You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
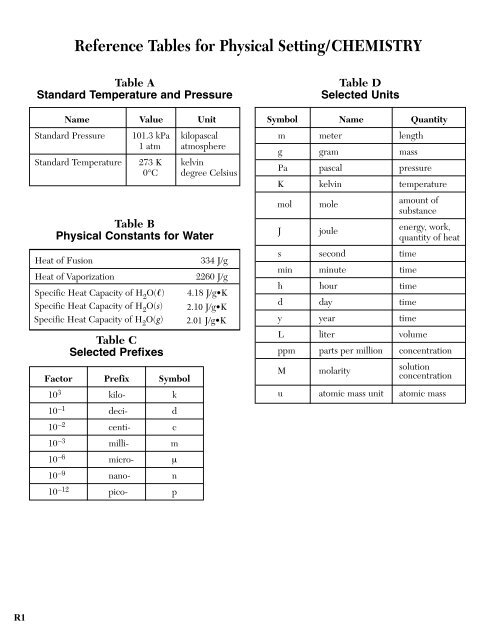
Reference Tables for Physical Setting/CHEMISTRY<br />
Table A<br />
Standard Temperature and Pressure<br />
Name Value Unit<br />
Standard Pressure 101.3 kPa kilopascal<br />
1 atm atmosphere<br />
Standard Temperature 273 K kelvin<br />
0°C degree Celsius<br />
Table D<br />
Selected Units<br />
Symbol Name Quantity<br />
m meter length<br />
g gram mass<br />
Pa pascal pressure<br />
K kelvin temperature<br />
Table B<br />
Physical Constants for Water<br />
mol<br />
J<br />
mole<br />
joule<br />
amount of<br />
substance<br />
energy, work,<br />
quantity of heat<br />
Heat of Fusion<br />
Heat of Vaporization<br />
Specific Heat Capacity of H 2<br />
O()<br />
Specific Heat Capacity of H 2<br />
O(s)<br />
Specific Heat Capacity of H 2<br />
O(g)<br />
Table C<br />
Selected Prefixes<br />
Factor Prefix Symbol<br />
10 3 kilo- k<br />
334 J/g<br />
2260 J/g<br />
4.18 J/g•K<br />
2.10 J/g•K<br />
2.01 J/g•K<br />
s second time<br />
min minute time<br />
h hour time<br />
d day time<br />
y year time<br />
L liter volume<br />
ppm parts per million concentration<br />
M<br />
molarity<br />
solution<br />
concentration<br />
u atomic mass unit atomic mass<br />
10 –1 deci- d<br />
10 –2 centi- c<br />
10 –3 milli- m<br />
10 –6 micro- μ<br />
10 –9 nano- n<br />
10 –12 pico- p<br />
R1
Table E<br />
Selected Polyatomic Ions<br />
Formula Name Formula Name<br />
H 3<br />
O +<br />
hydronium<br />
CrO 4<br />
2–<br />
chromate<br />
Hg 2<br />
2+<br />
mercury(I)<br />
Cr 2<br />
O 7<br />
2–<br />
dichromate<br />
NH 4<br />
+<br />
C 2<br />
H 3<br />
O<br />
–<br />
2 –}<br />
CH 3<br />
COO<br />
CN –<br />
CO 3<br />
2–<br />
HCO<br />
–<br />
3<br />
C 2<br />
O<br />
2–<br />
4<br />
ClO –<br />
ammonium<br />
acetate<br />
cyanide<br />
carbonate<br />
hydrogen<br />
carbonate<br />
oxalate<br />
hypochlorite<br />
MnO 4<br />
–<br />
NO<br />
–<br />
2<br />
NO<br />
–<br />
3<br />
O<br />
2–<br />
2<br />
OH –<br />
PO 4<br />
3–<br />
SCN –<br />
SO 3<br />
2–<br />
permanganate<br />
nitrite<br />
nitrate<br />
peroxide<br />
hydroxide<br />
phosphate<br />
thiocyanate<br />
sulfite<br />
ClO 2<br />
–<br />
chlorite<br />
SO 4<br />
2–<br />
sulfate<br />
ClO 3<br />
–<br />
chlorate<br />
HSO 4<br />
–<br />
hydrogen sulfate<br />
ClO 4<br />
–<br />
perchlorate<br />
S 2<br />
O 3<br />
2–<br />
thiosulfate<br />
Table F<br />
Solubility Guidelines for Aqueous Solutions<br />
Ions That Form<br />
Soluble Compounds<br />
Group 1 ions<br />
(Li + , Na + , etc.)<br />
ammonium (NH 4 + )<br />
nitrate (NO 3 – )<br />
acetate (C 2<br />
H 3<br />
O 2 – or<br />
CH 3<br />
COO – )<br />
hydrogen carbonate<br />
(HCO 3 – )<br />
chlorate (ClO 3 – )<br />
halides (Cl – , Br – , I – )<br />
Exceptions<br />
when combined with<br />
Ag + , Pb 2+ , or Hg 2<br />
2+<br />
sulfates (SO 4 2– ) when combined with Ag + ,<br />
Ca 2+ , Sr 2+ , Ba 2+ , or Pb 2+<br />
Ions That Form<br />
Insoluble Compounds*<br />
Exceptions<br />
carbonate (CO 3 2– ) when combined with Group 1<br />
ions or ammonium (NH 4 + )<br />
chromate (CrO 4 2– ) when combined with Group 1<br />
ions, Ca 2+ , Mg 2+ , or<br />
ammonium (NH 4 + )<br />
phosphate (PO 4 3– ) when combined with Group 1<br />
ions or ammonium (NH 4 + )<br />
sulfide (S 2– ) when combined with Group 1<br />
ions or ammonium (NH 4 + )<br />
hydroxide (OH – ) when combined with Group 1<br />
ions, Ca 2+ , Ba 2+ , Sr 2+ , or<br />
ammonium (NH 4 + )<br />
*compounds having very low solubility in H 2 O<br />
R2
150.<br />
140.<br />
Table G<br />
Solubility Curves at Standard Pressure<br />
KI<br />
NaNO 3<br />
130.<br />
120.<br />
KNO 3<br />
110.<br />
100.<br />
Solubility (g solute/100. g H 2<br />
O)<br />
90.<br />
80.<br />
70.<br />
60.<br />
HCl<br />
NH 4<br />
Cl<br />
KCl<br />
50.<br />
40.<br />
30.<br />
NaCl<br />
KClO 3<br />
NH 3<br />
20.<br />
10.<br />
SO 2<br />
0<br />
0 10. 20. 30. 40. 50. 60. 70. 80. 90. 100.<br />
Temperature (°C)<br />
R3
Table H<br />
Vapor Pressure of Four Liquids<br />
200.<br />
propanone<br />
ethanol<br />
150.<br />
water<br />
Vapor Pressure (kPa)<br />
100.<br />
101.3 kPa<br />
ethanoic<br />
acid<br />
50.<br />
0<br />
0 25 50. 75 100. 125<br />
R4
Table I<br />
Heats of Reaction at 101.3 kPa and 298 K<br />
Reaction<br />
ΔH (kJ)*<br />
CH 4<br />
(g) + 2O 2<br />
(g) CO 2<br />
(g) + 2H 2<br />
O() –890.4<br />
C 3<br />
H 8<br />
(g) + 5O 2<br />
(g) 3CO 2<br />
(g) + 4H 2<br />
O() –2219.2<br />
2C 8<br />
H 18<br />
() + 25O 2<br />
(g) 16CO 2<br />
(g) + 18H 2<br />
O() –10943<br />
2CH 3<br />
OH() + 3O 2<br />
(g) 2CO 2<br />
(g) + 4H 2<br />
O() –1452<br />
C 2<br />
H 5<br />
OH() + 3O 2<br />
(g) 2CO 2<br />
(g) + 3H 2<br />
O() –1367<br />
C 6<br />
H 12<br />
O 6<br />
(s) + 6O 2<br />
(g) 6CO 2<br />
(g) + 6H 2<br />
O() –2804<br />
2CO(g) + O 2<br />
(g) 2CO 2<br />
(g) –566.0<br />
C(s) + O 2<br />
(g) CO 2<br />
(g) –393.5<br />
4Al(s) + 3O 2<br />
(g) 2Al 2<br />
O 3<br />
(s) –3351<br />
N 2<br />
(g) + O 2<br />
(g) 2NO(g) +182.6<br />
N 2<br />
(g) + 2O 2<br />
(g) 2NO 2<br />
(g) +66.4<br />
2H 2<br />
(g) + O 2<br />
(g) 2H 2<br />
O(g) –483.6<br />
2H 2<br />
(g) + O 2<br />
(g) 2H 2<br />
O() –571.6<br />
N 2<br />
(g) + 3H 2<br />
(g) 2NH 3<br />
(g) –91.8<br />
2C(s) + 3H 2<br />
(g) C 2<br />
H 6<br />
(g) –84.0<br />
2C(s) + 2H 2<br />
(g) C 2<br />
H 4<br />
(g) +52.4<br />
2C(s) + H 2<br />
(g) C 2<br />
H 2<br />
(g) +227.4<br />
H 2<br />
(g) + I 2<br />
(g) 2HI(g) +53.0<br />
KNO 3<br />
(s) H 2 O K + (aq) + NO 3 – (aq) +34.89<br />
NaOH(s) H 2 O Na + (aq) + OH – (aq) –44.51<br />
NH 4<br />
Cl(s) H 2 O NH 4 + (aq) + Cl – (aq) +14.78<br />
NH 4<br />
NO 3<br />
(s) H 2 O NH 4 + (aq) + NO 3 – (aq) +25.69<br />
NaCl(s) H 2 O Na + (aq) + Cl – (aq) +3.88<br />
LiBr(s) H 2 O Li + (aq) + Br – (aq) –48.83<br />
H + (aq) + OH – (aq) H 2<br />
O() –55.8<br />
*The ΔH values are based on molar quantities represented in the equations.<br />
A minus sign indicates an exothermic reaction.<br />
Most<br />
Active<br />
Least<br />
Active<br />
Table J<br />
Activity Series**<br />
Metals Nonmetals Most<br />
Active<br />
Li<br />
Rb<br />
K<br />
Cs<br />
Ba<br />
Sr<br />
Ca<br />
Na<br />
Mg<br />
Al<br />
Ti<br />
Mn<br />
Zn<br />
Cr<br />
Fe<br />
Co<br />
Ni<br />
Sn<br />
Pb<br />
H 2<br />
Cu<br />
Ag<br />
Au<br />
F 2<br />
Cl 2<br />
Br 2<br />
I 2<br />
**Activity Series is based on the hydrogen<br />
standard. H 2 is not a metal.<br />
Least<br />
Active<br />
R5
Table K<br />
Common Acids<br />
Table N<br />
Selected Radioisotopes<br />
HCl(aq)<br />
Formula<br />
HNO 2<br />
(aq)<br />
HNO 3<br />
(aq)<br />
H 2<br />
SO 3<br />
(aq)<br />
H 2<br />
SO 4<br />
(aq)<br />
H 3<br />
PO 4<br />
(aq)<br />
H 2<br />
CO 3<br />
(aq)<br />
or<br />
CO 2<br />
(aq)<br />
CH 3<br />
COOH(aq)<br />
or<br />
HC 2<br />
H 3<br />
O 2<br />
(aq)<br />
Name<br />
hydrochloric acid<br />
nitrous acid<br />
nitric acid<br />
sulfurous acid<br />
sulfuric acid<br />
phosphoric acid<br />
carbonic acid<br />
ethanoic acid<br />
(acetic acid)<br />
Nuclide Half-Life Decay<br />
Mode<br />
Nuclide<br />
Name<br />
198 Au 2.695 d β – gold-198<br />
14 C 5715 y β – carbon-14<br />
37 Ca 182 ms β + calcium-37<br />
60 Co 5.271 y β – cobalt-60<br />
137 Cs 30.2 y β – cesium-137<br />
53 Fe 8.51 min β + iron-53<br />
220 Fr 27.4 s α francium-220<br />
