et. al. J. Am. Chem. Soc.
et. al. J. Am. Chem. Soc.
et. al. J. Am. Chem. Soc.
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
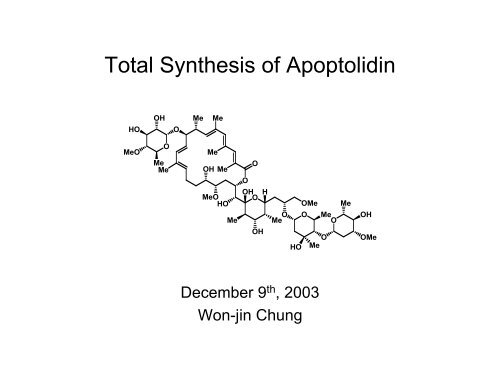
Tot<strong>al</strong> Synthesis of Apoptolidin<br />
HO<br />
MeO<br />
OH<br />
O<br />
Me<br />
Me<br />
O<br />
Me<br />
Me<br />
Me OH Me<br />
MeO HO<br />
Me<br />
O<br />
December 9 th , 2003<br />
O<br />
OH<br />
O<br />
OH<br />
H<br />
O O<br />
Me<br />
HO<br />
Won-jin Chung<br />
OMe<br />
O<br />
Me<br />
Me<br />
O<br />
Me<br />
OH<br />
OMe
HO<br />
MeO<br />
OH<br />
O<br />
Me<br />
Me<br />
O<br />
Me<br />
Me<br />
Me OH Me<br />
MeO HO<br />
Me<br />
O<br />
O<br />
OH<br />
O<br />
OH<br />
H<br />
Apoptolidin<br />
O O<br />
Me<br />
HO<br />
OMe<br />
O<br />
Me<br />
Me<br />
O<br />
Me<br />
• Formula : C 58 H 96 O 21<br />
• Molecular Weight : 1129.37<br />
• Induced apoptotic cell death in rat glia cells transformed with a oncogene<br />
OH<br />
OMe<br />
(IC 50 = 11 ng/mL) but not in norm<strong>al</strong> cells (IC 50 > 100 mg/mL)<br />
• Isolated from Nocardiopsis sp.<br />
• Stereochemistry was d<strong>et</strong>ermined by<br />
NMR and degradation studies.<br />
• The top 0.1% most selective agents tested in the NCI's 60 human cancer cell<br />
line panel out of the more than 37,000 compounds an<strong>al</strong>yzed to date
HO<br />
MeO<br />
OH<br />
O<br />
Me<br />
Me<br />
O<br />
Me<br />
Me<br />
Me OH Me<br />
MeO HO<br />
Me<br />
O<br />
O<br />
OH<br />
O<br />
OH<br />
H<br />
Apoptolidin<br />
O O<br />
Me<br />
HO<br />
OMe<br />
O<br />
Me<br />
Me<br />
O<br />
• 20 membered macrolide with a side chain containing a 6 membered cyclic hemik<strong>et</strong><strong>al</strong>.<br />
Me<br />
OH<br />
OMe<br />
• A disaccharide and a novel 6-deoxy-glucose residue<br />
• 25 stereocenters and 5 geom<strong>et</strong>ric<strong>al</strong> sites<br />
• Formula : C 58 H 96 O 21<br />
• Molecular Weight : 1129.37<br />
• Isolated from Nocardiopsis sp.<br />
• Stereochemistry was d<strong>et</strong>ermined by<br />
NMR and degradation studies.
Apoptosis<br />
There are two ways in which cells die:<br />
1. they are killed by injurious agents<br />
2. they are induced to commit suicide<br />
-Programmed Cell Death, Apoptosis<br />
Why suicide?<br />
- for proper development<br />
- to destroy cells that represent a threat to the integrity of the organism
Key Disconnections<br />
Koert, U. <strong>et</strong>. <strong>al</strong>. Angew. <strong>Chem</strong>. Int. Ed. 2001, 40, 2063.
BnO<br />
MeO<br />
O<br />
O O<br />
1. Li TMS<br />
2. LiHMDS, MeI<br />
3. TBAF<br />
88 % over three steps<br />
OMe<br />
Synthesis of the Southern H<strong>al</strong>f<br />
Ru-(S)-BINAP, H 2<br />
BnO<br />
MeOH, DMF, 95 o C<br />
86 % yield, 97 % ee<br />
OMe<br />
MeO<br />
H<br />
O OH<br />
Cp 2ZrCl 2, LiEt 3BH, NIS<br />
THF, 88 %<br />
OMe<br />
1. TBSCl,<br />
imidazole, 95 %<br />
2. DIBAL-H<br />
hexane, 90 %<br />
BnO<br />
H<br />
OMe<br />
O OTBS<br />
I<br />
OMe<br />
Koert, U. <strong>et</strong>. <strong>al</strong>. Angew. <strong>Chem</strong>. Int. Ed. 2001, 40, 2063.<br />
Koert, U. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron L<strong>et</strong>t. 2000, 41, 621.
H<br />
O OTBS<br />
Me 4NBH(OAc) 3<br />
HOAc, MeCN<br />
74 %, dr >95 : 5<br />
OMe<br />
O<br />
O<br />
O<br />
Bn<br />
N<br />
O<br />
Synthesis of the Southern H<strong>al</strong>f<br />
O<br />
N<br />
Bn<br />
O<br />
Me<br />
O<br />
Sn(OTf) 2, Et 3N<br />
97 %, dr 96 : 4<br />
Me<br />
OH<br />
Me<br />
OH<br />
Me<br />
OTBS<br />
OMe<br />
O<br />
O<br />
Bn<br />
N<br />
O<br />
Me<br />
O<br />
Me<br />
OH<br />
OTBS<br />
1. AlMe 3, MeNH(OMe) HCl<br />
HOAc, CH 2Cl 2, 81 %<br />
2. TMSCl, imidazole, 86 %<br />
OMe<br />
Me<br />
OMe<br />
N<br />
O<br />
Me<br />
O<br />
Me<br />
O<br />
TMS TMS<br />
OTBS<br />
OMe<br />
Koert, U. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron L<strong>et</strong>t. 2000, 41, 621.
