Understanding and predicting dose dependent and idiosyncratic events
12-07-2016-1330-Paul-Watkins-YES
12-07-2016-1330-Paul-Watkins-YES
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
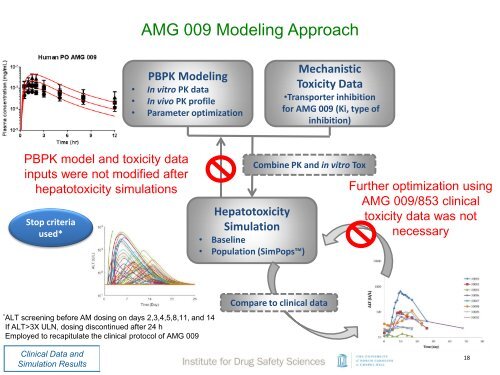
AMG 009 Modeling Approach<br />
PBPK Modeling<br />
• In vitro PK data<br />
• In vivo PK profile<br />
• Parameter optimization<br />
Mechanistic<br />
Toxicity Data<br />
•Transporter inhibition<br />
for AMG 009 (Ki, type of<br />
inhibition)<br />
PBPK model <strong>and</strong> toxicity data<br />
inputs were not modified after<br />
hepatotoxicity simulations<br />
Stop criteria<br />
used*<br />
Hepatotoxicity<br />
Simulation<br />
• Baseline<br />
• Population (SimPops)<br />
Combine PK <strong>and</strong> in vitro Tox<br />
Further optimization using<br />
AMG 009/853 clinical<br />
toxicity data was not<br />
necessary<br />
*<br />
ALT screening before AM dosing on days 2,3,4,5,8,11, <strong>and</strong> 14<br />
If ALT>3X ULN, dosing discontinued after 24 h<br />
Employed to recapitulate the clinical protocol of AMG 009<br />
Compare to clinical data<br />
Clinical Data <strong>and</strong><br />
Simulation Results<br />
18