3 H 12.31 y β – hydrogen-3<br />
131 I 8.021 d β – iodine-131<br />
37 K 1.23 s β + potassium-37<br />
42 K 12.36 h β – potassium-42<br />
Table L<br />
Common Bases<br />
85 Kr 10.73 y β – krypton-85<br />
16 N 7.13 s β – nitrogen-16<br />
Formula<br />
NaOH(aq)<br />
KOH(aq)<br />
Ca(OH) 2<br />
(aq)<br />
NH 3<br />
(aq)<br />
Name<br />
sodium hydroxide<br />
potassium hydroxide<br />
calcium hydroxide<br />
aqueous ammonia<br />
19 Ne 17.22 s β + neon-19<br />
32 P 14.28 d β – phosphorus-32<br />
239 Pu 2.410 × 10 4 y α plutonium-239<br />
226 Ra 1599 y α radium-226<br />
222 Rn 3.823 d α radon-222<br />
90 Sr 29.1 y β – strontium-90<br />
Table M<br />
Common Acid–Base Indicators<br />
Approximate<br />
Indicator pH Range Color<br />
for Color Change<br />
Change<br />
methyl orange 3.1–4.4 red to yellow<br />
bromthymol blue 6.0–7.6 yellow to blue<br />
phenolphthalein 8–9 colorless to pink<br />
litmus 4.5–8.3 red to blue<br />
bromcresol green 3.8–5.4 yellow to blue<br />
thymol blue 8.0–9.6 yellow to blue<br />
99 Tc 2.13 × 10 5 y β – technetium-99<br />
232 Th 1.40 × 10 10 y α thorium-232<br />
233 U 1.592 × 10 5 y α uranium-233<br />
235 U 7.04 × 10 8 y α uranium-235<br />
238 U 4.47 × 10 9 y α uranium-238<br />
Source: CRC Handbook of Chemistry and Physics, 91 st ed., 2010–2011,<br />
CRC Press<br />
Source: The Merck Index, 14 th ed., 2006, Merck Publishing Group<br />
R6
Table O<br />
Symbols Used in Nuclear Chemistry<br />
Name Notation Symbol<br />
alpha particle<br />
4<br />
2<br />
He or 4 2 α α<br />
beta particle<br />
0<br />
–1<br />
e or 0<br />
–1 β β–<br />
gamma radiation<br />
0<br />
0<br />
γ γ<br />
neutron<br />
1<br />
0<br />
n n<br />
proton<br />
1<br />
1<br />
H or 1 1 p p<br />
positron<br />
0<br />
+1<br />
e or 0<br />
+1 β β+<br />
Table P<br />
Organic Prefixes<br />
Prefix<br />
meth- 1<br />
eth- 2<br />
prop- 3<br />
but- 4<br />
pent- 5<br />
hex- 6<br />
hept- 7<br />
oct- 8<br />
non- 9<br />
dec- 10<br />
Number of<br />
Carbon Atoms<br />
Table Q<br />
Homologous Series of Hydrocarbons<br />
Name General Examples<br />
Formula Name Structural Formula<br />
R7<br />
alkanes C n<br />
H 2n+2<br />
ethane<br />
alkenes C n<br />
H 2n<br />
ethene<br />
alkynes C n<br />
H 2n–2<br />
ethyne<br />
Note: n = number of carbon atoms<br />
H H<br />
H C C H<br />
H H<br />
H<br />
H<br />
C C<br />
H<br />
H<br />
H C C H
Table R<br />
Organic Functional Groups<br />
Class of<br />
Compound<br />
Functional<br />
Group<br />
General<br />
Formula<br />
Example<br />
halide<br />
(halocarbon)<br />
F (fluoro-)<br />
Cl (chloro-)<br />
Br (bromo-)<br />
I (iodo-)<br />
R X<br />
(X represents<br />
any halogen)<br />
CH 3<br />
CHClCH 3<br />
2-chloropropane<br />
alcohol<br />
OH<br />
R<br />
OH<br />
CH 3<br />
CH 2<br />
CH 2<br />
OH<br />
1-propanol<br />
ether<br />
O<br />
R O R′<br />
CH 3<br />
OCH 2<br />
CH 3<br />
methyl ethyl ether<br />
aldehyde<br />
O<br />
C H<br />
R<br />
O<br />
C H<br />
O<br />
CH 3<br />
CH 2<br />
C H<br />
propanal<br />
ketone<br />
O<br />
C<br />
O<br />
R C R′<br />
O<br />
CH 3<br />
CCH 2<br />
CH 2<br />
CH 3<br />
2-pentanone<br />
organic acid<br />
O<br />
C OH<br />
R<br />
O<br />
C OH<br />
O<br />
CH 3<br />
CH 2<br />
C OH<br />
propanoic acid<br />
ester<br />
O<br />
C O<br />
O<br />
R C O R′<br />
O<br />
CH 3<br />
CH 2<br />
COCH 3<br />
methyl propanoate<br />
amine<br />
N<br />
R<br />
R′<br />
N R′′<br />
CH 3<br />
CH 2<br />
CH 2<br />
NH 2<br />
1-propanamine<br />
amide<br />
O<br />
C NH<br />
R<br />
O R′<br />
C NH<br />
O<br />
CH 3<br />
CH 2<br />
C NH 2<br />
propanamide<br />
Note: R represents a bonded atom or group of atoms.<br />
R8
0<br />
6.941<br />
+1<br />
Li<br />
3<br />
2-1<br />
Na<br />
39.0983<br />
K +1<br />
19<br />
2-8-8-1<br />
85.4678 +1<br />
Rb<br />
Cs<br />
(223)<br />
Fr<br />
87<br />
-18-32-18-8-1<br />
+1<br />
Ra<br />
88<br />
-18-32-18-8-2<br />
39<br />
138.9055<br />
La<br />
57<br />
2-8-18-18-9-2<br />
+2 (227)<br />
Ac<br />
89<br />
-18-32-18-9-2<br />
47.867<br />
Ti<br />
22<br />
2-8-10-2<br />
91.224<br />
Zr<br />
40<br />
2-8-18-10-2<br />
+3 178.49<br />
Hf<br />
72<br />
*18-32-10-2<br />
+3 (261)<br />
Rf<br />
104<br />
+2<br />
+3<br />
+4<br />
+4<br />
+4<br />
50.9415<br />
V<br />
23<br />
2-8-11-2<br />
+2<br />
+3<br />
+4<br />
+5<br />
51.996<br />
Cr<br />
24<br />
2-8-13-1<br />
95.94<br />
Mo<br />
42<br />
2-8-18-13-1<br />
183.84<br />
W<br />
74<br />
-18-32-12-2<br />
+2<br />
+3<br />
+6<br />
+6<br />
+6<br />
54.9380<br />
Mn<br />
25<br />
2-8-13-2<br />
+2<br />
+3<br />
+4<br />
+7<br />
55.845<br />
Fe<br />
26<br />
2-8-14-2<br />
+2<br />
+3 58.9332<br />
Co<br />
27<br />
2-8-15-2<br />
+2<br />
+3<br />
58.693<br />
Ni<br />
28<br />
2-8-16-2<br />
+2<br />
+3 63.546 Cu<br />
2-8-18-1<br />
107.868<br />
Ag<br />
47<br />
2-8-18-18-1<br />
79<br />
+1<br />
+2<br />
+1<br />
65.409<br />
Zn<br />
30<br />
2-8-18-2<br />
10.81<br />
+3 12.011<br />
B<br />
5<br />
2-3<br />
26.98154<br />
Al<br />
13<br />
2-8-3<br />
+2 69.723<br />
Ga<br />
31<br />
2-8-18-3<br />
+3<br />
+3<br />
–4<br />
+2<br />
+4<br />
C<br />
6<br />
2-4<br />
28.0855<br />
Si<br />
14<br />
2-8-4<br />
72.64<br />
Ge<br />
32<br />
2-8-18-4<br />
Pb<br />
–4<br />
+2<br />
+4<br />
+2<br />
+4<br />
74.9216<br />
As<br />
33<br />
2-8-18-5<br />
Sb<br />
–3<br />
+3<br />
15.9994 O<br />
–2 18.9984<br />
8<br />
2-6 2-7<br />
78.96<br />
Se<br />
34<br />
2-8-18-6<br />
127.60<br />
Te<br />
52<br />
2-8-18-18-6<br />
(209)<br />
Po<br />
84<br />
-18-32-18-6<br />
–2<br />
+4<br />
+6<br />
–2<br />
+4<br />
+6<br />
+2<br />
+4<br />
F<br />
79.904<br />
Br<br />
35<br />
2-8-18-7<br />
126.904<br />
l<br />
53<br />
2-8-18-18-7<br />
(210)<br />
At<br />
85<br />
-18-32-18-7<br />
( ? )<br />
Uus<br />
117<br />
4.00260 0<br />
He<br />
2<br />
2<br />
–1 20.180<br />
Ne<br />
10<br />
2-8<br />
0<br />
22.98977<br />
11<br />
2-8-1<br />
1<br />
1.00794 +1<br />
–1<br />
H<br />
1<br />
1<br />
1<br />
37<br />
2-8-18-8-1<br />
–1<br />
+1<br />
+5<br />
–1<br />
+1<br />
+5<br />
+7<br />
83.798<br />
Kr<br />
36<br />
2-8-18-8<br />
131.29<br />
Xe<br />
54<br />
2-8-18-18-8<br />
(222)<br />
Rn<br />
86<br />
-18-32-18-8<br />
0<br />
+2<br />
0<br />
+2<br />
+4<br />
+6<br />
0<br />
132.905<br />
55<br />
2-8-18-18-8-1<br />
Symbol<br />
Relative atomic masses are based<br />
Group on 12 C = 12 (exact)<br />
Group<br />
2<br />
13 14 15 16 17 18<br />
Atomic Number<br />
+1<br />
+1<br />
9.01218 +2<br />
Be<br />
4<br />
2-2<br />
24.305<br />
Mg<br />
12<br />
2-8-2<br />
40.08<br />
Ca<br />
20<br />
2-8-8-2<br />
87.62<br />
Sr<br />
38<br />
2-8-18-8-2<br />
137.33<br />
Ba<br />
56<br />
2-8-18-18-8-2<br />
(226)<br />
+2<br />
+2<br />
+2<br />
+2<br />
3<br />
44.9559<br />
Sc<br />
21<br />
2-8-9-2<br />
88.9059<br />
Y<br />
2-8-18-9-2<br />
+3<br />
+3<br />
4<br />
KEY<br />
92.9064<br />
Nb +3<br />
+5<br />
41<br />
2-8-18-12-1<br />
180.948<br />
Ta<br />
73<br />
-18-32-11-2<br />
(262)<br />
105<br />
5<br />
Periodic Table of the Elements<br />
Atomic Mass<br />
Electron Configuration<br />
+4<br />
Db<br />
+5<br />
6<br />
(266)<br />
Sg<br />
106<br />
12.011 2-4<br />
–4<br />
6<br />
C<br />
+2<br />
+4<br />
(98)<br />
Tc<br />
43<br />
2-8-18-13-2<br />
186.207<br />
Re<br />
75<br />
-18-32-13-2<br />
(272)<br />
Bh<br />
107<br />
7<br />
Group<br />
+4<br />
+6<br />
