surface pretreatment by phosphate conversion coatings – a review
surface pretreatment by phosphate conversion coatings – a review
surface pretreatment by phosphate conversion coatings – a review
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Surface <strong>pretreatment</strong> <strong>by</strong> <strong>phosphate</strong> <strong>conversion</strong> <strong>coatings</strong> <strong>–</strong> a <strong>review</strong><br />
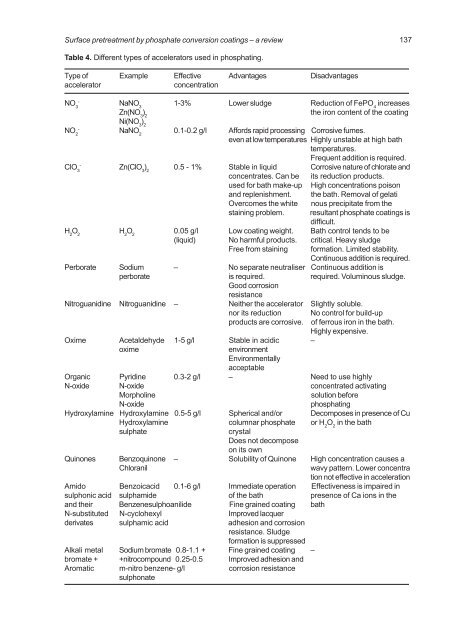
Table 4. Different types of accelerators used in phosphating.<br />
Type of Example Effective Advantages Disadvantages<br />
accelerator concentration<br />
- NO NaNO 1-3% Lower sludge Reduction of FePO increases<br />
3<br />
3 4<br />
Zn(NO ) the iron content of the coating<br />
3 2<br />
Ni(NO ) 3 2<br />
NO 2<br />
ClO 3<br />
- NaNO 2 0.1-0.2 g/l Affords rapid processing Corrosive fumes.<br />
137<br />
even at low temperatures Highly unstable at high bath<br />
temperatures.<br />
Frequent addition is required.<br />
- Zn(ClO 3 ) 2 0.5 - 1% Stable in liquid Corrosive nature of chlorate and<br />
concentrates. Can be its reduction products.<br />
used for bath make-up High concentrations poison<br />
and replenishment. the bath. Removal of gelati<br />
Overcomes the white nous precipitate from the<br />
staining problem. resultant <strong>phosphate</strong> <strong>coatings</strong> is<br />
difficult.<br />
H 2 O 2 H 2 O 2 0.05 g/l Low coating weight. Bath control tends to be<br />
(liquid) No harmful products. critical. Heavy sludge<br />
Free from staining formation. Limited stability.<br />
Continuous addition is required.<br />
Perborate Sodium <strong>–</strong> No separate neutraliser Continuous addition is<br />
perborate is required. required. Voluminous sludge.<br />
Good corrosion<br />
resistance<br />
Nitroguanidine Nitroguanidine <strong>–</strong> Neither the accelerator Slightly soluble.<br />
nor its reduction No control for build-up<br />
products are corrosive. of ferrous iron in the bath.<br />
Highly expensive.<br />
Oxime Acetaldehyde 1-5 g/l Stable in acidic <strong>–</strong><br />
oxime environment<br />
Environmentally<br />
acceptable<br />
Organic Pyridine 0.3-2 g/l <strong>–</strong> Need to use highly<br />
N-oxide N-oxide concentrated activating<br />
Morpholine solution before<br />
N-oxide phosphating<br />
Hydroxylamine Hydroxylamine 0.5-5 g/l Spherical and/or Decomposes in presence of Cu<br />
Hydroxylamine columnar <strong>phosphate</strong> or H 2 O 2 in the bath<br />
sulphate crystal<br />
Does not decompose<br />
on its own<br />
Quinones Benzoquinone <strong>–</strong> Solubility of Quinone High concentration causes a<br />
Chloranil wavy pattern. Lower concentra<br />
tion not effective in acceleration<br />
Amido Benzoicacid 0.1-6 g/l Immediate operation Effectiveness is impaired in<br />
sulphonic acid sulphamide of the bath presence of Ca ions in the<br />
and their Benzenesulphoanilide Fine grained coating bath<br />
N-substituted N-cyclohexyl Improved lacquer<br />
derivates sulphamic acid adhesion and corrosion<br />
resistance. Sludge<br />
formation is suppressed<br />
Alkali metal Sodium bromate 0.8-1.1 + Fine grained coating <strong>–</strong><br />
bromate + +nitrocompound 0.25-0.5 Improved adhesion and<br />
Aromatic m-nitro benzene- g/l corrosion resistance<br />
sulphonate