BnO<br />
PPTS<br />
OMe<br />
MeOH, CH 2Cl 2<br />
87 % two steps<br />
1. Ac 2O, py,<br />
DMAP, 69 %<br />
2. H 2, Pd(OH) 2/C<br />
EtOAc, 97 %<br />
3. Dess-Martin<br />
periodinane<br />
py, CH 2Cl 2, 91 %<br />
OMe<br />
Me<br />
Me<br />
N<br />
Me<br />
OMe<br />
t-BuLi<br />
+<br />
O O O OTBS Et2O, -78<br />
TMS TMS<br />
o I BnO<br />
C<br />
BnO<br />
H<br />
O<br />
OMe<br />
OMe<br />
Synthesis of the Southern H<strong>al</strong>f<br />
Me<br />
OMe H<br />
O<br />
OH<br />
AcO<br />
OMe H<br />
O<br />
OAc<br />
Me<br />
OTBS<br />
Me OTBS<br />
Me OTBS<br />
OMe<br />
OMe<br />
1. TBSOTf<br />
2,6-lutidine<br />
CH 2Cl 2, 98 %<br />
2. K 2OsO 2(OH) 4<br />
NMO, 90 days<br />
78 %, dr 6 : 1<br />
BnO<br />
OMe<br />
OMe<br />
O<br />
Me<br />
O<br />
Me<br />
O<br />
TMS TMS<br />
HO<br />
OMe H<br />
O<br />
OH<br />
Me<br />
OTBS<br />
OTBS<br />
Me OTBS<br />
OMe<br />
OMe<br />
Koert, U. <strong>et</strong>. <strong>al</strong>. Angew. <strong>Chem</strong>. Int. Ed. 2001, 40, 2063.
H<br />
O<br />
OMe<br />
AcO<br />
OMe H<br />
O<br />
OAc<br />
Me<br />
OTBS<br />
Synthesis of the Southern H<strong>al</strong>f<br />
Me OTBS<br />
OMe<br />
n-Bu 3Sn<br />
Me<br />
58 %, dr 93 : 7<br />
MgBr<br />
n-Bu 3Sn<br />
Me<br />
OH<br />
OMe<br />
AcO<br />
Me<br />
OAc<br />
OMe H<br />
O<br />
OTBS<br />
Me OTBS<br />
OMe<br />
Koert, U. <strong>et</strong>. <strong>al</strong>. Angew. <strong>Chem</strong>. Int. Ed. 2001, 40, 2063.
HO<br />
O<br />
Me<br />
O<br />
1. DIBAL-H<br />
2. MnO 2<br />
3. Me CO2Et PPh 3<br />
86 % three steps<br />
1. DIBAL-H, 80 %<br />
2. MnO 2<br />
3. Me CO2Et PPh 3<br />
60 % two steps<br />
1. TBSOTf<br />
2,6-lutidine<br />
CH 2Cl 2, 90 %<br />
2. DIBAL-H<br />
CH 2Cl 2, 95 %<br />
TBSO<br />
TESO<br />
TBSO<br />
Synthesis of the Northern H<strong>al</strong>f<br />
I<br />
Me<br />
Me<br />
TBSO<br />
Me<br />
Me<br />
Me<br />
Me<br />
O<br />
CO 2Et<br />
Me<br />
Me CO 2Et<br />
OH<br />
1.<br />
Me CO 2Et<br />
PPh 3<br />
2. TESCl<br />
imidazole<br />
82 % two steps<br />
1. camphorsulfonic acid<br />
MeOH, CH 2Cl 2, 95 %<br />
2. Dess-Martin periodinane<br />
3. CrCl 2, CHI 3, hydroquinone<br />
69 % two steps<br />
TBSO<br />
TESO<br />
TBSO<br />
I<br />
Me<br />
Me<br />
Me<br />
CO 2Et<br />
Me<br />
Me<br />
CO 2Et<br />
Koert, U. <strong>et</strong>. <strong>al</strong>. Angew. <strong>Chem</strong>. Int. Ed. 2001, 40, 2063.
n-Bu 3Sn<br />
Me<br />
TBSO<br />
I<br />
OH<br />
Me<br />
OMe<br />
AcO<br />
Me<br />
Me<br />
Me<br />
+<br />
Compl<strong>et</strong>ion of the Synthesis<br />
Me CO 2Et<br />
OAc<br />
OMe H<br />
O<br />
OTBS<br />
Me OTBS<br />
Cu I -thiophene-2-carboxylate<br />
N-m<strong>et</strong>hylpyrrolidinone, 80 %<br />
OMe<br />
TBSO<br />
Me<br />
Me<br />
OH<br />
Me<br />
Me<br />
Me CO 2Et<br />
OMe<br />
AcO<br />
Me<br />
OAc<br />
OMe H<br />
O<br />
OTBS<br />
Me OTBS<br />
OMe<br />
Koert, U. <strong>et</strong>. <strong>al</strong>. Angew. <strong>Chem</strong>. Int. Ed. 2001, 40, 2063.
TBSO<br />
Me<br />
HF, py<br />
Me<br />
THF, 55 %<br />
OH<br />
Me<br />
Me<br />
Me CO 2Et<br />
OMe<br />
AcO<br />
Me<br />
Me<br />
HO<br />
OAc<br />
OMe H<br />
O<br />
Compl<strong>et</strong>ion of the Synthesis<br />
OTBS<br />
Me<br />
Me OTBS<br />
OH<br />
Me<br />
Me<br />
Me<br />
OMe<br />
HO<br />
Me<br />
OMe<br />
O<br />
O<br />
OMe H<br />
O<br />
OH<br />
1. LiOH, 87 %<br />
2. Cl<br />
O<br />
Cl Cl<br />
Cl<br />
Et 3N, DMAP, 74 %<br />
Me OH<br />
OMe<br />
TBSO<br />
Me<br />
Me<br />
Me<br />
Me<br />
OH Me<br />
OMe<br />
HO<br />
Me<br />
O<br />
O<br />
OMe H<br />
O<br />
OTBS<br />
Me OTBS<br />
19 steps (the longest linear)<br />
2.8 % yield<br />
OMe<br />
Koert, U. <strong>et</strong>. <strong>al</strong>. Angew. <strong>Chem</strong>. Int. Ed. 2001, 40, 2063.
Key Disconnections<br />
Toshima, K. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron L<strong>et</strong>t. 2001, 42, 8873.