+7<br />
+4<br />
+6<br />
+7<br />
8<br />
101.07<br />
Ru<br />
44<br />
2-8-18-15-1<br />
190.23<br />
Os<br />
76<br />
-18-32-14-2<br />
(277)<br />
Hs<br />
108<br />
+3<br />
+3<br />
+4<br />
Selected Oxidation States<br />
Note: Numbers in parentheses<br />
are mass numbers of the most<br />
stable or common isotope.<br />
9<br />
102.906<br />
Rh<br />
45<br />
2-8-18-16-1<br />
192.217<br />
Ir<br />
77<br />
-18-32-15-2<br />
(276)<br />
Mt<br />
109<br />
+3<br />
+3<br />
+4<br />
106.42<br />
Pd<br />
46<br />
2-8-18-18<br />
195.08<br />
Pt<br />
78<br />
-18-32-17-1<br />
+2<br />
+4<br />
+2<br />
+4<br />
196.967<br />
Au<br />
-18-32-18-1<br />
(281)<br />
Ds (280) Rg<br />
110<br />
10<br />
29<br />
111<br />
11 12<br />
+1<br />
+3<br />
112.41<br />
Cd<br />
48<br />
2-8-18-18-2<br />
200.59<br />
Hg<br />
80<br />
-18-32-18-2<br />
(285)<br />
Cn<br />
112<br />
+2 114.818<br />
In<br />
+1<br />
+2<br />
49<br />
2-8-18-18-3<br />
204.383<br />
Tl<br />
81<br />
-18-32-18-3<br />
(284)<br />
Uut<br />
113**<br />
+3<br />
+1<br />
+3<br />
118.71<br />
Sn<br />
50<br />
2-8-18-18-4<br />
207.2<br />
82<br />
-18-32-18-4<br />
(289)<br />
Uuq<br />
114<br />
+2<br />
+4<br />
+2<br />
+4<br />
14.0067 –3<br />
–2<br />
N<br />
–1<br />
7<br />
2-5<br />
30.97376<br />
P<br />
15<br />
2-8-5<br />
121.760<br />
51<br />
2-8-18-18-5<br />
208.980<br />
Bi<br />
83<br />
-18-32-18-5<br />
(288)<br />
Uup<br />
115<br />
+1<br />
+2<br />
+3<br />
+4<br />
+5<br />
–3<br />
+3<br />
+5<br />
+5<br />
–3<br />
+3<br />
+5<br />
+3<br />
+5<br />
32.065<br />
S<br />
16<br />
2-8-6<br />
(292)<br />
Uuh<br />
116<br />
–2<br />
+4<br />
+6<br />
35.453<br />
Cl<br />
17<br />
2-8-7<br />
–1<br />
+1<br />
+5<br />
+7<br />
39.948<br />
Ar<br />
18<br />
2-8-8<br />
18<br />
(294)<br />
Uuo<br />
118<br />
140.116<br />
Ce<br />
58<br />
232.038<br />
Th<br />
90<br />
+3<br />
+4<br />
140.908<br />
Pr +3<br />
59<br />
144.24<br />
Nd<br />
60<br />
+4 231.036<br />
Pa +4 238.029 +5<br />
U +3<br />
+4<br />
+5<br />
+6<br />
91<br />
92<br />
+3<br />
(145)<br />
Pm<br />
61<br />
+3<br />
150.36<br />
Sm<br />
62<br />
+2<br />
+3<br />
151.964<br />
Eu<br />
63<br />
+2<br />
+3<br />
157.25<br />
Gd<br />
64<br />
+3<br />
158.925<br />
(237)Np (244) Pu (243) Am (247) Cm +3 (247) Bk +3<br />
+3<br />
+4<br />
+5<br />
+6<br />
93 94<br />
+3<br />
+4<br />
+5<br />
+6<br />
65<br />
+3<br />
+4<br />
+5<br />
+6<br />
95 96 97<br />
Tb<br />
+3<br />
+4<br />
162.500<br />
Dy<br />
66<br />
(251)<br />
+3<br />
164.930<br />
Ho<br />
67<br />
+3<br />
167.259<br />
Er<br />
68<br />
Cf +3 (252) Es (257) Fm<br />
100<br />
98 99<br />
+3<br />
+3<br />
+3<br />
168.934<br />
Tm +3<br />
69<br />
(258)<br />
Md<br />
101<br />
+2<br />
+3<br />
173.04<br />
Yb<br />
70<br />
(259)<br />
No<br />
102<br />
+2<br />
+3<br />
+2<br />
+3<br />
174.9668<br />
Lu<br />
71<br />
(262)<br />
Lr<br />
103<br />
+3<br />
+3<br />
*denotes the presence of (2-8-) for elements 72 and above<br />
**The systematic names and symbols for elements of atomic numbers 113 and above<br />
will be used until the approval of trivial names by IUPAC.<br />
Source: CRC Handbook of Chemistry and Physics, 91 st ed., 2010–2011, CRC Press<br />
9<br />
R9<br />
Period<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
7
Table S<br />
Properties of Selected Elements<br />
First<br />
Atomic Symbol Name Ionization<br />
Electro- Melting Boiling* Density** Atomic<br />
Number Energy negativity Point Point (g/cm 3 ) Radius<br />
(kJ/mol) (K) (K) (pm)<br />
1 H hydrogen 1312 2.2 14 20. 0.000082 32<br />
2 He helium 2372 — — 4 0.000164 37<br />
3 Li lithium 520. 1.0 454 1615 0.534 130.<br />
4 Be beryllium 900. 1.6 1560. 2744 1.85 99<br />
5 B boron 801 2.0 2348 4273 2.34 84<br />
6 C carbon 1086 2.6 — — .— 75<br />
7 N nitrogen 1402 3.0 63 77 0.001145 71<br />
8 O oxygen 1314 3.4 54 90. 0.001308 64<br />
9 F fluorine 1681 4.0 53 85 0.001553 60.<br />
10 Ne neon 2081 — 24 27 0.000825 62<br />
11 Na sodium 496 0.9 371 1156 0.97 160.<br />
12 Mg magnesium 738 1.3 923 1363 1.74 140.<br />
13 Al aluminum 578 1.6 933 2792 2.70 124<br />
14 Si silicon 787 1.9 1687 3538 2.3296 114<br />
15 P phosphorus (white) 1012 2.2 317 554 1.823 109<br />
16 S sulfur (monoclinic) 1000. 2.6 388 718 2.00 104<br />
17 Cl chlorine 1251 3.2 172 239 0.002898 100.<br />
18 Ar argon 1521 — 84 87 0.001633 101<br />
19 K potassium 419 0.8 337 1032 0.89 200.<br />
20 Ca calcium 590. 1.0 1115 1757 1.54 174<br />
21 Sc scandium 633 1.4 1814 3109 2.99 159<br />
22 Ti titanium 659 1.5 1941 3560. 4.506 148<br />
23 V vanadium 651 1.6 2183 3680. 6.0 144<br />
24 Cr chromium 653 1.7 2180. 2944 7.15 130.<br />
25 Mn manganese 717 1.6 1519 2334 7.3 129<br />
26 Fe iron 762 1.8 1811 3134 7.87 124<br />
27 Co cobalt 760. 1.9 1768 3200. 8.86 118<br />
28 Ni nickel 737 1.9 1728 3186 8.90 117<br />
29 Cu copper 745 1.9 1358 2835 8.96 122<br />
30 Zn zinc 906 1.7 693 1180. 7.134 120.<br />
31 Ga gallium 579 1.8 303 2477 5.91 123<br />
32 Ge germanium 762 2.0 1211 3106 5.3234 120.<br />
33 As arsenic (gray) 944 2.2 1090. — 5.75 120.<br />
34 Se selenium (gray) 941 2.6 494 958 4.809 118<br />
35 Br bromine 1140. 3.0 266 332 3.1028 117<br />
36 Kr krypton 1351 — 116 120. 0.003425 116<br />
37 Rb rubidium 403 0.8 312 961 1.53 215<br />
38 Sr strontium 549 1.0 1050. 1655 2.64 190.<br />
39 Y yttrium 600. 1.2 1795 3618 4.47 176<br />
40 Zr zirconium 640. 1.3 2128 4682 6.52 164<br />
R10
First<br />
Atomic Symbol Name Ionization<br />
Electro- Melting Boiling* Density** Atomic<br />
Number Energy negativity Point Point (g/cm 3 ) Radius<br />
(kJ/mol) (K) (K) (pm)<br />
41 Nb niobium 652 1.6 2750. 5017 8.57 156<br />
42 Mo molybdenum 684 2.2 2896 4912 10.2 146<br />
43 Tc technetium 702 2.1 2430. 4538 11 138<br />
44 Ru ruthenium 710. 2.2 2606 4423 12.1 136<br />
45 Rh rhodium 720. 2.3 2237 3968 12.4 134<br />
46 Pd palladium 804 2.2 1828 3236 12.0 130.<br />
47 Ag silver 731 1.9 1235 2435 10.5 136<br />
48 Cd cadmium 868 1.7 594 1040. 8.69 140.<br />
49 In indium 558 1.8 430. 2345 7.31 142<br />
50 Sn tin (white) 709 2.0 505 2875 7.287 140.<br />
51 Sb antimony (gray) 831 2.1 904 1860. 6.68 140.<br />
52 Te tellurium 869 2.1 723 1261 6.232 137<br />
53 I iodine 1008 2.7 387 457 4.933 136<br />
54 Xe xenon 1170. 2.6 161 165 0.005366 136<br />
55 Cs cesium 376 0.8 302 944 1.873 238<br />
56 Ba barium 503 0.9 1000. 2170. 3.62 206<br />
57 La lanthanum 538 1.1 1193 3737 6.15 194<br />
Elements 58–71 have been omitted.<br />
72 Hf hafnium 659 1.3 2506 4876 13.3 164<br />
73 Ta tantalum 728 1.5 3290. 5731 16.4 158<br />
74 W tungsten 759 1.7 3695 5828 19.3 150.<br />
75 Re rhenium 756 1.9 3458 5869 20.8 141<br />
76 Os osmium 814 2.2 3306 5285 22.587 136<br />
77 Ir iridium 865 2.2 2719 4701 22.562 132<br />
78 Pt platinum 864 2.2 2041 4098 21.5 130.<br />
79 Au gold 890. 2.4 1337 3129 19.3 130.<br />
80 Hg mercury 1007 1.9 234 630. 13.5336 132<br />
81 Tl thallium 589 1.8 577 1746 11.8 144<br />
82 Pb lead 716 1.8 600. 2022 11.3 145<br />
83 Bi bismuth 703 1.9 544 1837 9.79 150.<br />
84 Po polonium 812 2.0 527 1235 9.20 142<br />
85 At astatine — 2.2 575 — — 148<br />
86 Rn radon 1037 — 202 211 0.009074 146<br />
87 Fr francium 393 0.7 300. — — 242<br />
88 Ra radium 509 0.9 969 — 5 211<br />
89 Ac actinium 499 1.1 1323 3471 10. 201<br />
Elements 90 and above have been omitted.<br />
*boiling point at standard pressure<br />
