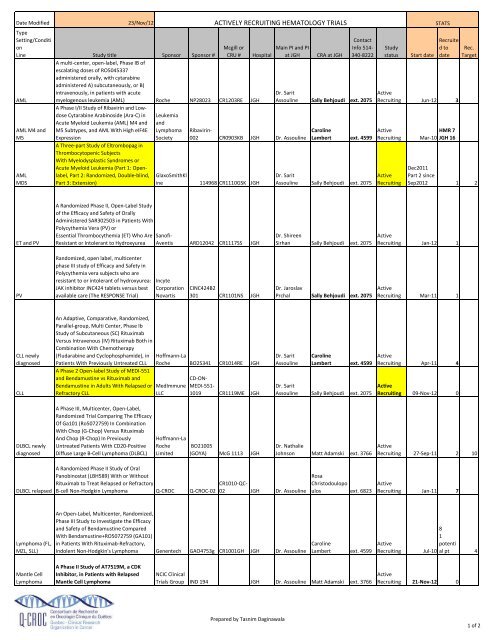
Table of ongoing and upcoming clinical trials
Table of ongoing and upcoming clinical trials
Table of ongoing and upcoming clinical trials
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Date Modified 23/Nov/12<br />
Type<br />
Setting/Conditi<br />
on<br />
Line Study title<br />
A multi-center, open-label, Phase IB <strong>of</strong><br />
escalating doses <strong>of</strong> RO5045337<br />
administered orally, with cytarabine<br />
administered A) subcutaneously, or B)<br />
intravenously, in patients with acute<br />
Sponsor Sponsor #<br />
AML<br />
myelogenous leukemia (AML) Roche NP28023 CR1203RE JGH<br />
AML M4 <strong>and</strong><br />
M5<br />
AML<br />
MDS<br />
ET <strong>and</strong> PV<br />
PV<br />
CLL newly<br />
diagnosed<br />
CLL<br />
DLBCL newly<br />
diagnosed<br />
DLBCL relapsed<br />
Lymphoma (FL,<br />
MZL, SLL)<br />
Mantle Cell<br />
Lymphoma<br />
A Phase I/II Study <strong>of</strong> Ribavirin <strong>and</strong> Lowdose<br />
Cytarabine Arabinoside (Ara-C) in<br />
Acute Myeloid Leukemia (AML) M4 <strong>and</strong><br />
M5 Subtypes, <strong>and</strong> AML With High eIF4E<br />
Expression<br />
A Three-part Study <strong>of</strong> Eltrombopag in<br />
Thrombocytopenic Subjects<br />
With Myelodysplastic Syndromes or<br />
Acute Myeloid Leukemia (Part 1: Openlabel,<br />
Part 2: R<strong>and</strong>omized, Double-blind,<br />
Part 3: Extension)<br />
Leukemia<br />
<strong>and</strong><br />
Lymphoma<br />
Society<br />
Mcgill or<br />
CRU # Hospital<br />
Ribavirin-<br />
002 CR0903KB JGH Dr. Assouline<br />
GlaxoSmithKl<br />
ine 114968 CR1110GSK JGH<br />
A R<strong>and</strong>omized Phase II, Open-Label Study<br />
<strong>of</strong> the Efficacy <strong>and</strong> Safety <strong>of</strong> Orally<br />
Administered SAR302503 in Patients With<br />
Polycythemia Vera (PV) or<br />
Essential Thrombocythemia (ET) Who Are San<strong>of</strong>i-<br />
Resistant or Intolerant to Hydroxyurea Aventis ARD12042 CR1117SS JGH<br />
R<strong>and</strong>omized, open label, multicenter<br />
phase III study <strong>of</strong> Efficacy <strong>and</strong> Safety in<br />
Polycythemia vera subjects who are<br />
resistant to or intolerant <strong>of</strong> hydroxyurea:<br />
JAK inhibitor INC424 tablets versus best<br />
available care (The RESPONSE Trial)<br />
An Adaptive, Comparative, R<strong>and</strong>omized,<br />
Parallel-group, Multi Center, Phase Ib<br />
Study <strong>of</strong> Subcutaneous (SC) Rituximab<br />
Versus Intravenous (IV) Rituximab Both in<br />
Combination With Chemotherapy<br />
(Fludarabine <strong>and</strong> Cyclophosphamide), in<br />
Patients With Previously Untreated CLL<br />
Incyte<br />
Corporation<br />
Novartis<br />
A Phase 2 Open-label Study <strong>of</strong> MEDI-551<br />
<strong>and</strong> Bendamustine vs Rituximab <strong>and</strong><br />
Bendamustine in Adults With Relapsed or MedImmune<br />
Refractory CLL<br />
LLC<br />
A Phase III, Multicenter, Open-Label,<br />
R<strong>and</strong>omized Trial Comparing The Efficacy<br />
Of Ga101 (Ro5072759) In Combination<br />