H<br />
O<br />
Me<br />
1. TIPSOTf<br />
py, 90%<br />
OTr<br />
2. CSA, 87 %<br />
H<br />
TIPSO<br />
THF, 60 %<br />
H<br />
Me<br />
Synthesis of the Northern H<strong>al</strong>f<br />
TMS, n-BuLi<br />
Cram : anti-Cram = 2 : 1<br />
OH<br />
HO<br />
Me<br />
TMS<br />
(COCl) 2, DMSO, Et 3N<br />
CH 2Cl 2<br />
OTr<br />
TIPSO<br />
H<br />
K 2CO 3<br />
MeOH, 99 %<br />
Me<br />
O<br />
H<br />
HO<br />
H<br />
Me CO 2Et<br />
PPh 3<br />
toluene, 96 %<br />
E : Z = 100 : 0<br />
Me<br />
OTr<br />
TIPSO<br />
H<br />
Me<br />
Me<br />
CO 2Et<br />
Toshima, K. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron L<strong>et</strong>t. 2001, 42, 8873.
TIPSO<br />
H<br />
Me<br />
1. DIBAL-H, 97%<br />
2. MnO 2, 98 %<br />
3. Me CO 2Et<br />
P(O)(OEt) 2<br />
n-BuLi, 99 %<br />
E : Z = 100 : 0<br />
Me<br />
CO 2Et<br />
TIPSO<br />
H<br />
Synthesis of the Northern H<strong>al</strong>f<br />
Me<br />
1. DIBAL-H, 98%<br />
2. MnO 2, 98 %<br />
3. Me CO 2Et<br />
PPh 3<br />
toluene, 99 %<br />
E : Z = 97 : 3<br />
Me<br />
Me<br />
Me CO 2Et<br />
TIPSO<br />
H<br />
Me<br />
Me<br />
Me<br />
1. n-Bu 3SnH, PdCl 2(PPh 3) 2<br />
toluene, 0 o C, 92 %<br />
2. aq. LiOH<br />
1,4-dioxane, 80 o C, 79 %<br />
CO 2Et<br />
TIPSO<br />
n-Bu 3Sn<br />
Me Me<br />
Me<br />
Me CO 2H<br />
Toshima, K. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron L<strong>et</strong>t. 2001, 42, 8873.
HO<br />
Me<br />
O<br />
Me<br />
OMPM<br />
O<br />
Dess-Martin<br />
periodinane<br />
py, rt, 73 %<br />
H<br />
Me<br />
O<br />
O<br />
Me<br />
OMPM<br />
(c-Hex) 2BH<br />
HO OMPM Dess-Martin<br />
periodinane<br />
H OMPM<br />
THF, rt, 88 % O<br />
py, rt, 99 %<br />
O<br />
O<br />
Me<br />
O<br />
Me<br />
Me<br />
O<br />
Me<br />
1. MeOTf<br />
2,6-di-t-butylpyridine, 90 %<br />
2. (c-Hex) 2BH, THF, 89 %<br />
Synthesis of the Northern H<strong>al</strong>f<br />
OH<br />
OTBS<br />
OMe<br />
O<br />
Me<br />
Me<br />
O<br />
OMPM<br />
O<br />
Ph 3P=CH 2<br />
benzene, 81 %<br />
Me<br />
O<br />
Me<br />
OTBS<br />
OMPM<br />
n-Bu3Sn OH<br />
MgBr2 Et2O, 83 % O<br />
1. TsCl, Et 3N, TMEDA<br />
MeCN, 95 %<br />
2. Li H<br />
DMSO, 86 %<br />
H<br />
O<br />
OTBS<br />
OMe<br />
O<br />
Me<br />
OTBS<br />
Me<br />
Me<br />
O<br />
Me<br />
OMPM<br />
OMPM<br />
Toshima, K. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron L<strong>et</strong>t. 2001, 42, 8873.<br />
O
H<br />
OTBS<br />
OMe<br />
O<br />
Me<br />
Me<br />
OMPM<br />
O<br />
Synthesis of the Northern H<strong>al</strong>f<br />
1. MeI, n-BuLi<br />
THF, 96 %<br />
2. Cp 2ZrHCl, NIS<br />
THF, 80 %<br />
Me<br />
I<br />
OTBS<br />
OMe<br />
O<br />
Me<br />
Me<br />
OMPM<br />
O<br />
DDQ<br />
CH 2Cl 2, 99 %<br />
Me<br />
I<br />
OTBS<br />
OMe<br />
O<br />
Me<br />
Me<br />
OH<br />
Toshima, K. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron L<strong>et</strong>t. 2001, 42, 8873.<br />
O
TIPSO<br />
n-Bu 3Sn<br />
Me<br />
I<br />
Me Me<br />
+<br />
Me<br />
OTBS<br />
Me CO 2H<br />
OMe<br />
O<br />
Me<br />
Compl<strong>et</strong>ion of the Synthesis<br />
Me<br />
OH<br />
O<br />
1.<br />
Cl<br />
Cl Cl<br />
Et3N, DMAP, 89 %<br />
2. TBAF, 67 %<br />
O<br />
Cl<br />
HO<br />
n-Bu 3Sn<br />
I<br />
Me<br />
Me Me<br />
OH<br />
Me<br />
Me<br />
OMe<br />
O<br />
Toshima, K. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron L<strong>et</strong>t. 2001, 42, 8873.<br />
Me<br />
Me<br />
O<br />
O<br />
O
HO<br />
n-Bu 3Sn<br />
I<br />
Me<br />
Me Me<br />
OH<br />
Me<br />
OMe<br />
O<br />
Me<br />
Compl<strong>et</strong>ion of the Synthesis<br />
Me<br />
Me<br />
O<br />
O<br />
O<br />
PdCl2(MeCN) 2<br />
Ph2PO2NBu4, LiCl<br />
DMF, 30 %<br />
Me<br />
HO<br />
Me Me<br />
OH<br />
Me<br />
Me<br />
OMe<br />
O<br />
Me<br />
Me<br />
17 steps (the longest linear)<br />
5.2 % yield<br />
Toshima, K. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron L<strong>et</strong>t. 2001, 42, 8873.<br />