**density of solids and liquids at room temperature and density of gases at 298 K and 101.3 kPa<br />
— no data available<br />
Source: CRC Handbook for Chemistry and Physics, 91 st ed., 2010–2011, CRC Press<br />
R11
Table T<br />
Important Formulas and Equations<br />
d = density<br />
m<br />
Density d = m = mass<br />
V<br />
V = volume<br />
Mole Calculations number of moles =<br />
given mass<br />
gram-formula mass<br />
measured value – accepted value<br />
Percent Error % error = × 100<br />
accepted value<br />
mass of part<br />
Percent Composition % composition by mass = × 100<br />
mass of whole<br />
mass of solute<br />
parts per million = × 1000000<br />
mass of solution<br />
Concentration<br />
molarity =<br />
moles of solute<br />
liter of solution<br />
Combined Gas Law<br />
P<br />
P = pressure<br />
1<br />
V 1<br />
P<br />
= 2<br />
V 2<br />
V = volume<br />
T 1<br />
T 2 T = temperature<br />
M A<br />
= molarity of H + M B<br />
= molarity of OH –<br />
Titration M A<br />
V A<br />
= M B<br />
V B<br />
V A<br />
= volume of acid V B<br />
= volume of base<br />
q = mCΔT q = heat H f<br />
= heat of fusion<br />
Heat q = mH f<br />
m = mass H v<br />
= heat of vaporization<br />
q = mH v<br />
C=specific heat capacity<br />
ΔT = change in temperature<br />
Temperature<br />
K = °C + 273<br />
K = kelvin<br />
°C = degree Celsius<br />
R12
Collier County CHEMISTRY EXAM FORMULA AND RESOURCE PACKET<br />
GENERAL<br />
D m V<br />
[ ExperimentalValue AcceptedVa lue]<br />
% error <br />
x100<br />
AcceptedVa lue<br />
% yield <br />
ExperimentalYield<br />
TheoreticalYield<br />
x100<br />
CONCENTRATIONS<br />
moles of solute<br />
M = Molarity <br />
liters of solution<br />
KEY<br />
P = pressure<br />
V = volume<br />
T = temperature<br />
n = number of moles<br />
m = mass<br />
M = molar mass (grams/mole)<br />
D = density<br />
KE = kinetic energy<br />
Avogadro’s Number = 6.02 x 10 23<br />
GASES, LIQUIDS, SOLUTIONS<br />
m = Molality <br />
M1V1 M2V2<br />
S1<br />
P1<br />
S 2<br />
P 2<br />
ACID/BASE<br />
pH = - log[H + ]<br />
[H + ]=10 -pH<br />
moles of solute<br />
kilograms of solvent<br />
<br />
Gas constant<br />
R 8.314 L kPa L atm L mmHg<br />
0.0821 62.4<br />
K mol K mol K mol<br />
1 atm = 760 mmHg = 760 torr = 101.3 kPa<br />
K = o C + 273<br />
o C = K - 273<br />
STP = Standard Temperature and Pressure = 0 o C<br />
and 1 atm<br />
P V<br />
1 1<br />
<br />
P2V<br />
2<br />
pOH = - log [OH - ]<br />
[OH - ]= 10 -pOH<br />
pH + pOH = 14<br />
Kw = [H + ] x [OH - ] = 1.0 x 10 -14 M 2<br />
V<br />
T<br />
1<br />
1<br />
P<br />
T<br />
1<br />
1<br />
V<br />
<br />
T<br />
P<br />
<br />
T<br />
2<br />
2<br />
2<br />
2<br />
Or V1T2 = V2T1<br />
Or P1T2 = P2T1<br />
THERMOCHEMISTRY<br />
ΔH= mCΔT, where ΔT = T f - T<br />
P1V<br />
1<br />
T<br />
1<br />
<br />
P2V<br />
2<br />
T<br />
2<br />
Or<br />
P1V1T2=P2V2T1<br />
q = mCΔT<br />
PV<br />
nRT<br />
Specific Heat of Water = 4.18 J/g*˚C or 1.0 cal/g*˚C<br />
Specific Heat of Ice = 2.1 J/g*˚C or 0.5 cal/g*˚C<br />
Specific Heat of Steam = 2.0 J/g*˚C or 0.4 cal/g*˚C<br />
P<br />
Total<br />
P<br />
1<br />
P<br />
2<br />
Rate A<br />
Rate B<br />
...<br />
<br />
Molar MassB<br />
Molar MassA<br />
R13
CHEMISTRY EXAM FORMULA AND RESOURCE PACKET<br />
Solubility of Compounds at 25 o C and 1 atm<br />
acetate<br />
bromide<br />
carbonate<br />
chlorate<br />
chloride<br />
hydroxide<br />
iodide<br />
nitrate<br />
oxide<br />
perchlorate<br />
phosphate<br />
sulfate<br />
sulfide<br />
aluminum S S - S S I S S I S I S d<br />
ammonium S S S S S S S S - S S S S<br />
barium S S I S S S S S sS S I I d<br />
calcium S S I S S S S S sS S I sS I<br />
copper(II) S S - S S I S S I S I S I<br />
iron(II) S S I S S I S S I S I S I<br />
iron(III) S S - S S I S S I S I sS d<br />
lithium S S sS S S S S S S S sS S S<br />
magnesium S S I S S I S S I S I S d<br />
potassium S S S S S S S S S S S S S<br />
silver sS I I S I - I S I S I sS I<br />
sodium S S S S S S S S S S S S S<br />
strontium S S I S S S S S S S I I I<br />
zinc S S I S S I S S I S I S I<br />
S=soluble<br />
sS = slightly soluble in water<br />
I = insoluble in water<br />
d = decomposes in water<br />
- = no such compound<br />
R14
CHEMISTRY EXAM FORMULA AND RESOURCE PACKET<br />
Common Polyatomic Ions<br />
1- Charge 2- Charge 3- Charge<br />
Formula Name Formula Name Formula Name<br />
Dihydrogen<br />
Phosphate<br />
Hydrogen<br />
phosphate<br />
Phosphite<br />
Acetate Oxalate Phosphate<br />
Hydrogen<br />
sulfite<br />
Sulfite<br />
Hydrogen<br />
sulfate<br />
Sulfate<br />
Hydrogen<br />
carbonate<br />
Carbonate<br />
Nitrite Chromate 1+ Charge<br />
Nitrate Dichromate Formula Name<br />
Cyanide Silicate Ammonium<br />
Hydroxide<br />
Permanganate<br />
Hypochlorite<br />
Chlorite<br />
Chlorate<br />
Perchlorate<br />
R15
Activity Series of Metals<br />
Name<br />
Symbol<br />
D<br />
Lithium<br />
Li<br />
e<br />
Potassium<br />
K<br />
c<br />
r<br />
Barium<br />
Ba<br />
e<br />
Calcium<br />
Ca<br />
a<br />
Sodium<br />
Na<br />
s<br />
i<br />
Magnesium<br />
Mg<br />
n<br />
Aluminum<br />
Al<br />
g<br />
Zinc<br />
Zn<br />
Iron<br />
Fe<br />
A<br />
c<br />
Nickel<br />
Ni<br />
t<br />
Tin<br />
Sn<br />
i<br />
v<br />
Lead<br />
Pb<br />
i<br />
(Hydrogen)<br />
(H)*<br />
t<br />
Copper<br />
Cu<br />
y<br />
Mercury<br />
Hg<br />
Silver<br />
Ag<br />
Gold<br />
Au<br />
*Metals from Li to Na will replace H from acids and water; from Mg to<br />
Pb they will replace H from acids only.<br />
Decreasing<br />
Activity<br />
Activity Series of Nonmetal (Halogens)<br />
Name<br />
Symbol<br />
Fluorine F 2<br />
Chlorine Cl 2<br />
Bromine Br 2<br />
Iodine I 2<br />
R16
Honors Chemistry<br />
Class Policies and Grading<br />
The students will receive a Unit Outline at the beginning of each Unit. It will<br />
have information about the assignments that they will do, what it’s grade<br />
classification will be, what action they will need to do to complete the<br />
assignment and when it is due.<br />
The students will receive a Weekly Memo of the activities they will be<br />
responsible for that week. It will serve to inform the students of the learning<br />
goal for the week. It will also give the students any special information<br />
about that week.<br />
The students will also receive daily lectures and assignments that are<br />
designed to teach and re-enforce information related to the learning goal.<br />
This will be time in which new material will be taught and reviewed and will<br />
give the students the opportunity to ask questions regarding the concepts<br />
being taught.<br />
The students will work with a Lab partner and also be in a Lab group, but it<br />
will be up to the individual student to do his or her part of all assignments<br />
and the individual student will ultimately be responsible for all information<br />
presented in the class.<br />
The students will be required to follow all District and School Policies and to<br />
follow all Lab Safety Procedures, which they will be given and will sign,<br />
while performing labs. Students should come to class on time and with the<br />
supplies needed for that class.<br />
The following grading policy will be used.<br />
Assignment Categories<br />
Notebook<br />
Test/Projects<br />
Labs/Quizzes<br />
Work<br />
The students will be given a teacher generated Mid Term and a District<br />
Final.