With Chop (G-Chop) Versus Rituximab<br />
And Chop (R-Chop) In Previously<br />
Untreated Patients With CD20-Positive<br />
Diffuse Large B-Cell Lymphoma (DLBCL)<br />
CINC424B2<br />
301 CR1101NS JGH<br />
H<strong>of</strong>fmann-La<br />
Roche BO25341 CR1014RE JGH<br />
H<strong>of</strong>fmann-La<br />
Roche<br />
Limited<br />
CD-ON-<br />
MEDI-551-<br />
1019 CR1119ME JGH<br />
BO21005<br />
(GOYA) McG 1113 JGH<br />
Main PI <strong>and</strong> PI<br />
at JGH CRA at JGH<br />
Contact<br />
Info 514-<br />
340-8222<br />
Dr. Sarit<br />
Assouline Sally Behjoudi ext. 2075<br />
Caroline<br />
Lambert ext. 4599<br />
Dr. Sarit<br />
Assouline Sally Behjoudi ext. 2075<br />
Dr. Shireen<br />
Sirhan Sally Behjoudi ext. 2075<br />
Dr. Jaroslav<br />
Prchal Sally Behjoudi ext. 2075<br />
Dr. Sarit<br />
Assouline<br />
A R<strong>and</strong>omized Phase II Study <strong>of</strong> Oral<br />
Panobinostat (LBH589) With or Without<br />
Rituximab to Treat Relapsed or Refractory<br />
CR1010-QC-<br />
B-cell Non-Hodgkin Lymphoma Q-CROC Q-CROC-02 02 JGH Dr. Assouline<br />
An Open-Label, Multicenter, R<strong>and</strong>omized,<br />
Phase III Study to Investigate the Efficacy<br />
<strong>and</strong> Safety <strong>of</strong> Bendamustine Compared<br />
With Bendamustine+RO5072759 (GA101)<br />
in Patients With Rituximab-Refractory,<br />
Indolent Non-Hodgkin's Lymphoma Genentech GAO4753g CR1001GH JGH Dr. Assouline<br />
A Phase II Study <strong>of</strong> AT7519M, a CDK<br />
Inhibitor, in Patients with Relapsed<br />
Mantle Cell Lymphoma<br />
ACTIVELY RECRUITING HEMATOLOGY TRIALS<br />
Caroline<br />
Lambert ext. 4599<br />
Dr. Sarit<br />
Assouline Sally Behjoudi ext. 2075<br />
Dr. Nathalie<br />
Johnson Matt Adamski ext. 3766<br />
Rosa<br />
Christodoulopo<br />
ulos ext. 6823<br />
Caroline<br />
Lambert ext. 4599<br />
NCIC Clinical<br />
Trials Group IND 194 JGH Dr. Assouline Matt Adamski ext. 3766<br />
Prepared by Tasnim Daginawala<br />
Study<br />
status Start date<br />
Recruite<br />
d to<br />
date<br />
Active<br />
Recruiting Jun-12 3<br />
Active<br />
HMR 7<br />
Recruiting Mar-10 JGH 16<br />
Active<br />
Recruiting<br />
STATS<br />
Rec.<br />
Target<br />
Dec2011<br />
Part 2 since<br />
Sep2012 1 2<br />
Active<br />
Recruiting Jan-12 1<br />
Active<br />
Recruiting Mar-11 1<br />
Active<br />
Recruiting Apr-11 4<br />
Active<br />
Recruiting 09-Nov-12 0<br />
Active<br />
Recruiting 27-Sep-11 2 10<br />
Active<br />
Recruiting Jan-11 7<br />
Active<br />
8<br />
1<br />
potenti<br />
Recruiting Jul-10 al pt 4<br />
Active<br />
Recruiting 21-Nov-12 0<br />
1 <strong>of</strong> 2
Type<br />
Setting/Conditi<br />
on<br />
Line Study title Sponsor Sponsor #<br />
PTCL relapsed<br />
AML<br />
CML<br />
CLL<br />
CLL<br />
CLL<br />
NHL<br />
DLBCL relapsed<br />
A Phase 3, R<strong>and</strong>omized, Two-Arm, Open-<br />
Label, Multicenter, International Trial <strong>of</strong><br />
Alisertib (MLN8237) or Investigator’s<br />
Choice (Selected Single Agent) in Patients<br />
with Relapsed or Refractory Peripheral T-<br />
Cell Lymphoma<br />
A Phase III R<strong>and</strong>omised, Double-Blind,<br />