O<br />
O<br />
O
MeO<br />
Stille coupling<br />
HO<br />
OH<br />
O<br />
O<br />
Key Disconnections<br />
Glycosidation<br />
OH<br />
MeO HO<br />
O<br />
O<br />
OH H<br />
O<br />
OH<br />
O O<br />
HO<br />
Yamaguchi macrolactonization<br />
OMe<br />
O<br />
O<br />
Glycosidation<br />
OH<br />
OMe
R<strong>et</strong>rosynth<strong>et</strong>ic An<strong>al</strong>ysis<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP
TES<br />
TBSOTf<br />
2,6-lutidine<br />
Synthesis of Vinylstannane M<strong>et</strong>hyl Ester (C 1 ~ C 11)<br />
O<br />
H<br />
(+) (Ipc) 2B<br />
BF 3 OEt 2<br />
CH 2Cl 2, 0 o C, 2 h, 97 % TES<br />
Me CO 2Et<br />
PPh 3<br />
toluene, 100 o C, 12 h<br />
E : Z > 9 : 1,<br />
90 % over two steps<br />
THF, -78 o C, 6 h<br />
TES<br />
TBSO<br />
TBSO<br />
Me<br />
Me<br />
Me Me<br />
CO 2Et<br />
NaBO 3 4H 2O<br />
THF / H 2O (2 / 1), rt, 12 h, 82 %<br />
1. O 3, CH 2Cl 2, -78 o C<br />
2. PPh 3, -78 o C then, rt, 12 h<br />
1. DIBAL-H<br />
toluene, -78 o C, 2 h, 90 %<br />
2. 0.05 eq. TPAP, NMO<br />
4 MS, CH 2Cl 2, rt, 30 min<br />
Å<br />
TES<br />
TES<br />
TES<br />
TBSO<br />
TBSO<br />
OH<br />
Me<br />
Me<br />
O<br />
H<br />
Me Me<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP<br />
O<br />
H
TES<br />
TBSO<br />
TBAF<br />
Synthesis of Vinylstannane M<strong>et</strong>hyl Ester (C 1 ~ C 11)<br />
Me Me<br />
O<br />
H<br />
Me<br />
CO 2Et<br />
PO(OEt) 2<br />
HO<br />
H<br />
, NaH<br />
THF, 0 o C then rt, 1 h<br />
E : Z > 10 : 1,<br />
81 % over two steps<br />
Me<br />
TBSO<br />
Me<br />
TES<br />
Me<br />
Me<br />
CO 2Et<br />
THF, 0<br />
Me<br />
oC then rt, 1 h, 98 % THF, 0 oC, 30 min, 69 %<br />
Me<br />
Me<br />
CO 2Me<br />
n-Bu 3SnH<br />
1. DIBAL-H, 89 %<br />
2. TPAP, NMO<br />
3.<br />
Me<br />
CO 2Me<br />
PO(OEt) 2<br />
0.05 eq. Pd(PPh 3) 2Cl 2<br />
, NaH<br />
E : Z > 10 : 1,<br />
95 % over two steps<br />
TBSO<br />
HO<br />
n-Bu 3Sn<br />
Me<br />
TES<br />
Me<br />
Me<br />
Me<br />
Me<br />
Me<br />
Me<br />
Me<br />
CO 2Me<br />
CO 2Me<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP
HO<br />
OH<br />
H<br />
O<br />
Synthesis of Ac<strong>et</strong>ylenic Aldehyde (C 12 ~ C 20)<br />
O<br />
OH<br />
1. Me 2C(OMe) 2<br />
0.02 eq. TsOH<br />
CH 2Cl 2, rt, 3 h<br />
2.<br />
Me<br />
1. Me 2SO 4, BnEt 3NCl<br />
50 % aq. NaOH, CH 2Cl 2<br />
0 o C then rt, 86 %<br />
2. PPTS, MeOH, rt, 90 %<br />
3. SEMCl, TBAI, DIPEA<br />
CH 2Cl 2, 40 o C, 99 %<br />
OMe<br />
CH 2Cl 2, rt, 7 h<br />
Me<br />
O<br />
Me<br />
O<br />
Me<br />
OMe<br />
O<br />
Me<br />
O<br />
O<br />
OSEM<br />
H<br />
N<br />
O<br />
O<br />
O<br />
OMIP<br />
morpholine<br />
Me<br />
65 o C, 15 h,<br />
97 % over three steps<br />
1. LiBHEt 3, THF, 0 o C, 81 %<br />
2. PMBCl, NaH, TBAI<br />
DMF, rt, 83 %<br />
3. 60 % aq. AcOH, rt, 88 %<br />
O<br />
HO<br />
Me<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP<br />
O<br />
OH<br />
OH<br />
OMe<br />
O<br />
OMIP<br />
OSEM<br />
N<br />
O<br />
OPMB
HO<br />
OH<br />
OMe<br />
Synthesis of Ac<strong>et</strong>ylenic Aldehyde (C 12 ~ C 20)<br />
OSEM<br />
OPMB<br />
1. 1.5 % HCl in MeOH<br />
MeOH, rt, 30 min, 95 %<br />
2. 1.2 eq. DDQ<br />
4 MS, CH2Cl2, 0 o Å<br />
C<br />
then rt, 2 h, 78 %<br />
1. DIBAL-H<br />
CH 2Cl 2, 72 %<br />
2. SO 3 py, Et 3N<br />
DMSO / CH 2Cl 2 (2 / 1)<br />
95 %<br />
CH 3C(OCH 3) 3<br />
PPTS<br />
CH 2Cl 2, rt, 45 min<br />
then, AcCl<br />
CH 2Cl 2, rt, 5 h<br />
then, K 2CO 3<br />
MeOH, rt, 12 h, 88 %<br />
H<br />
CH 3<br />
OH<br />
OMe<br />
OTBS<br />
OMe<br />
O<br />
O<br />
O<br />
O<br />
PMB<br />
H<br />
OPMB<br />
OMe<br />
OSEM<br />
OPMB<br />
MgBr<br />
Et 2O, - 78 o C<br />
1 h, 94 %<br />
1. TBSOTf, 2,6-lutidine<br />
CH 2Cl 2, 98 %<br />
2. n-BuLi, MeI<br />
THF, 99 %<br />
H<br />
CH 3<br />
OH<br />
OTBS<br />
OMe<br />
OMe<br />
OSEM<br />