Unit 1<br />
Measurement Lab<br />
Separation of Mixtures Lab with Lab Write Up<br />
Unit 2<br />
Flame Test Lab<br />
Nuclear Decay Lab<br />
Element Marketing Project<br />
Unit 3<br />
Golden Penny Lab with Lab Write Up<br />
Molecular Geometry<br />
Research Presentation on a Chemical<br />
Mid Term<br />
Unit 4<br />
Double Displacement Lab<br />
Stoichiometry Lab with Lab Write Up<br />
Mole Educational Demonstration Project<br />
Unit 5<br />
Gas Laws Lab with Lab Write Up<br />
States of Matter Lab<br />
Teach a Gas Law Project<br />
Unit 6<br />
Dilutions Lab<br />
Titration Lab<br />
District Final<br />
1
Unit 1 (22 days)<br />
Chapter 1 Introduction to Chemistry<br />
Honors Chemistry<br />
2017/2018 Syllabus<br />
3 days<br />
1.1 The Scope of Chemistry 1.3 Thinking Like a Scientist<br />
1.2 Chemistry and You 1.4 Problem Solving in Chemistry<br />
Chapter 2 Matter and Change<br />
2.1 Properties of Matter 2.3 Elements and Compounds<br />
2.2 Mixtures 2.4 Chemical Reactions<br />
Chapter 3 Scientific Measurement<br />
9 days<br />
10 days<br />
3.1 Using and Expressing Measurements 3.3 Solving Conversion Problems<br />
3.2 Units of Measurement<br />
Unit 2 (15 days)<br />
Chapter 4 Atomic Structure<br />
5 days<br />
4.1 Defining the Atom 4.3 Distinguishing Among Atoms<br />
4.2 Structure of the Nuclear Atom<br />
Chapter 5 Electrons in Atoms<br />
5 days<br />
5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms<br />
5.3 Atomic Emission Spectrum and the Quantum Mechanical Model<br />
Chapter 6 The Periodic Table<br />
6.1 Organizing the Elements 6.3 Periodic Trends<br />
6.2 Classifying Elements<br />
5 days<br />
Unit 3 (28 days)<br />
Chapter 25 Nuclear Chemistry<br />
25.1 Nuclear Radiation 25.3 Fission and Fusion<br />
25.2 Nuclear Transformations 25.4 Radiation in Your Life<br />
Chapter 7 Ionic and Metallic Bonding<br />
7.1 Ions 7.3 Bonding in Metals<br />
7.2 Ionic Bonds and Ionic Compounds<br />
Chapter 8 Covalent Bonding<br />
6 days<br />
8 days<br />
8 days<br />
8.1 Molecular Compounds 8.3 Bonding Theories<br />
8.2 The Nature of Covalent Bonding 8.4 Polar Bonds and Molecules<br />
Chapter 9 Chemical Names and Formulas<br />
6 days<br />
9.1 Naming Ions 9.3 Naming & Writing Formulas Molecular Compounds<br />
9.2 Naming and Writing Formulas for Ionic Compounds 9.4 Names for Acids and Bases<br />
Unit 4 (8 days)<br />
Chapter 22 Hydrocarbons Compounds<br />
22.1 Hydrocarbons 22.4 Hydrocarbon Rings<br />
Chapter 23 Functional Groups<br />
4 days<br />
4 days<br />
23.1 Introduction to Functional Groups 23.4 Alcohols, Ethers, and Amines<br />
2
Unit 5 (28 days)<br />
Chapter 10 Chemical Quantities 8 days<br />
10.1 The Mole: A Measurement of Matter 10.3 % Composition & Chem. Formulas<br />
10.2 Mole-Mass and Mole-Volume Relationships<br />
Chapter 11 Chemical Reactions 8 days<br />
11.1 Describing Chemical Reactions 11.3 Reactions in Aqueous Solutions<br />
11.2 Types of Chemical Reactions<br />
Chapter 12 Stoichiometry 12 days<br />
12.1 The Arithmetic of Equations 12.3 Limiting Reagent and % Yield<br />
12.2 Chemical Calculations<br />
Unit 6 (22 days)<br />
Chapter 13 States of Matter 6 days<br />
13.1 The Nature of Gases 13.3 The Nature of Solids<br />
13.2 The Nature of Liquids 13.4 Changes in State<br />
Chapter 14 The Behavior of Gases 10 days<br />
14.1 Properties of Gases 14.3 Ideal Gases<br />
14.2 The Gas Laws 14.4 Gases: Mixtures and Movement<br />
Chapter 15 Water and Aqueous Systems 6 days<br />
15.1 Water and its Properties 15.3 Heterogeneous Aqueous Systems<br />
15.2 Homogeneous Aqueous Systems<br />
Unit 7 (18 days)<br />
Chapter 16 Solutions 8 days<br />
16.1 Properties of Solutions 16.3 Colligative Properties of Solutions<br />
16.2 Concentrations of Solutions 16.4 Calc. Involving Colligative Property<br />
Chapter 17 Thermochemistry 5 days<br />
17.1 The Flow of Energy 17.3 Heat in Changes of State<br />
17.2 Measuring and Expressing Enthalpy Change 17.4 Calculating Heats in Reactions<br />
Chapter 18 Reaction Rates and Equilibrium 5 days<br />
18.1 Rates of Reactions 18.3 Reversible Reaction & Equilibrium<br />
18.2 The Progress of Chemical Reactions 18.5 Free Energy and Entropy<br />
Unit 8 (14 days)<br />
Chapter 19 Acid and Bases 10 days<br />
19.1 Acid-Base Theories 19.4 Neutralization Reactions<br />
19.2 Hydrogen Ions and Acidity 19.5 Salts in Solutions<br />
19.3 Strengths of Acids and Bases<br />
Chapter 20 Oxidation-Reduction Reactions 4 days<br />
20.1 The Meaning of Oxidation and Reduction 20.3 Describing Redox Equations<br />
20.2 Oxidation Numbers<br />
3
Lorenzo Walker Technical High School<br />
MUSTANG LABORATORIES<br />
Chemistry Safety<br />
Safety in the MUSTANG LABORATORIES - Chemistry Laboratory<br />
Working in the chemistry laboratory is an interesting and rewarding experience. During your labs, you will be actively<br />
involved from beginning to end—from setting some change in motion to drawing some conclusion. In the laboratory, you<br />
will be working with equipment and materials that can cause injury if they are not handled properly.<br />
However, the laboratory is a safe place to work if you are careful. Accidents do not just happen; they are caused—by<br />
carelessness, haste, and disregard of safety rules and practices. Safety rules to be followed in the laboratory are listed<br />
below. Before beginning any lab work, read these rules, learn them, and follow them carefully.<br />
General<br />
1. Be prepared to work when you arrive at the lab. Familiarize yourself with the lab procedures before beginning the lab.<br />
2. Perform only those lab activities assigned by your teacher. Never do anything in the laboratory that is not called for in<br />
the laboratory procedure or by your teacher. Never work alone in the lab. Do not engage in any horseplay.<br />
3. Work areas should be kept clean and tidy at all times. Only lab manuals and <strong>notebook</strong>s should be brought to the work<br />
area. Other books, purses, brief cases, etc. should be left at your desk or placed in a designated storage area.<br />
4. Clothing should be appropriate for working in the lab. Jackets, ties, and other loose garments should be removed. Open<br />
shoes should not be worn.<br />
5. Long hair should be tied back or covered, especially in the vicinity of open flame.<br />
6. Jewelry that might present a safety hazard, such as dangling necklaces, chains, medallions, or bracelets should not be<br />
worn in the lab.<br />
7. Follow all instructions, both written and oral, carefully.<br />
8. Safety goggles and lab aprons should be worn at all times.<br />
9. Set up apparatus as described in the lab manual or by your teacher. Never use makeshift arrangements.<br />
10. Always use the prescribed instrument (tongs, test tube holder, forceps, etc.) for handling apparatus or equipment.<br />
11. Keep all combustible materials away from open flames.<br />
12. Never touch any substance in the lab unless specifically instructed to do so by your teacher.<br />
13. Never put your face near the mouth of a container that is holding chemicals.<br />
14. Never smell any chemicals unless instructed to do so by your teacher. When testing for odors, use a wafting motion to<br />
direct the odors to your nose.<br />
15. Any activity involving poisonous vapors should be conducted in the fume hood.<br />
16. Dispose of waste materials as instructed by your teacher.<br />
17. Clean up all spills immediately.<br />
18. Clean and wipe dry all work surfaces at the end of class. Wash your hands thoroughly.<br />
19. Know the location of emergency equipment (first aid kit, fire extinguisher, fire shower, fire blanket, etc.) and how to use them.<br />
20. Report all accidents to the teacher immediately.<br />
Handling Chemicals<br />
21. Read and double check labels on reagent bottles before removing any reagent. Take only as much reagent as you<br />
need.<br />
22. Do not return unused reagent to stock bottles.<br />
23. When transferring chemical reagents from one container to another, hold the containers out away from your body.<br />
24. When mixing an acid and water, always add the acid to the water.<br />
25. Avoid touching chemicals with your hands. If chemicals do come in contact with your hands, wash them immediately.<br />
26. Notify your teacher if you have any medical problems that might relate to lab work, such as allergies or asthma.<br />
27. If you will be working with chemicals in the lab, avoid wearing contact lenses. Change to glasses, if possible, or notify<br />
the teacher.<br />
Handling Glassware<br />
28. Glass tubing, especially long pieces, should be carried in a vertical position to minimize the likelihood of breakage and<br />
to avoid stabbing anyone.<br />
29. Never handle broken glass with your bare hands. Use a brush and dustpan to clean up broken glass. Dispose of the<br />
glass as directed by your teacher.<br />
4
30. Always lubricate glassware (tubing, thistle tubes, thermometers, etc.) with water or glycerin before attempting to insert<br />
it into a rubber stopper.<br />
31. Never apply force when inserting or removing glassware from a stopper. Use a twisting motion. If a piece of glassware<br />
becomes "frozen" in a stopper, take it to your teacher.<br />
32. Do not place hot glassware directly on the lab table. Always use an insulating pad of some sort.<br />
33. Allow plenty of time for hot glass to cool before touching it. Hot glass can cause painful burns. (Hot glass looks cool.)<br />
Heating Substances<br />
34. Exercise extreme caution when using a gas burner. Keep your head and clothing away from the flame.<br />
35. Always turn the burner off when it is not in use.<br />
36. Do not bring any substance into contact with a flame unless instructed to do so.<br />
37. Never heat anything without being instructed to do so.<br />
38. Never look into a container that is being heated.<br />
39. When heating a substance in a test tube, make sure that the mouth of the tube is not pointed at yourself or anyone<br />
