THUYẾT VÂN ĐẠO PHÂN TỬ MOLECULAR ORBITAL (MO)
https://drive.google.com/file/d/1b4JflWGDKGIc0QUSzX9TL5LSSCAL10aA/view?usp=sharing
https://drive.google.com/file/d/1b4JflWGDKGIc0QUSzX9TL5LSSCAL10aA/view?usp=sharing
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
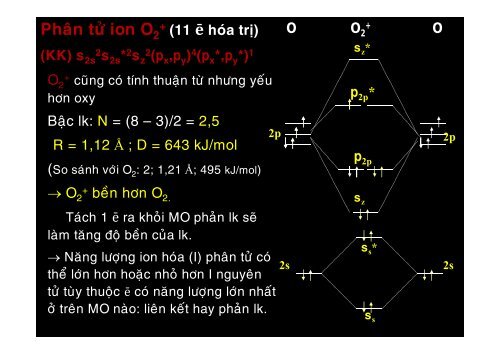
Phaân töû ion O 2 + (11 ē hoùa trò)<br />
O<br />
O 2<br />
+<br />
O<br />
(KK) 2s2 2s * 2 z2 ( x , y ) 4 ( x *, y *) 1<br />
z *<br />
O 2 + cuõng coù tính thuaän töø nhöng yeáu<br />
hôn oxy<br />
2p *<br />
Baäc lk: N = (8 – 3)/2 = 2,5<br />
R = 1,12 Å ; D = 643 kJ/mol<br />
2p<br />
2p<br />
(So saùnh vôùi O 2 : 2; 1,21 Å; 495 kJ/mol)<br />
2p<br />
O 2+ beàn hôn O 2.<br />
z<br />
Taùch 1 ē ra khoûi <strong>MO</strong> phaûn lk seõ<br />
laøm taêng ñoä beàn cuûa lk.<br />
Naêng löôïng ion hoùa (I) phaân töû coù<br />
theå lôùn hôn hoaëc nhoû hôn I nguyeân<br />
töû tuøy thuoäc ē coù naêng löôïng lôùn nhaát<br />
ôû treân <strong>MO</strong> naøo: lieân keát hay phaûn lk.<br />
2s<br />
s *<br />
s<br />
77<br />
2s