OPMB<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP<br />
O<br />
O<br />
PMB
O<br />
OH<br />
Ac<strong>et</strong>ylenic Aldehyde – The Second Generation<br />
1. DDQ<br />
CH 2Cl 2 / H 2O (18 / 1)<br />
97 %<br />
2. SO 3 py, Et 3N<br />
DMSO / CH 2Cl 2 (2 / 1)<br />
96 %<br />
H 3C<br />
1. MeOTf<br />
2,6-di-t-butyl-4-m<strong>et</strong>hylpyridine<br />
CH 2Cl 2, 85 %<br />
2. AD-Mix-a<br />
dr 6 : 1, 85 %<br />
1. PMBCl, NaH, TBAI<br />
DMF, 71 %<br />
2.<br />
MgBr<br />
Et 2O, 90 %<br />
H<br />
CH 3<br />
OTBS<br />
OTBS<br />
OH<br />
O<br />
OMe<br />
H<br />
OH<br />
OPMB<br />
OH<br />
1. TBSOTf, 2,6-lutidine<br />
CH 2Cl 2, 97 %<br />
2. n-BuLi, MeI<br />
THF, 95 %<br />
(+) (Ipc) 2B<br />
Et 2O, -100 o C, 2 h<br />
then 30 % aq. H 2O 2<br />
3 M aq. NaOH, rt, 15 h<br />
dr 10 : 1, 95 %<br />
1. DMBA, CSA<br />
toluene, 110 o C, 99 %<br />
2. DIBAL-H<br />
CH 2Cl 2, 70 %<br />
3. SO 3 py, Et 3N<br />
DMSO / CH 2Cl 2 (2 / 1)<br />
90 %<br />
H 3C<br />
CH 3<br />
CH 3<br />
OTBS<br />
OH<br />
OTBS<br />
OTBS<br />
OMe<br />
OPMB<br />
ODMB<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP<br />
O<br />
H
O<br />
(+) (Ipc) 2B<br />
Synthesis of Dithiane (C 21 ~ C 28)<br />
1,3-dithiane<br />
n-BuLi<br />
OMe<br />
THF, -78<br />
S<br />
oC then rt<br />
30 min, 91 %<br />
Me<br />
BF 3 OEt 2, THF, -78 o C<br />
then, NaBO 3 4H 2O<br />
THF / H 2O (1 / 1), rt, 98 %<br />
Me<br />
OH<br />
S<br />
OPMB<br />
OMe<br />
OH<br />
OMe<br />
1. PMBCl, NaH<br />
DMF, 99 %<br />
2. MeI, K 2CO 3<br />
MeCN / H 2O (6 / 1)<br />
92 %<br />
1. TBSOTf, 2,6-lutidine<br />
CH 2Cl 2, 97 %<br />
H<br />
O<br />
H<br />
Me<br />
2. OsO4, NMO, rt, 12 h<br />
O O<br />
then NaIO4, rt, 2 h, 94 % TBS<br />
OPMB<br />
OMe<br />
OPMB<br />
OMe<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP
H<br />
O<br />
Me<br />
O<br />
MeNHOMe HCl<br />
AlMe 3<br />
CH 2Cl 2, -20 o C<br />
then rt, 12 h 90 %<br />
1. HS SH<br />
BF 3 OEt 2, 78 %<br />
2. TBSOTf<br />
TBS<br />
OPMB<br />
2,6-lutidine, 97 %<br />
OMe<br />
Synthesis of Dithiane (C 21 ~ C 28)<br />
O<br />
Me<br />
N OH<br />
MeO Me<br />
S<br />
S<br />
O<br />
Me<br />
O O<br />
O<br />
N<br />
Me<br />
Me<br />
O<br />
Ph<br />
O<br />
Me<br />
n-Bu 2BOTf, Et 3N<br />
CH 2Cl 2, -78 o C<br />
then 0 o C, 2 h<br />
TBS<br />
TBS<br />
TBS<br />
OPMB<br />
OPMB<br />
OMe<br />
30 % aq. H 2O 2<br />
0 o C, 2 h<br />
dr 10 : 1, 83 %<br />
OMe<br />
O<br />
O<br />
O<br />
N<br />
Me<br />
1. TMSOTf<br />
2,6-lutidine<br />
CH 2Cl 2, -30 o C, 1h<br />
2. DIBAL-H<br />
CH 2Cl 2, -78 o C, 2 h<br />
89 % two steps<br />
OH<br />
Me<br />
Ph<br />
H<br />
O<br />
TBS<br />
O<br />
Me<br />
OPMB<br />
O<br />
Me<br />
TMS<br />
OMe<br />
O<br />
TBS<br />
OPMB<br />
OMe<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP
Me<br />
HO<br />
O<br />
NaBH 4<br />
O<br />
Me<br />
SPh<br />
O<br />
Me<br />
Synthesis of Carbohydrate Unit A<br />
1. MeI, NaH<br />
DMF, rt, 1.5 h, 92 %<br />
2.<br />
HO<br />
TsOH<br />
MeOH, 0 o C, 5 min, 70 %<br />
OH<br />
MeOH, rt, 12 h, 91 %<br />
Me<br />
MeO<br />
O<br />
OTBS<br />
Me<br />
MeO<br />
SPh<br />
OH<br />
O<br />
OH<br />
SPh<br />
OH<br />
1. TBSOTf<br />
1. TBSOTf<br />
2,6-lutidine, 100 %<br />
2. mCPBA<br />
CH 2Cl 2, -78 o C<br />
5 h, 67 %<br />
2,6-lutidine, 95 %<br />
2. (COCl) 2, DMSO, Et 3N<br />
100 %<br />
Me<br />
MeO<br />
O<br />
A<br />
OTBS<br />
O<br />
SPh<br />
Me<br />
MeO<br />
OTBS<br />
O<br />
OTBS<br />
SPh<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP<br />
O
Me<br />
HO<br />
O<br />
O<br />
Me<br />
SPh<br />
O<br />
Me<br />
PMBCl, TBAI, NaH<br />
DMF, 83 %<br />
1. TBSOTf<br />
Synthesis of Carbohydrate Unit D<br />
2,6-lutidine, 95 %<br />
2. BCl 3 SMe 2<br />
CH 2Cl 2, 0 o C<br />
30 min, 92 %<br />
Me<br />
TBSO<br />
O<br />
O<br />
SPh<br />
Me<br />
TBSO<br />
OPMB<br />
O<br />
OH<br />
SPh<br />
OH<br />
n-Bu2SnO<br />
toluene<br />
reflux, 6h<br />
