Dale Doughty
Dale Doughty
Dale Doughty
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
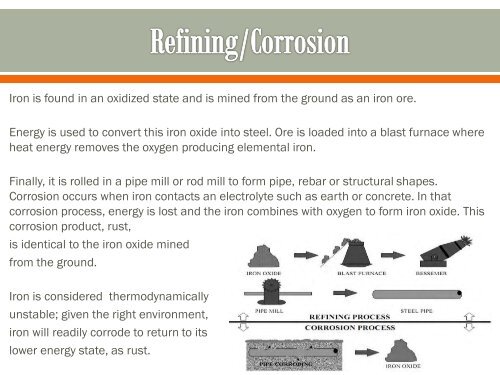
Iron is found in an oxidized state and is mined from the ground as an iron ore.<br />
Energy is used to convert this iron oxide into steel. Ore is loaded into a blast furnace where<br />
heat energy removes the oxygen producing elemental iron.<br />
Finally, it is rolled in a pipe mill or rod mill to form pipe, rebar or structural shapes.<br />
Corrosion occurs when iron contacts an electrolyte such as earth or concrete. In that<br />
corrosion process, energy is lost and the iron combines with oxygen to form iron oxide. This<br />
corrosion product, rust,<br />
is identical to the iron oxide mined<br />
from the ground.<br />
Iron is considered thermodynamically<br />
unstable; given the right environment,<br />
iron will readily corrode to return to its<br />
lower energy state, as rust.
Corrosion can be defined as the deterioration of metal due to<br />
interaction with the environment.<br />
Corrosion is a natural phenomenon that is expected to occur.<br />
Metals as high energy materials exist because heat energy<br />
was added to natural iron ores during the smelting process.<br />
Environmental contact constantly attacks these high energy<br />
materials and breaks them back down to the natural elements<br />
from which they were derived.
The anode and the cathode can be on different metals or on<br />
the same metal as shown:
For many metals, the rate of corrosion increases appreciably<br />
below a pH of about 4.<br />
Between 4 and 8 corrosion rate is fairly independent of pH.<br />
Above 8, the environment becomes passive and corrosion<br />
rates tend to decrease.
Corrosion cells may form because of differences in the<br />
electrolyte. For example, when a single metal structure spans<br />
an electrolyte made up of different types of soils, different<br />
chemical substances, different concentrations of the same<br />
substance, or temperature variations, the structure may<br />
experience voltage differences.
Potential Difference Between Anode and Cathode (Galvanic<br />
Series)<br />
Circuit resistance – Resistivity of the Electrolyte<br />
Chemical Activity
Example connecting zinc with carbon will produce a corrosion cell with a potential of<br />
about 1.4 volts.<br />
METAL<br />
VOLTS (CSE)<br />
Commercially Pure Magnesium -1.75<br />
Magnesium Alloy -1.60<br />
Zinc -1.10<br />
Aluminum Alloy -1.05<br />
Commercially Pure Aluminum -0.80<br />
Mild Steel (clean & shiny) -0.50 to -0.80<br />
Mild Steel (rusted) -0.20 to -0.50<br />
Cast Iron (not graphitized) -0.50<br />
Lead -0.50<br />
Mild Steel in Concrete -0.20<br />
Copper, Brass, Bronze -0.20<br />
High Silicon Cast Iron -0.20<br />
Carbon, Graphite, Coke +0.30
Circuit resistance includes the following:<br />
Resistance of the anode<br />
Resistance of the cathode<br />
Resistance of the electrolyte<br />
Resistance of the metallic path<br />
Increasing the resistance will reduce the corrosion rate.