else.<br />
40. Never leave unattended anything that is being heated or is visibly reacting.<br />
First Aid in the MUSTANG LABORATORIES - Chemistry Laboratory<br />
Accidents do not often happen in well-equipped chemistry laboratories if students understand safe laboratory procedures<br />
and are careful in following them. When an occasional accident does occur, it is likely to be a minor one.<br />
The instructor will assist in treating injuries such as minor cuts and burns. However, for some types of injuries, you must<br />
take action immediately. The following information will be helpful to you if an accident occurs.<br />
1. Shock. People who are suffering from any severe injury (for example, a bad burn or major loss of blood) may be in a<br />
state of shock. A person in shock is usually pale and faint. The person may be sweating, with cold, moist skin and a weak,<br />
rapid pulse. Shock is a serious medical condition. Do not allow a person in shock to walk anywhere—even to the campus<br />
security office. While emergency help is being summoned, place the victim face up in a horizontal position, with the feet<br />
raised about 30 centimeters. Loosen any tightly fitting clothing and keep him or her warm.<br />
2. Chemicals in the Eyes. Getting any kind of a chemical into the eyes is undesirable, but certain chemicals are<br />
especially harmful. They can destroy eyesight in a matter of seconds. Because you will be wearing safety goggles at all<br />
times in the lab, the likelihood of this kind of accident is remote. However, if it does happen, flush your eyes with water<br />
immediately. Do NOT attempt to go to the campus office before flushing your eyes. It is important that flushing with water<br />
be continued for a prolonged time—about 15 minutes.<br />
3. Clothing or Hair on Fire. A person whose clothing or hair catches on fire will often run around hysterically in an<br />
unsuccessful effort to get away from the fire. This only provides the fire with more oxygen and makes it burn faster. For<br />
clothing fires, throw yourself to the ground and roll around to extinguish the flames. For hair fires, use a fire blanket to<br />
smother the flames. Notify campus security immediately.<br />
4. Bleeding from a Cut. Most cuts that occur in the chemistry laboratory are minor. For minor cuts, apply pressure to the<br />
wound with a sterile gauze. Notify campus security of all injuries in the lab. If the victim is bleeding badly, raise the<br />
bleeding part, if possible, and apply pressure to the wound with a piece of sterile gauze. While first aid is being given,<br />
someone else should notify the campus security officer.<br />
5. Chemicals in the Mouth. Many chemicals are poisonous to varying degrees. Any chemical taken into the mouth<br />
should be spat out and the mouth rinsed thoroughly with water. Note the name of the chemical and notify the campus<br />
office immediately. If the victim swallows a chemical, note the name of the chemical and notify campus security<br />
immediately.<br />
If necessary, the campus security officer or administrator will contact the Poison Control Center, a hospital emergency<br />
room, or a physician for instructions.<br />
6. Acid or Base Spilled on the Skin.<br />
Flush the skin with water for about 15 minutes. Take the victim to the campus office to report the injury.<br />
7. Breathing Smoke or Chemical Fumes.<br />
All experiments that give off smoke or noxious gases should be conducted in a well-ventilated fume hood. This will make<br />
an accident of this kind unlikely. If smoke or chemical fumes are present in the laboratory, all persons—even those who<br />
do not feel ill—should leave the laboratory immediately. Make certain that all doors to the laboratory are closed after the<br />
last person has left. Since smoke rises, stay low while evacuating a smoke-filled room. Notify campus security<br />
immediately.<br />
5
MUSTANG LABORATORIES<br />
COMMITMENT TO SAFETY IN THE LABORATORY<br />
As a student enrolled in Chemistry at Lorenzo Walker Technical High<br />
School, I agree to use good laboratory safety practices at all times. I<br />
also agree that I will:<br />
1. Conduct myself in a professional manner, respecting both my personal safety and the safety of<br />
others in the laboratory.<br />
2. Wear proper and approved safety glasses or goggles in the laboratory at all times.<br />
3. Wear sensible clothing and tie back long hair in the laboratory. Understand that open-toed shoes<br />
pose a hazard during laboratory classes and that contact lenses are an added safety risk.<br />
4. Keep my lab area free of clutter during an experiment.<br />
5. Never bring food or drink into the laboratory, nor apply makeup within the laboratory.<br />
6. Be aware of the location of safety equipment such as the fire extinguisher, eye wash station, fire<br />
blanket, first aid kit. Know the location of the nearest telephone and exits.<br />
7. Read the assigned lab prior to coming to the laboratory.<br />
8. Carefully read all labels on all chemical containers before using their contents, remove a small<br />
amount of reagent properly if needed, do not pour back the unused chemicals into the original<br />
container.<br />
9. Dispose of chemicals as directed by the instructor only. At no time will I pour anything down the<br />
sink without prior instruction.<br />
10. Never inhale fumes emitted during an experiment. Use the fume hood when instructed to do so.<br />
11. Report any accident immediately to the instructor, including chemical spills.<br />
12. Dispose of broken glass and sharps only in the designated containers.<br />
13. Clean my work area and all glassware before leaving the laboratory.<br />
14. Wash my hands before leaving the laboratory.<br />
NAME Cristopher __________________________<br />
Crisostomo<br />
5th<br />
PERIOD ________________________<br />
PARENT NAME Genarina ____________________________<br />
Tavarez<br />
PARENT NUMBER _________________________<br />
862-899-6625<br />
SIGNATURE ____________________________<br />
DATE ____________________________________<br />
6
7
Chapter 1<br />
Unit 1<br />
Introduction to Chemistry<br />
The students will learn why and how to solve problems using<br />
chemistry.<br />
Identify what is science, what clearly is not science, and what superficially<br />
resembles science (but fails to meet the criteria for science).<br />
Students will identify a phenomenon as science or not science.<br />
Science<br />
Inference<br />
Observation<br />
Hypothesis<br />
Identify which questions can be answered through science and which<br />
questions are outside the boundaries of scientific investigation, such as<br />
questions addressed by other ways of knowing, such as art, philosophy, and<br />
religion.<br />
Students will differentiate between problems and/or phenomenon that can and<br />
those that cannot be explained or answered by science.<br />
Students will differentiate between problems and/or phenomenon that can and<br />
those that cannot be explained or answered by science.<br />
Observation<br />
Inference<br />
Hypothesis<br />
Theory<br />
Controlled experiment<br />
Describe how scientific inferences are drawn from scientific observations<br />
and provide examples from the content being studied.<br />
Students will conduct and record observations.<br />
Students will make inferences.<br />
Students will identify a statement as being either an observation or inference.<br />
Students will pose scientific questions and make predictions based on<br />
inferences.<br />
Inference<br />
Hypothesis<br />
Observation<br />
Controlled experiment<br />
Identify sources of information and assess their reliability according to the<br />
strict standards of scientific investigation.<br />
8<br />
<br />
Students will compare and assess the validity of known scientific information<br />
from a variety of sources:
Print vs. print<br />
Online vs. online<br />
Print vs. online<br />
Students will conduct an experiment using the scientific method and compare<br />
with other groups.<br />
Controlled experiment<br />
Investigation<br />
Peer Review<br />
Accuracy<br />
Precision<br />
Percentage Error<br />
Chapter 2<br />
Matter and Change<br />
The students will learn what properties are used to describe<br />
matter and how matter can change its form.<br />
Differentiate between physical and chemical properties and physical and<br />
chemical changes of matter.<br />
Students will be able to identify physical and chemical properties of various<br />
substances.<br />
Students will be able to identify indicators of physical and chemical changes.<br />
Students will be able to calculate density.<br />
mass<br />
mixture<br />
physical property<br />
intensive property<br />
volume<br />
solution<br />
chemical property<br />
element<br />
vapor<br />
compound<br />
extensive property<br />
Chapter 3<br />
Scientific Measurements<br />
The students will be able to solve conversion problems using<br />
measurements.<br />
Determine appropriate and consistent standards of measurement for the<br />
data to be collected in a survey or experiment.<br />
Students will participate in activities to collect data using standardized<br />
measurement.<br />
Students will be able to manipulate/convert data collected and apply the data<br />
to scientific situations.<br />
Scientific notation<br />
Experimental value<br />
International System of Units (SI)<br />
Percent error<br />
Significant figures<br />
Dimensional analysis<br />
Accepted value<br />
9
The Learning Goal for this assignment is:<br />
Determine appropriate and consistent standards of measurement for the data to be collected in a survey or<br />
experiment<br />
Notes Section<br />
kilo 1000<br />
Hecto 100<br />
Deca 10<br />
Base Meter,Gram,Liter=1<br />
Deci .1<br />
Centi .01<br />
Milli .001<br />
10