Controlled, Parallel Group Study Of<br />
Intravenous Volasertib In Combination<br />
With Subcutaneous Low-Dose<br />
Cytarabine Vs. Placebo + Low-Dose<br />
Cytarabine In Patients ≥ 65 Years With<br />
Previously Untreated Acute Myeloid<br />
Leukaemia, Who Are Ineligible For<br />
Intensive Remission Induction Therapy<br />
A Phase 3 R<strong>and</strong>omized, Open-Label Study<br />
Of Ponatinib Versus Imatinib In Adult<br />
Patients With Newly Diagnosed Chronic<br />
Myeloid Leukemia In Chronic Phase<br />
R<strong>and</strong>omized, Double-blind, Placebocontrolled<br />
Phase 3 Study <strong>of</strong> Ibrutinib, a<br />
Bruton's Tyrosine Kinase (BTK) Inhibitor,<br />
in Combination with Bendamustine <strong>and</strong><br />
Rituximab (BR) in Subjects With Relapsed<br />
or Refractory Chronic Lymphocytic<br />
Leukemia / Small Lymphocytic<br />
Lymphoma.<br />
Millennium<br />
Pharmaceuti<br />
cals C14012 McG 1218<br />
Mcgill or<br />
CRU # Hospital<br />
RVH<br />
JGH<br />
Boehringer<br />
Ingelheim 1230.14 McG 1222 JGH<br />
ARIAD<br />
Pharmaceuti<br />
cals<br />
Janssen<br />
Research &<br />
Development<br />
LLC<br />
A Study To Evaluate The Efficacy And<br />
Safety Of Dinaciclib Or Ofatumumab In<br />
Subjects With Refractory Chronic<br />
Lymphocytic Leukemia (CLL) Merck<br />
An Open-Label, Multicenter, Phase I Trial<br />
<strong>of</strong> the Safety <strong>and</strong> Pharmacokinetics <strong>of</strong><br />
Escalating Doses <strong>of</strong> DCDS4501A in<br />
Patients With Relapsed or Refractory B-<br />
Cell Non Hodgkin's Lymphoma <strong>and</strong><br />
Chronic Lymphocytic Leukemia<br />
Genentech/R<br />
oche<br />
AP24534-<br />
12-301 McG 1221 JGH<br />
PCI-32765-<br />
CLL3001 (“<br />
HELIOS”<br />
trial) McG 1230 JGH<br />
MK-7965-<br />
012-02 McG 1232 JGH<br />
DCS4968g<br />
GO01294 CR1104GN JGH<br />
A R<strong>and</strong>omized, Open Label, Multicenter,<br />
Phase II Trial Evaluating the Safety <strong>and</strong><br />
Activity <strong>of</strong> DCDT2980S in Combination<br />
with Rituximab or DCDS4501A in<br />
combination with Rituximab in patients<br />
with Relapsed or Refractory B-Cell Non-<br />
Hodgkins Lymphoma Genentech GO27834 CR1206 JGH<br />
Main PI <strong>and</strong> PI<br />
at JGH CRA at JGH<br />
Contact<br />
Info 514-<br />
340-8222<br />
Dr. Sarit<br />
Assouline Matt Adamski ext. 3766<br />
UPCOMING HEMATOLOGY TRIALS<br />
Dr. John<br />
Storring Matt Adamski ext. 3766<br />
Study<br />
status Start date<br />
Recruite<br />
d to<br />
date<br />
Rec.<br />
Target<br />
Active<br />
Recruiting 15-Oct-12 2 in scr 10<br />
Pending IRB<br />
approval<br />
Dr. Sarit<br />
Assouline Matt Adamski ext. 3766 Pending SIV<br />
Dr. Sarit<br />
Assouline Matt Adamski ext. 3766<br />
Dr. John<br />
Storring Matt Adamski ext. 3766<br />
Dr. Sarit<br />
Assouline Dennis Baltzis ext. 3628<br />
Pending IRB<br />
approval<br />
Pending IRB<br />
approval<br />
On hold<br />
(CLL on<br />
hold, other<br />
cohorts<br />
closed) Jun-11 10<br />
Dr. Sarit<br />
Assouline Dennis Baltzis ext. 3628 Pending SIV 23-Nov-12<br />
Prepared by Tasnim Daginawala 2 <strong>of</strong> 2