Vol 34 No 2 suppli final.pmd - IDA KERALA STATE ::. idakerala.com
Vol 34 No 2 suppli final.pmd - IDA KERALA STATE ::. idakerala.com
Vol 34 No 2 suppli final.pmd - IDA KERALA STATE ::. idakerala.com
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Efficacy of bioglass (HABG) grafting over non grafting on mandibular third molar extraction sites<br />
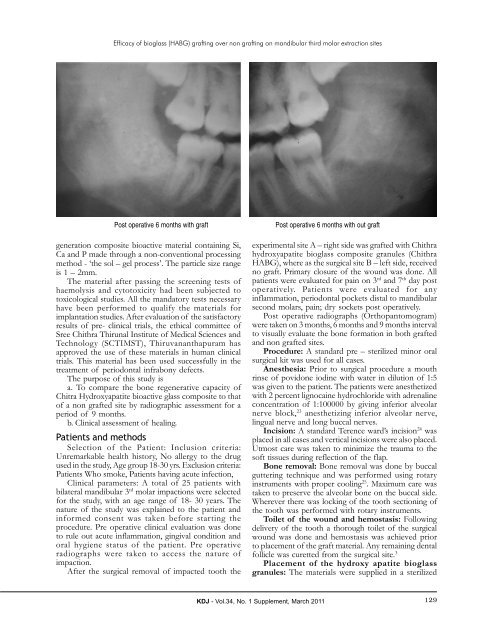
Post operative 6 months with graft Post operative 6 months with out graft<br />
generation <strong>com</strong>posite bioactive material containing Si,<br />
Ca and P made through a non-conventional processing<br />
method - ‘the sol – gel process’. The particle size range<br />
is 1 – 2mm.<br />
The material after passing the screening tests of<br />
haemolysis and cytotoxicity had been subjected to<br />
toxicological studies. All the mandatory tests necessary<br />
have been performed to qualify the materials for<br />
implantation studies. After evaluation of the satisfactory<br />
results of pre- clinical trials, the ethical <strong>com</strong>mittee of<br />
Sree Chithra Thirunal Institute of Medical Sciences and<br />
Technology (SCTIMST), Thiruvananthapuram has<br />
approved the use of these materials in human clinical<br />
trials. This material has been used successfully in the<br />
treatment of periodontal infrabony defects.<br />
The purpose of this study is<br />
a. To <strong>com</strong>pare the bone regenerative capacity of<br />
Chitra Hydroxyapatite bioactive glass <strong>com</strong>posite to that<br />
of a non grafted site by radiographic assessment for a<br />
period of 9 months.<br />
b. Clinical assessment of healing.<br />
Patients and methods<br />
Selection of the Patient: Inclusion criteria:<br />
Unremarkable health history, <strong>No</strong> allergy to the drug<br />
used in the study, Age group 18-30 yrs. Exclusion criteria:<br />
Patients Who smoke, Patients having acute infection,<br />
Clinical parameters: A total of 25 patients with<br />
bilateral mandibular 3rd molar impactions were selected<br />
for the study, with an age range of 18- 30 years. The<br />
nature of the study was explained to the patient and<br />
informed consent was taken before starting the<br />
procedure. Pre operative clinical evaluation was done<br />
to rule out acute inflammation, gingival condition and<br />
oral hygiene status of the patient. Pre operative<br />
radiographs were taken to access the nature of<br />
impaction.<br />
After the surgical removal of impacted tooth the<br />
experimental site A – right side was grafted with Chithra<br />
hydroxyapatite bioglass <strong>com</strong>posite granules (Chithra<br />
HABG), where as the surgical site B – left side, received<br />
no graft. Primary closure of the wound was done. All<br />
patients were evaluated for pain on 3 rd and 7 th day post<br />
operatively. Patients were evaluated for any<br />
inflammation, periodontal pockets distal to mandibular<br />
second molars, pain; dry sockets post operatively.<br />
Post operative radiographs (Orthopantomogram)<br />
were taken on 3 months, 6 months and 9 months interval<br />
to visually evaluate the bone formation in both grafted<br />
and non grafted sites.<br />
Procedure: A standard pre – sterilized minor oral<br />
surgical kit was used for all cases.<br />
Anesthesia: Prior to surgical procedure a mouth<br />
rinse of povidone iodine with water in dilution of 1:5<br />
was given to the patient. The patients were anesthetized<br />
with 2 percent lignocaine hydrochloride with adrenaline<br />
concentration of 1:100000 by giving inferior alveolar<br />
nerve block, 23 anesthetizing inferior alveolar nerve,<br />
lingual nerve and long buccal nerves.<br />
Incision: A standard Terence ward’s incision 24 was<br />
placed in all cases and vertical incisions were also placed.<br />
Utmost care was taken to minimize the trauma to the<br />
soft tissues during reflection of the flap.<br />
Bone removal: Bone removal was done by buccal<br />
guttering technique and was performed using rotary<br />
instruments with proper cooling 25 . Maximum care was<br />
taken to preserve the alveolar bone on the buccal side.<br />
Wherever there was locking of the tooth sectioning of<br />
the tooth was performed with rotary instruments.<br />
Toilet of the wound and hemostasis: Following<br />
delivery of the tooth a thorough toilet of the surgical<br />
wound was done and hemostasis was achieved prior<br />
to placement of the graft material. Any remaining dental<br />
follicle was curetted from the surgical site. 3<br />
Placement of the hydroxy apatite bioglass<br />
granules: The materials were <strong>suppli</strong>ed in a sterilized<br />
KDJ - <strong>Vol</strong>.<strong>34</strong>, <strong>No</strong>. 1 Supplement, March 2011<br />
129