To use the Stair-Step method, find the prefix the original measurement starts with. (ex. milligram)<br />
If there is no prefix, then you are starting with a base unit.<br />
Find the step which you wish to make the conversion to. (ex. decigram)<br />
Count the number of steps you moved, and determine in which direction you moved (left or right).<br />
The decimal in your original measurement moves the same number of places as steps you moved and in the<br />
same direction. (ex. milligram to decigram is 2 steps to the left, so 40 milligrams = .40 decigrams)<br />
If the number of steps you move is larger than the number you have, you will have to add zeros to hold the<br />
places. (ex. kilometers to meters is three steps to the right, so 10 kilometers would be equal to 10,000 m)<br />
That’s all there is to it! You need to be able to count to 6, and know your left from your right!<br />
1) Write the equivalent<br />
a) 5 dm =_______m .5<br />
.004 8000<br />
b) 4 mL = ______L c) 8 g = _______mg<br />
d) 9 mg =_______g .009<br />
e) 2 mL = ______L .002 f) 6 kg = _____g 6000<br />
g) 4 cm =_______m .04 h) 12 mg = ______ .012 g i) 6.5 cm 3 = _______L .0065<br />
j) 7.02 mL =_____cm 7.02 3 k) .03 hg = _______ 30<br />
dg l) 6035 mm _____cm 603.5<br />
m) .32 m = _______cm 320<br />
n) 38.2 g = .0382 _____kg<br />
11
2. One cereal bar has a mass of 37 g. What is the mass of 6 cereal bars? Is that more than or less<br />
than 1 kg? Explain your answer.<br />
6 cereal bars is 222g while 1kg would be 1000g since kilo means thousand<br />
3. Wanda needs to move 110 kg of rocks. She can carry l0 hg each trip. How many trips must she<br />
make? Explain your answer.<br />
110 trips since 110kg is 110,000g and 10hg is 1000g so she would need to make 110 trips 110000/10000=110<br />
4. Dr. O is playing in her garden again She needs 1 kg of potting soil for her plants. She has 750 g.<br />
How much more does she need? Explain your answer.<br />
1000-750=250 she needs 250 to complete the 1000g's<br />
5. Weather satellites orbit Earth at an altitude of 1,400,000 meters. What is this altitude in kilometers?<br />
1400<br />
6. Which unit would you use to measure the capacity? Write milliliter or liter.<br />
a) a bucket __________<br />
liter<br />
b) a thimble __________<br />
milliliter<br />
c) a water storage tank__________<br />
liter<br />
d) a carton of juice__________<br />
liter<br />
7. Circle the more reasonable measure:<br />
a) length of an ant 5mm or 5cm<br />
b) length of an automobile 5 m or 50 m<br />
KHDBDCM<br />
c) distance from NY to LA 450 km or 4,500 km<br />
d) height of a dining table 75 mm or 75 cm<br />
8. Will a tablecloth that is 155 cm long cover a table that is 1.6 m long? Explain your answer.<br />
no 155cm would be 1.55 so it would be covering everything except .05<br />
9. A dollar bill is 15.6 cm long. If 200 dollar bills were laid end to end, how many meters long would<br />
the line be?<br />
31.2 meters long if 200 bills were laid end to end<br />
10. The ceiling in Jan’s living room is 2.5 m high. She has a hanging lamp that hangs down 41 cm.<br />
Her husband is exactly 2 m tall. Will he hit his head on the hanging lamp? Why or why not?<br />
no he wont hit his head since 41cm is .41m there will be 9cm of space<br />
12
Using SI Units<br />
Match the terms in Column II with the descriptions in Column I. Write the letters of the correct term in<br />
the blank on the left.<br />
Column I Column II<br />
_____ k 1. distance between two points<br />
a. time<br />
e<br />
_____ 2. SI unit of length<br />
m<br />
_____ 3. tool used to measure length<br />
b. volume<br />
c. mass<br />
_____ g 4. units obtained by combining other units<br />
_____<br />
b<br />
5. amount of space occupied by an object<br />
h<br />
_____ 6. unit used to express volume<br />
f<br />
_____ 7. SI unit of mass<br />
_____ c 8. amount of matter in an object<br />
d<br />
_____ 9. mass per unit of volume<br />
_____<br />
o<br />
10. temperature scale of most laboratory thermometers<br />
1<br />
_____ 11. instrument used to measure mass<br />
a<br />
_____ 12. interval between two events<br />
j<br />
_____ 13. SI unit of temperature<br />
_____ i 14. SI unit of time<br />
_____ n 15. instrument used to measure temperature<br />
d. density<br />
e. meter<br />
f. kilogram<br />
g. derived<br />
h. liter<br />
i. second<br />
j. Kelvin<br />
k. length<br />
1. balance<br />
m. meterstick<br />
n. thermometer<br />
o. Celsius<br />
Circle the two terms in each group that are related. Explain how the terms are related.<br />
16. Celsius degree, mass, Kelvin _____________________________________________________<br />
Both are related to temperature<br />
________________________________________________________________________________<br />
17. balance, second, mass __________________________________________________________<br />
a balance is used to find the mass in something<br />
________________________________________________________________________________<br />
18. kilogram, liter, cubic centimeter __________________________________________________<br />
cm cubed can be turned into ml<br />
________________________________________________________________________________<br />
19. time, second, distance __________________________________________________________<br />
seconds are a measurement of time<br />
________________________________________________________________________________<br />
20. decimeter, kilometer, Kelvin _____________________________________________________<br />
the base of both of them is meter<br />
________________________________________________________________________________<br />
13
1. How many meters are in one kilometer? 1000m __________<br />
2. What part of a liter is one milliliter? __________<br />
.001<br />
3. How many grams are in two dekagrams? __________<br />
20g<br />
4. If one gram of water has a volume of one milliliter, what would the mass of one liter of water be in<br />
kilograms?__________<br />
1kg<br />
5. What part of a meter is a decimeter? __________<br />
.1m<br />
In the blank at the left, write the term that correctly completes each statement. Choose from the terms<br />
listed below.<br />
Metric SI standard ten<br />
prefixes ten tenth<br />
6. An exact quantity that people agree to use for comparison is a ______________ standard ten tenth .<br />
7. The system of measurement used worldwide in science is _______________<br />
si ten<br />
.<br />
8. SI is based on units of _______________ Metric prefixes .<br />
9. The system of measurement that was based on units of ten was the _______________ si ten<br />
system.<br />
10. In SI, _______________ ,Metric prefixes are used with the names of the base unit to indicate the multiple of ten<br />
that is being used with the base unit.<br />
11. The prefix deci- means _______________ standard ten tenth .<br />
14
Standards of Measurement<br />
Fill in the missing information in the table below.<br />
S.I prefixes and their meanings<br />
Prefix<br />
Meaning<br />
0.001<br />
0.01<br />
deci- 0.1<br />
10<br />
hecto- 100<br />
1000<br />
Circle the larger unit in each pair of units.<br />
1. millimeter, kilometer 4. centimeter, millimeter<br />
2. decimeter, dekameter 5. hectogram, kilogram<br />
3. hectogram, decigram<br />
6. In SI, the base unit of length is the meter. Use this information to arrange the following units of<br />
measurement in the correct order from smallest to largest.<br />
Write the number 1 (smallest) through 7 - (largest) in the spaces provided.<br />
_____ a. kilometer<br />
_____ b. centimeter<br />
_____ c. meter<br />
_____ e. hectometer<br />
_____ f. millimeter<br />
_____ g. decimeter<br />
_____ d. dekameter<br />
Use your knowledge of the prefixes used in SI to answer the following questions in the spaces<br />
provided.<br />
7. One part of the Olympic games involves an activity called the decathlon. How many events do you<br />
think make up the decathlon?_____________________________________________________<br />
8. How many years make up a decade? _______________________________________________<br />
9. How many years make up a century? ______________________________________________<br />
10. What part of a second do you think a millisecond is? __________________________________<br />
15
The Learning Goal for this assignment is:<br />
Notes Section<br />
1. 7,485 6. 1.683<br />
2. 884.2 7. 3.622<br />
3. 0.00002887 8. 0.00001735<br />
4. 0.05893 9. 0.9736<br />
5. 0.006162 10. 0.08558<br />
11. 6.633 X 10−⁴ 16. 1.937 X 10⁴<br />
12. 4.445 X 10−⁴ 17. 3.457 X 10⁴<br />
13. 2.182 X 10−³ 18. 3.948 X 10−⁵<br />
14. 4.695 X 10² 19. 8.945 X 10⁵<br />
15. 7.274 X 10⁵ 20. 6.783 X 10²<br />
15
16<br />
SCIENTIFIC NOTATION RULES<br />
How to Write Numbers in Scientific Notation<br />
Scientific notation is a standard way of writing very large and very small numbers so that they're<br />
easier to both compare and use in computations. To write in scientific notation, follow the form<br />
N X 10 ᴬ<br />
where N is a number between 1 and 10, but not 10 itself, and A is an integer (positive or negative<br />
number).<br />
RULE #1: Standard Scientific Notation is a number from 1 to 9 followed by a decimal and the<br />
remaining significant figures and an exponent of 10 to hold place value.<br />
Example:<br />
5.43 x 10 2 = 5.43 x 100 = 543<br />
8.65 x 10 – 3 = 8.65 x .001 = 0.00865<br />
****54.3 x 10 1 is not Standard Scientific Notation!!!<br />
RULE #2: When the decimal is moved to the Left the exponent gets Larger, but the value of the<br />
number stays the same. Each place the decimal moves Changes the exponent by one (1). If you<br />
move the decimal to the Right it makes the exponent smaller by one (1) for each place it is moved.<br />
Example:<br />
6000. x 10 0 = 600.0 x 10 1 = 60.00 x 10 2 = 6.000 x 10 3 = 6000<br />
(Note: 10 0 = 1)<br />
All the previous numbers are equal, but only 6.000 x 10 3 is in proper Scientific Notation.
