire, “On the road again: Metal foams find favor,” Physics Today 55, 37-42 (July 2002).<br />
Thanks <strong>for</strong> the images:<br />
John Banhart, Ken Brakke, Jan Cilliers, R<strong>and</strong>y Kamien, Andy Kraynik, John Sullivan, Paul Thomas, Denis Weaire, Eric Weeks,…
special thanks to collaborators<br />
• Students<br />
– Alex Gittings<br />
– Anthony Gopal<br />
– Pierre-Anthony Lemieux<br />
– Rajesh Ojha<br />
– Ian Ono<br />
– Sidney Park<br />
– Moin Vera<br />
• Postdocs<br />
– Ranjini B<strong>and</strong>yopadhyay<br />
– Narayanan Menon<br />
– Corey O’Hern<br />
– Arnaud Saint-Jalmes<br />
– Shubha Tewari<br />
– LoicVanel<br />
• Colleagues<br />
– Chuck Knobler<br />
– Steve Langer<br />
– Andrea Liu<br />
– Sid Nagel<br />
– Dave Pine<br />
– Joe Rudnick<br />
– Dave Weitz
• Shake, blend, stir, agitate, etc.<br />
– Uncontrolled / irreproducible<br />
– Unwanted foaming of multicomponent liquids<br />
• Sparge = blow bubbles<br />
– Polydisperse or monodisperse<br />
– Uncontrolled/non-uni<strong>for</strong>m liquid fraction<br />
solution<br />
porous plate<br />
gas<br />
foam<br />
gas<br />
<strong>Foam</strong> production I.<br />
bubble raft<br />
gas<br />
foam
• in-situ release / production of gas<br />
– nucleation<br />
• eg CO 2 in beer<br />
– aerosol:<br />
• eg propane in shaving cream<br />
• small bubbles!<br />
–active:<br />
• eg H 2 in molten zinc<br />
• eg CO 2 from yeast in bread<br />
<strong>Foam</strong> production II.
<strong>Foam</strong> production III.<br />
• turbulent mixing of thin liquid jet with gas<br />
– vast quantities<br />
– small polydisperse bubbles<br />
– controlled liquid fraction<br />
• lab samples<br />
• firefighting<br />
• distributing pesticides/dyes/etc.<br />
• covering l<strong>and</strong>fills<br />
• supressing dust<br />
• …<br />
solution<br />
gas<br />
jet<br />
foam
• many materials can be similarly foamed<br />
– nonaqueous liquids (oil, ferrofluids,…)<br />
– polymers (styrofoam, polyurethane,…)<br />
– metals<br />
–glass<br />
– concrete<br />
• variants found in nature<br />
– cork<br />
– bone<br />
– sponge<br />
– honeycomb<br />
<strong>Foam</strong> production IV.
• spittle bug:<br />
• cuckoo spit / froghoppers:<br />
• stickleback-fish’s nest<br />
<strong>Foam</strong>s produced by animals
• antifoaming agents<br />
– prevent foaming or break an existing foam<br />
<strong>Foam</strong> production V.<br />
• mysterious combination of surfactants, oils, particles,…
Microscopic behavior<br />
• look at progressively larger length scales…<br />
– surfactant solutions<br />
– soap films<br />
– local equilibrium & topology
• bubbles quickly coalesce – no foam<br />
Pure liquid<br />
– van der Waals <strong>for</strong>ce prefers monotonic dielectric profile;<br />
there<strong>for</strong>e, bubbles attract:<br />
a b a<br />
l<br />
“effective interface potential”<br />
is free energy cost per unit area:<br />
V vdw(l)= -A/12πl 2 , A=Hamaker constant<br />
l
Surfactant solution<br />
• surface active agent – adsorbs at air/water interface<br />
– head: hydrophilic (eg salt)<br />
– tail: hydrophobic (eg hydrocarbon chain)<br />
surface tension, σ<br />
• lore <strong>for</strong> good foams…<br />
– chain length: short enough that the surfactant is soluble<br />
– concentration: just above the “critical micelle concentration”<br />
eg sodium dodecylsulfate (SDS)<br />
80 erg/cm 2<br />
CMC<br />
30 erg/cm 2<br />
Log [surfactant concentation, c]<br />
{NB: lower σ doesn’t stabilize the foam…
Electrostatic “double-layer”<br />
• adsorbed surfactants dissociate, cause repulsion necessary to<br />