0.05 eq. RhCl(PPh 3) 3<br />
1.5 eq. DABCO<br />
MeOH / H 2O (10 / 1)<br />
reflux, 2 h<br />
Br , CeF<br />
DMF, 70 o C, 12 h<br />
regioisomers 3 : 1<br />
90 %<br />
0.05 eq. OsO 4<br />
1.2 eq. NMO<br />
ac<strong>et</strong>one / H 2O (10 / 1)<br />
rt, 12 h, 92 %<br />
Me<br />
TBSO<br />
Me<br />
TBSO<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP<br />
O<br />
O<br />
O<br />
OH<br />
SPh<br />
OH<br />
SPh<br />
OPMB
Me<br />
TBSO<br />
O<br />
OH<br />
1. TBSOTf<br />
SPh<br />
OPMB<br />
2,6-lutidine, 95 %<br />
2. 1.2 eq. DDQ<br />
4 MS, CH2Cl2, 0 o Å<br />
C<br />
then rt, 2 h, 78 %<br />
BnOH, SnCl 2<br />
Et 2O, 0 o C<br />
then rt, 5 h, 90 %<br />
Synthesis of Carbohydrate Unit D<br />
DMP, NaHCO 3<br />
CH 2Cl 2, rt, 1 h, 96 %<br />
Me<br />
Me<br />
TBSO<br />
Me<br />
O<br />
O<br />
TBSO SPh<br />
Me OTBS<br />
Me<br />
TBSO<br />
SPh<br />
OH<br />
OTBS<br />
OBn TBAF<br />
O<br />
O<br />
DAST<br />
SPh<br />
OPMB<br />
MeMgBr<br />
CH 2Cl 2, 0 o C, 20 min<br />
a : b = 1 : 10, 100 %<br />
THF, rt, 6 h, 98 %<br />
Et 2O, -78 o C,10 min<br />
dr 7.5 : 1, 91 %<br />
Me<br />
O<br />
Me<br />
TBSO<br />
Me<br />
OBn<br />
HO SPh<br />
Me OH<br />
Me<br />
TBSO<br />
Me<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP<br />
O<br />
F<br />
SPh<br />
OTBS<br />
O<br />
SPh<br />
OPMB<br />
OH
Me<br />
TBSO<br />
Me<br />
TBSO<br />
O<br />
OH<br />
O<br />
OMe<br />
SPh<br />
OH<br />
F<br />
1. TESOTf<br />
2,6-lutidine<br />
CH 2Cl 2, 84 %<br />
2. Raney Ni<br />
EtOH, 55 o C<br />
5 h, 92 %<br />
SPh<br />
Synthesis of Carbohydrate Unit D-E<br />
n-Bu 2SnO<br />
toluene<br />
reflux, 6 h<br />
Me<br />
O<br />
MeI, CeF<br />
DMF, 55 o C<br />
1 h, 83 %<br />
OBn<br />
HO SPh<br />
Me OH<br />
SnCl 2<br />
Me<br />
TBSO<br />
O<br />
OMe<br />
SPh<br />
OH<br />
DAST<br />
D +<br />
D<br />
Et 2O, 0 o C<br />
then rt, 12 h, 45 %<br />
TBSO<br />
Me<br />
Me<br />
O<br />
O OH<br />
DAST<br />
MeO<br />
O<br />
Me<br />
OTES<br />
TBSO<br />
MeO<br />
CH 2Cl 2, 0 o C, 30 min<br />
a : b = 1 : 7, 100 %<br />
Me<br />
Me<br />
O<br />
E E<br />
CH 2Cl 2, 0 o C, 15 min<br />
a : b = 15 : 1, 100 %<br />
SPh<br />
O<br />
Me<br />
TBSO<br />
OBn<br />
O SPh<br />
Me OH<br />
TBSO<br />
Me<br />
Me<br />
O<br />
O F<br />
D E D E<br />
MeO<br />
O<br />
Me<br />
OTES<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP<br />
O<br />
D<br />
OMe<br />
F<br />
SPh
S<br />
S<br />
1. TBAF<br />
Me<br />
O<br />
Me<br />
TBS<br />
THF, rt, 90 %<br />
Dithiane Coupling to Synthesize Vinyl Iodide<br />
O<br />
2. PhI(OCOCF 3) 2<br />
0 o C, 10 min<br />
TBS<br />
OPMB<br />
CH 3<br />
OMe<br />
OH<br />
+<br />
MeO<br />
HO<br />
Me<br />
CH 3<br />
OTBS<br />
ODMB<br />
OH H<br />
O<br />
OH<br />
OMe<br />
O<br />
H<br />
ODMB<br />
Me OPMB<br />
OMe<br />
t-BuLi, HMPA<br />
THF, 96 %<br />
dr 1.5 : 1<br />
1. TBSOTf<br />
CH 3<br />
2,6-lutidine, 95 %<br />
2. (MeO) 3CMe, PPTS<br />
CH 2Cl 2, rt, 95 %<br />
OTBS<br />
OMe<br />
CH 3<br />
O<br />
DMB<br />
OH<br />
OTBS<br />
MeO<br />
Me<br />
S S<br />
O<br />
Me Me<br />
Me<br />
O<br />
Me<br />
TBS<br />
ODMB<br />
O<br />
O H<br />
O<br />
TBS<br />
OTBS<br />
OPMB<br />
Me OPMB<br />
OMe<br />
OMe<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP
(Cp) 2ZrHCl, I 2<br />
THF, 90 %<br />
regioisomer 6 : 1<br />
Dithiane Coupling to Synthesize Vinyl Iodide<br />
Me<br />
I<br />
OTBS<br />
MeO<br />
HO<br />
1. DDQ, LiOH, 85 %<br />
Me<br />
2. triphosgene, py, 88 %<br />
3. TESOTf, 95 %<br />
Me<br />
I<br />
OTBS<br />
MeO<br />
O<br />
O<br />
Me<br />
ODMB<br />
OMe H<br />
O<br />
OTBS<br />
O<br />
OMe H<br />
O<br />
OTBS<br />
Me OPMB<br />
Me OTES<br />
OMe<br />
OMe<br />
+<br />
Me<br />
Me<br />
I<br />
NaBH 4, 86 %<br />
I<br />
OTBS<br />
MeO<br />
HO<br />
OTBS<br />
MeO<br />
O<br />
Me<br />
Me<br />
ODMB<br />
OMe H<br />
O<br />
OTBS<br />
ODMB<br />
OMe H<br />
O<br />
