1st_Semester_Notebook
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
http://www.bbc.co.uk/education/guides/zm9hvcw/revision<br />
LG: The student will learn how Hydrocarbons are named and the<br />
general properties of Hydrocarbons.<br />
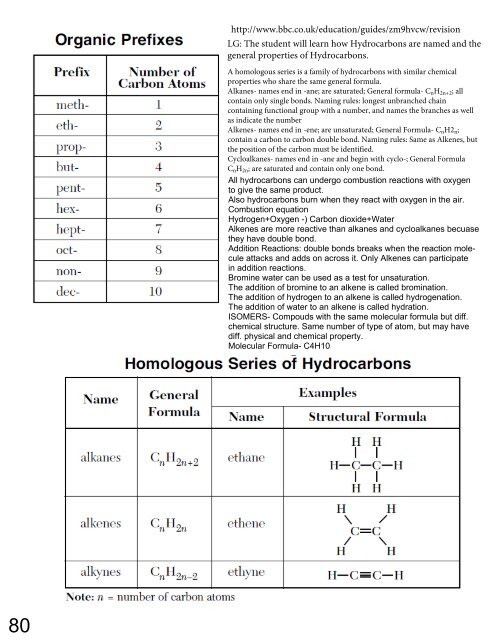
A homologous series is a family of hydrocarbons with similar chemical<br />
properties who share the same general formula.<br />
Alkanes- names end in -ane; are saturated; General formula- C n H 2n+2 ; all<br />
contain only single bonds. Naming rules: longest unbranched chain<br />
containing functional group with a number, and names the branches as well<br />
as indicate the number<br />
Alkenes- names end in -ene; are unsaturated; General Formula- C n H2 n ;<br />
contain a carbon to carbon double bond. Naming rules: Same as Alkenes, but<br />
the position of the carbon must be identified.<br />
Cycloalkanes- names end in -ane and begin with cyclo-; General Formula<br />
C n H 2n ; are saturated and contain only one bond.<br />
All hydrocarbons can undergo combustion reactions with oxygen<br />
to give the same product.<br />
Also hydrocarbons burn when they react with oxygen in the air.<br />
Combustion equation<br />
Hydrogen+Oxygen -) Carbon dioxide+Water<br />
Alkenes are more reactive than alkanes and cycloalkanes becuase<br />
they have double bond.<br />
Addition Reactions: double bonds breaks when the reaction molecule<br />
attacks and adds on across it. Only Alkenes can participate<br />
in addition reactions.<br />
Bromine water can be used as a test for unsaturation.<br />
The addition of bromine to an alkene is called bromination.<br />
The addition of hydrogen to an alkene is called hydrogenation.<br />
The addition of water to an alkene is called hydration.<br />
ISOMERS- Compouds with the same molecular formula but diff.<br />
chemical structure. Same number of type of atom, but may have<br />
diff. physical and chemical property.<br />
Molecular Formula- C4H10<br />
80