Soils – High resistivity soils reduce the corrosion rate,<br />
while low resistivity soils increase the corrosion rate.<br />
CLASSIFICATION<br />
ELECTROLYTE<br />
RESISTIVITY<br />
(ohm-cm)<br />
ANTICIPATED<br />
CORROSIVITY<br />
Low Resistance 50 to 2,000 Severe<br />
Medium 2,000 to 10,000 Moderate<br />
High 10,000 to 30,000 Mild<br />
Very High Above 30,000 Increasingly Less
Passive (less corrosive) Environment<br />
High pH (neutral or basic)<br />
Low Moisture Content<br />
Lack of Salts<br />
High Resistivity<br />
Low Temperature<br />
Homogenous Environment
Uniform or near uniform (slow)<br />
Localized (moderate)<br />
Pitting (can be very rapid)
Uniform or near uniform - Corrosion attacks all areas of the<br />
metal at the same or similar rate typically by atmospheric<br />
contact. Weathering steel alloy.
Localized - Some areas of metal corrode at different rates due<br />
to heterogeneities in the metal or environment. This type of<br />
attack can approach pitting.
Pitting - Very highly localized attack resulting in small pits that<br />
may quickly penetrate to perforation.
Reference electrodes, or half-cells, are important devices that<br />
permit measuring the potential of a metal exposed to an<br />
electrolyte. An example is a structure-to-soil potential<br />
measurement.<br />
Structure-to-soil potentials are measured in reference to an<br />
electrode.
Saturated CSE Reference
Over the center line of the buried structure.
Piping<br />
Underground or submerged steel, cast iron, aluminum, and<br />
pre-stressed concrete cylinder pipelines.
Buried Tanks<br />
Underground Storage Tanks (UST) and piping
Aboveground Storage Tanks<br />
Exterior tank bottoms (both primary and secondary) of<br />
Aboveground Storage Tanks (AST).
Foundation piles<br />
Piling
Concrete<br />
Concrete bridge deck reinforcement and substructures
Corrosion can occur at the steel/concrete interface, resulting<br />
in spalling.<br />
Reinforced concrete is susceptible to chlorides when used in<br />
marine environments.<br />
Sulfate exposure physically damages concrete.
Galvanic (or sacrificial) cathodic protection makes practical use of dissimilar<br />
metal corrosion. It requires a substantial potential difference, or driving voltage,<br />
between a galvanic anode and the structure to be protected.<br />
The galvanic anode is connected to the structure it is protecting, either directly or<br />
through a test station so it can be monitored.
There are several metals commonly used as galvanic anodes:<br />
• Aluminum<br />
• Magnesium<br />
• Zinc.
Typically most effective with electrically isolated and wellcoated<br />
structures.<br />
No external power source required but limited driving potential<br />
(driving potential based upon the galvanic series).<br />
Limited output makes it ineffective when trying to protect large<br />
surfaces.<br />
Requires a low resistivity electrolyte to function well.
IM PRESSED CURRENT SY STEM<br />
Anode Groundbed<br />
Rectifier<br />
AC Power Supply<br />
Pos itiv e Cable<br />
Negativ e Cable<br />
Pipeline
Graphite Anodes<br />
Conductive Polymer<br />
High-Silicon Chromium-Bearing Cast Iron<br />
Lead-silver<br />
Mixed-metal oxide (MMO)<br />
Platinum<br />
Scrap iron or steel<br />
Metallized titanium<br />
Thermal sprayed zinc and aluminum<br />
Magnetite<br />
Aluminum
Deep anode installations are those where anodes are installed<br />
at the bottom of a drilled hole.<br />
Deep anodes are at least 15.24 m (50 ft) deep
A horizontal groundbed is installed similar to a vertical installation.<br />
The anode installation details depend upon the spacing of the<br />
anodes. If the spacing is more than 10 feet, it is usually best to<br />
excavate a separate hole for each anode. With closer spacing, it is<br />
usually more economical to excavate one long trench for both the<br />
header cable and the anodes.
Potential measurement test stations are used to monitor the<br />
effectiveness of cathodic protection, check for stray current<br />
effects, and, on unprotected or partially protected pipelines, to<br />
locate areas of active corrosion.
Test station
CIS/DCVG
CIS
CIS
-