OTBS<br />
Me OPMB<br />
Me OPMB<br />
OMe<br />
DMP, NaHCO 3<br />
CH 2Cl 2, 88 %<br />
OMe<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP
Me<br />
I<br />
HO<br />
n-Bu 3Sn<br />
OTBS<br />
MeO<br />
Me<br />
+<br />
O<br />
Synthesis of Fully Protected Apoptolidin<br />
O<br />
Me<br />
1. Tf 2O, DTBMP,<br />
Me<br />
Me<br />
Me<br />
O<br />
OMe H<br />
O<br />
Me<br />
MeO<br />
Et 2O, -90 o C, 1.5 h<br />
2. KOH, 24 h<br />
OTBS<br />
O<br />
A<br />
CO 2Me<br />
Me OTES<br />
OTBS<br />
O<br />
SPh<br />
OTBS<br />
OMe<br />
0.1 eq. PdCl 2(MeCN) 2<br />
DMF, rt, 15 h, 86 %<br />
TBSO<br />
MeO<br />
OTBS<br />
A<br />
O<br />
Me<br />
O<br />
Me<br />
OTBS<br />
Me<br />
MeO<br />
HO<br />
HO<br />
Me<br />
Me<br />
Me Me Me<br />
OH<br />
OMe H<br />
O<br />
OTBS<br />
MeO<br />
CO 2H<br />
OTBS<br />
Me Me Me<br />
O<br />
O<br />
Me<br />
Me OTES<br />
O<br />
OMe H<br />
O<br />
OMe<br />
CO 2Me<br />
OTBS<br />
Me OTES<br />
OMe<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP
Cl<br />
Synthesis of Fully Protected Apoptolidin<br />
O<br />
Cl Cl<br />
Cl<br />
THF, 0 o C then rt, 5 h<br />
1. (Cl 2Ac) 2O, py<br />
0 o C, 5 min, 90 %<br />
2. PPTS<br />
0 oC, 15 h, 80 %<br />
, Et 3N DMAP<br />
toluene, rt, 12h<br />
27 % over 3 steps<br />
TBSO<br />
OTBS<br />
A<br />
O<br />
TBSO<br />
OTBS<br />
O<br />
Me<br />
Me<br />
MeO<br />
O<br />
Me<br />
Me<br />
Me<br />
TBSO Me<br />
Me<br />
Me<br />
A<br />
MeO<br />
O<br />
Me<br />
Me<br />
Me<br />
TBSO<br />
Me<br />
MeO<br />
Cl2AcO Me<br />
O<br />
O<br />
OMe H<br />
O<br />
OTBS<br />
MeO<br />
HO<br />
Me OH<br />
Me<br />
OMe<br />
O<br />
O<br />
OMe H<br />
O<br />
OTBS<br />
Me OTES<br />
OMe<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP
TBSO<br />
Synthesis of Fully Protected Apoptolidin<br />
OTBS<br />
A<br />
O<br />
Me<br />
Me<br />
MeO<br />
O<br />
Me<br />
Me<br />
Me<br />
TBSO<br />
Me<br />
TBSO<br />
MeO<br />
Cl2AcO Me<br />
OTBS<br />
A<br />
O<br />
O<br />
OMe H<br />
O<br />
O<br />
OTBS<br />
Me<br />
Me OH<br />
Me<br />
MeO<br />
O<br />
Me<br />
Me<br />
Me<br />
TBSO<br />
Me<br />
MeO<br />
Cl2AcO Me<br />
OMe<br />
O<br />
+<br />
O<br />
OMe H<br />
O<br />
OTBS<br />
SnCl 2<br />
Me O<br />
F<br />
TESO Me<br />
O Me<br />
O<br />
OMe<br />
TESO Me<br />
O<br />
O Me<br />
O<br />
O<br />
Me<br />
Me<br />
D E<br />
Et 2O, rt, 12 h, 70 %<br />
D E<br />
OTBS<br />
OMe<br />
OTBS<br />
OMe<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP
TBSO<br />
OTBS<br />
Synthesis of Fully Protected Apoptolidin<br />
O<br />
Me<br />
Me<br />
MeO<br />
O<br />
Me<br />
Me<br />
Me<br />
TBSO<br />
Me<br />
HO<br />
A<br />
OH<br />
A<br />
O<br />
MeO<br />
Cl2AcO Me<br />
Me<br />
Me<br />
MeO<br />
O<br />
Me<br />
Me<br />
Me OH Me<br />
MeO<br />
HO<br />
Me<br />
O<br />
O<br />
OMe H<br />
O<br />
OTBS<br />
O<br />
O<br />
OH H<br />
O<br />
OH<br />
Me O<br />
OMe<br />
TESO Me<br />
Me O<br />
O Me<br />
O<br />
OMe<br />
O<br />
O Me<br />
O<br />
HO Me<br />
O<br />
Me<br />
D E<br />
Me<br />
D E<br />
OTBS<br />
OMe<br />
OH<br />
OMe<br />
1. HF py<br />
THF, 96 h<br />
2. Et 3N<br />
MeOH, rt, 3.5 h<br />
40 % over two steps<br />
3. TsOH<br />
THF / H 2O (1 / 1)<br />
0 o C, 2.5 h, 60 %<br />
36 steps (the longest linear)<br />
0.28 % yield<br />
Nicolaou, K. C. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. ASAP
Hydrozirconation/iodination<br />
Lipshutz, B. H. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron L<strong>et</strong>t. 1990, 31, 7257.
Stereocontrolled Sn(OTf) 2 Mediated Aldol Reaction<br />
Bn<br />
O<br />
H<br />
N<br />
O<br />
R<br />
Me<br />
H<br />
O<br />
Me<br />
H<br />
O<br />
L<br />
O<br />
Sn<br />
L<br />
Evans, D. A. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. 1990, 112, 866.
Anti-Selective Reduction<br />
Evans, D. A. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. 1990, 110, 3560.