overcome van der Waals <strong>and</strong> hence stabilize the foam<br />
– electrostatic<br />
– entropic (dominant!)<br />
-<br />
-<br />
-<br />
-<br />
-<br />
-<br />
-<br />
-<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
-<br />
-<br />
-<br />
-<br />
-<br />
-<br />
-<br />
-<br />
free energy cost per unit area:<br />
V DL(l)= (64k BTρ/K D)Exp[-K Dl],<br />
ρ = electrolyte concentration<br />
K D -1 ~ ρ -1/2 = Debye screening length<br />
– NB: This is similar to the electrostatic stabilization of colloids
Soap film tension<br />
• film tension / interface potential / free energy per area:<br />
γ(l) = 2σ + V VDW (l) + V DL (l) ~ 2σ<br />
thickness, l<br />
• disjoining pressure: Π(l) = -dγ/dl<br />
– vanishes at equilibrium thickness, l eq ~ K D -1 (30-3000Å)
• Plateau border<br />
– scalloped-triangular channel where three films meet<br />
– the edge shared by three neighboring bubbles<br />
• Vertex<br />
– region where four Plateau borders meet<br />
– the point shared by four neighboring bubbles<br />
Film junctions
Liquid distribution<br />
• division of liquid between films-borders-vertices<br />
– repulsion vs surface tension<br />
– wet vs dry<br />
Π repulsion dominates<br />
=> maximize l<br />
dry<br />
=> polyhedral<br />
σ dominates<br />
=> minimize area<br />
wet<br />
=> spherical
Laplace’s law<br />
• the pressure is greater on the inside a curved interface<br />
due to surface tension, σ = energy / area = <strong>for</strong>ce / length<br />
σ P i = P o + 2σ/r {in general, ∆P= σ(1/r 1 +1/r 2 )}<br />
r<br />
– <strong>for</strong>ces on half-sphere:<br />
• ΣF up = P iπr 2 –P oπr 2 –2πσr = 0<br />
– energy change = pressure x volume change:<br />
• dU = (∆P)4πr 2 dr, where U(r)=4πr 2 σ<br />
P o<br />
P i<br />
σ
Liquid volume fraction<br />
• liquid redistributes until liquid pressure is same everywhere<br />
bubble radius, R<br />
• typically: film thickness l
Plateau’s rules <strong>for</strong> dry foams<br />
• <strong>for</strong> mechanical equilibrium:<br />
P<br />
– i.e. <strong>for</strong> zero net <strong>for</strong>ce on a Plateau border,<br />
– zero net <strong>for</strong>ce on a vertex,<br />
– <strong>and</strong> Σ∆P=0 going around a closed loop:<br />
(1) films have constant curvature & intersect three at a time at 120 o<br />
(2) borders intersect four at a time at cos -1 (1/3)=109.47 o<br />
• rule #2 follows from rule <strong>#1</strong><br />
• both are obviously correct if the films <strong>and</strong> borders are straight:<br />
P<br />
ΣF=0<br />
P
Rule <strong>#1</strong> <strong>for</strong> straight borders<br />
• choose r 1 <strong>and</strong> orientation of equilateral triangle<br />
• construct r 2 from extension down to axis<br />
• construct r 3 from inscribed equilateral triangle<br />
– NB: centers are on a line<br />
r 1 r 3<br />
Plateau border in/out of page<br />
– films meet at 120 o (triangles meet at 60 o -60 o -60 o , <strong>and</strong> are normal to PB’s)<br />
– similar triangles give (r 1+r 2)/r 1 = r 2/r 3, i.e. 1/r 1 + 1/r 2 = 1/r 3 <strong>and</strong> so ΣP=0<br />
r 2
• proof of Plateau’s rules is not obvious!<br />
– established in 1976 by Jean Taylor<br />
Curved Plateau borders
Decoration theorem <strong>for</strong> wet foams<br />
• <strong>for</strong> d=2 dimensions, an equilibrium wet foam can be<br />
constructed by decorating an equilibrium dry foam<br />
– can you construct an elementary proof?<br />
• PB’s are circular arcs that join tangentially to film<br />
• theorem fails in d=3 due to PB curvature
• periodic foam structures<br />
• disordered foam structures<br />
– experiment<br />
– simulation<br />
Next time…