O<br />
O<br />
Li<br />
t-BuLi<br />
95 %<br />
Cuprate<br />
O<br />
Bu 2Cu(CN)Li 2<br />
Li<br />
Preparation of Bromide<br />
(Bu 3Sn)(Bu)Cu(CN)Li 2<br />
O<br />
MeI, 75 %<br />
Cu CN<br />
R<br />
+<br />
2-<br />
Bu 3SnH<br />
O Cu R<br />
CN<br />
HO<br />
LiO<br />
Me<br />
SnBu 3<br />
Cu(CN)Li<br />
R<br />
1. MsCl, Et 3N<br />
2. LiBr, ac<strong>et</strong>one<br />
E +<br />
Br<br />
HO<br />
Me<br />
SnBu 3<br />
Pancrazi, A. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron 1996, 52, 6613.<br />
E<br />
R
Takai Reaction<br />
Takai, K. <strong>et</strong>. <strong>al</strong>. Synl<strong>et</strong>t 1999, 8, 1268.
Cu-Mediated Cross Coupling<br />
Liebeskind, L. S. <strong>et</strong>. <strong>al</strong>. J. <strong>Am</strong>. <strong>Chem</strong>. <strong>Soc</strong>. 1996, 118, 2748.
Cl<br />
Cl<br />
O<br />
Cl<br />
Yamaguchi Lactonization<br />
Cl<br />
R'OH, DMAP<br />
Benzene<br />
+<br />
R'O<br />
O<br />
RCOOH<br />
R<br />
+<br />
Cl<br />
Cl<br />
Et 3N<br />
THF<br />
O<br />
Cl<br />
O<br />
Cl<br />
Cl<br />
Yamaguchi, M. <strong>et</strong>. <strong>al</strong>. Bull. <strong>Chem</strong>. <strong>Soc</strong>. Jpn. 1979, 52, 1989.<br />
O<br />
Cl<br />
O<br />
O<br />
R
TrCl<br />
MeO<br />
Preparation of the Starting Aldehyde<br />
O<br />
Me<br />
OTr<br />
MeO<br />
O<br />
Me<br />
OH<br />
M<strong>et</strong>hyl (R)-(-)-3-hydroxy-2-m<strong>et</strong>hylpropionate<br />
LAH<br />
1 g $ 20.30 Aldrich<br />
HO<br />
Me<br />
OTr<br />
Swern<br />
H<br />
O<br />
Me<br />
OTr<br />
Terashima, S. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron L<strong>et</strong>t. 1986, 27, 6241.
Addition of Trim<strong>et</strong>hylsilylac<strong>et</strong>ylene – Cram’s rule<br />
TrO<br />
O<br />
H<br />
Me<br />
H<br />
TMS
EtO<br />
O<br />
OH<br />
OH<br />
O<br />
di<strong>et</strong>hyl L-tartrate<br />
BH 3THF<br />
THF, reflux, 94 %<br />
OEt<br />
Preparation of the Starting Alcohol<br />
HO<br />
MeO<br />
MeO<br />
TsOH, DMF, 99 %<br />
HO<br />
OMPM<br />
OH<br />
OMe<br />
EtO<br />
O<br />
O<br />
O<br />
O<br />
Ar<br />
3-pentanone, TsOH<br />
THF, 89 %<br />
OEt<br />
NaBH 4, LiCl<br />
THF, 100 %<br />
Et<br />
O<br />
O<br />
Et<br />
OMPM<br />
HO<br />
OH<br />
O<br />
O<br />
Ar<br />
OH<br />
Somfai, P. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron 1993, 49, 6645.
Addition of Allyl Stannane to Aldehyde – Chelation<br />
OTBS<br />
s-BuLi<br />
n-Bu 2SnCl, 77 % n-Bu 3Sn<br />
TBSO<br />
H<br />
n-Bu 3Sn<br />
O<br />
H<br />
H<br />
Mg ++<br />
OMPM<br />
R<br />
OTBS<br />
100 % cis<br />
Keck, G. E. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron L<strong>et</strong>t. 1987, 28, 139.
Preparation of the Propagyl Aldehyde Derivative<br />
H<br />
PTS<br />
OH<br />
MeOH, 92 %<br />
DHP, PPTS<br />
CH 2Cl 2, 80 %<br />
TES<br />
OH<br />
H<br />
OTHP<br />
PCC, NaOAc<br />
CH 2Cl 2, 76 %<br />
n-BuLi, TESCl<br />
THF, 71 %<br />
TES<br />
O<br />
H<br />
TES<br />
OTHP<br />
Chattopadhyay, S. <strong>et</strong>. <strong>al</strong>. J. Org. <strong>Chem</strong>. 1998, 63, 6128
Pd Cat<strong>al</strong>yzed Hydrostannylation<br />
Pd(PPh 3) 2Cl 2 + 2 n-Bu 3SnH<br />
R Br<br />
Pd(PPh3) 2 + H2 + 2 n-Bu3SnCl n-Bu 3SnH R Br<br />
Pd(PPh 3) 2Cl 2<br />
n-Bu 3SnH R H<br />
Pd(PPh 3) 2Cl 2<br />
H Snn-Bu 3<br />
H Snn-Bu 3<br />
Guibe, F. <strong>et</strong>. <strong>al</strong>. J. Org. <strong>Chem</strong>. 1990, 55, 1857
Transformation of 1,2-Diols into Epoxides<br />
Sharpless, K. B. <strong>et</strong>. <strong>al</strong>. T<strong>et</strong>rahedron 1992, 48, 10515
HO<br />
OH<br />
H<br />
HO<br />
O<br />
Preparation of the Starting Lactone<br />
O<br />
OH<br />
L-Ascorbic Acid<br />
H 2, Pd/C<br />
EtOAc, Et 3N, 95 %<br />
AcO<br />
H 2, Pd/C<br />
H 2O, 99 %<br />
OAc<br />
H<br />
O<br />
O<br />
OAc<br />
HO<br />
OH<br />
H<br />
HO<br />
KOH<br />
72 %<br />
O<br />
O<br />
OH<br />
HO<br />
AcCl<br />
90 %<br />
OH<br />
H<br />
O<br />
O<br />
OH<br />
AcO<br />
OAc<br />
H<br />
AcO<br />
O<br />
O<br />
OAc<br />
Andrews, G. C. <strong>et</strong>. <strong>al</strong>. J. Org. <strong>Chem</strong>. 1981, 46, 2976.<br />
Pedersen, C. <strong>et</strong>. <strong>al</strong>. Acta. <strong>Chem</strong>. Scand. B 1981, 35, 155.