RULE #3: To add/subtract in scientific notation, the exponents must first be the same.<br />
Example:<br />
(3.0 x 10 2 ) + (6.4 x 10 3 ); since 6.4 x 10 3 is equal to 64. x 10 2 . Now add.<br />
(3.0 x 10 2 )<br />
+ (64. x 10 2 )<br />
67.0 x 10 2 = 6.70 x 10 3 = 6.7 x 10 3<br />
67.0 x 10 2 is mathematically correct, but a number in standard scientific notation can only<br />
have one number to the left of the decimal, so the decimal is moved to the left one place and<br />
one is added to the exponent.<br />
Following the rules for significant figures, the answer becomes 6.7 x 10 3 .<br />
RULE #4: To multiply, find the product of the numbers, then add the exponents.<br />
Example:<br />
(2.4 x 10 2 ) (5.5 x 10 –4 ) = ? [2.4 x 5.5 = 13.2]; [2 + -4 = -2], so<br />
(2.4 x 10 2 ) (5.5 x 10 –4 ) = 13.2 x 10 –2 = 1.3 x 10 – 1<br />
RULE #5: To divide, find the quotient of the number and subtract the exponents.<br />
Example:<br />
(3.3 x 10 – 6 ) / (9.1 x 10 – 8 ) = ? [3.3 / 9.1 = .36]; [-6 – (-8) = 2], so<br />
(3.3 x 10 – 6 ) / (9.1 x 10 – 8 ) = .36 x 10 2 = 3.6 x 10 1<br />
17
Convert each number from Scientific Notation to real numbers:<br />
1. 7.485 X 10³ 7485<br />
6. 1.683 X 10⁰<br />
1<br />
2. 8.842 X 10² 884.2<br />
7. 3.622 10⁰<br />
1<br />
3. 2.887 X 10−⁵ .00002887<br />
8. 1.735 X 10−⁵<br />
.00001735<br />
4. 5.893 X 10−²<br />
.05893<br />
9. 9.736 X 10−¹<br />
.9736<br />
5. 6.162 X 10−³ .006162<br />
10. 8.558 X 10−²<br />
.08558<br />
Convert each number from a real number to Scientific Notation:<br />
11. 0.0006633 6.633 x 10^-4<br />
16. 1,937,000<br />
1.937x 10^6<br />
12. 0.0004445 4.445x 10^-4<br />
17. 34,570<br />
3.457x 10^4<br />
13. 0.002182 2.182x10^-3<br />
18. 0.00003948<br />
3.948x 10^-5<br />
14. 469.5 4.695x 10^2<br />
19. 894,500<br />
8.945x 10^5<br />
15. 727,400 7.274x10^5<br />
20. 678.3<br />
6.783x 10^2<br />
18
The Learning Goal for this assignment is:<br />
Notes Section:<br />
Question Sig Figs Question Add & Subtract Question Multiple & Divide<br />
1 4 1 55.36 1 20,000<br />
2 4 2 84.2 2 94<br />
3 3 3 115.4 3 300<br />
4 3 4 0.8 4 7<br />
5 4 5 245.53 5 62<br />
6 3 6 34.5 6 0.005<br />
7 3 7 74.0 7 4,000<br />
8 2 8 53.287 8 3,900,000<br />
9 2 9 54.876 9 2<br />
10 2 10 40.19 10 30,000,000<br />
11 3 11 7.7 11 1,200<br />
12 2 12 67.170 12 0.2<br />
13 3 13 81.0 13 0.87<br />
14 4 14 73.290 14 0.049<br />
15 4 15 29.789 15 2,000<br />
16 3 16 39.53 16 0.5<br />
17 4 17 70.58 17 1.9<br />
18 2 18 86.6 18 0.05<br />
19 2 19 64.990 19 230<br />
20 1 20 36.0 20 460,000<br />
19
Significant Figures Rules<br />
There are three rules on determining how many significant figures are in a<br />
number:<br />
1. Non-zero digits are always significant.<br />
1 2 3 4 5 6 7 8 9<br />
2. Any zeros between two significant digits are significant.<br />
101 8405 15.057 8450<br />
3. A final zero or trailing zeros in the DECIMAL PORTION ONLY are<br />
significant.<br />
15.240 .5220 .10010 .006040<br />
Please remember that, in science, all numbers are based upon measurements (except for a very few<br />
that are defined). Since all measurements are uncertain, we must only use those numbers that are<br />
meaningful.<br />
Not all of the digits have meaning (significance) and, therefore, should not be written down. In<br />
science, only the numbers that have significance (derived from measurement) are written.<br />
Rule 1: Non-zero digits are always significant.<br />
If you measure something and the device you use (ruler, thermometer, triple-beam, balance, etc.)<br />
returns a number to you, then you have made a measurement decision and that ACT of measuring<br />
gives significance to that particular numeral (or digit) in the overall value you obtain.<br />
Hence a number like 46.78 would have four significant figures and 3.94 would have three.<br />
Rule 2: Any zeros between two significant digits are significant.<br />
Suppose you had a number like 409. By the first rule, the 4 and the 9 are significant. However, to<br />
make a measurement decision on the 4 (in the hundred's place) and the 9 (in the one's place), you<br />
HAD to have made a decision on the ten's place. The measurement scale for this number would have<br />
hundreds, tens, and ones marked.<br />
Like the following example:<br />
These are sometimes called "captured zeros."<br />
If a number has a decimal at the end (after the one’s place) then all digits (numbers) are significant<br />
and will be counted.<br />
In the following example the zeros are significant digits and highlighted in blue.<br />
960.<br />
70050.<br />
20
Rule 3: A final zero or trailing zeros in the decimal portion ONLY are<br />
significant.<br />
This rule causes the most confusion among students.<br />
In the following example the zeros are significant digits and highlighted in blue.<br />
0.07030<br />
0.00800<br />
Here are two more examples where the significant zeros are highlighted in blue.<br />
When Zeros are Not Significant Digits<br />
4.7 0 x 10−³<br />
6.5 0 0 x 10⁴<br />
Zero Type # 1 : Space holding zeros in numbers less than one.<br />
In the following example the zeros are NOT significant digits and highlighted in red.<br />
0.09060<br />
0.00400<br />
These zeros serve only as space holders. They are there to put the decimal point in its correct<br />
location.<br />
They DO NOT involve measurement decisions.<br />
Zero Type # 2 : Trailing zeros in a whole number.<br />
In the following example the zeros are NOT significant digits and highlighted in red.<br />
200<br />
25000<br />
For addition and subtraction, look at the decimal portion (i.e., to the right of the decimal point)<br />
of the numbers ONLY. Here is what to do:<br />
1) Count the number of significant figures in the decimal portion of each number in the problem. (The<br />
digits to the left of the decimal place are not used to determine the number of decimal places in the<br />
final answer.)<br />
2) Add or subtract in the normal fashion.<br />
3) Round the answer to the LEAST number of places in the decimal portion of any number in the<br />
problem<br />
The following rule applies for multiplication and division:<br />
The LEAST number of significant figures in any number of the problem determines the number of<br />
significant figures in the answer.<br />
This means you MUST know how to recognize significant figures in order to use this rule.<br />
21
How Many Significant Digits for Each Number?<br />
1) 2359 = 4______<br />
2) 2.445 x 10−⁵= ______ 4<br />
3) 2.93 x 10⁴= ______ 3<br />
4) 1.30 x 10−⁷= ______ 3<br />
5) 2604 = ______ 4<br />
6) 9160 = ______ 3<br />
7) 0.0800 = ______ 3<br />
8) 0.84 = ______ 2<br />
9) 0.0080 = ______ 2<br />
10) 0.00040 = ______ 2<br />
11) 0.0520 = ______ 3<br />
12) 0.060 = ______ 2<br />
13) 6.90 x 10−¹= ______ 3<br />
14) 7.200 x 10⁵= ______ 2<br />
15) 5.566 x 10−²= ______ 4<br />
16) 3.88 x 10⁸= ______ 3<br />
17) 3004 = ______ 4<br />
18) 0.021 = ______ 2<br />
19) 240 = ______ 2<br />
20) 500 = ______ 1<br />
22
For addition and subtraction, look at the decimal portion (i.e., to the right of the decimal point) of the<br />
numbers ONLY. Here is what to do:<br />
1) Count the number of significant figures in the decimal portion of each number in the problem. (The<br />
digits to the left of the decimal place are not used to determine the number of decimal places in the<br />
final answer.)<br />
2) Add or subtract in the normal fashion.<br />
3) Round the answer to the LEAST number of places in the decimal portion of any number in the<br />
problem.<br />
Solve the Problems and Round Accordingly...<br />
1) 43.287 + 5.79 + 6.284 = _______ 55.36 55.361<br />
2) 87.54 - 3.3 = _______ 84.2<br />
3) 99.1498 + 6.5397 + 9.7 = _______ 11534<br />
84.24<br />
115.3895<br />
4) 5.868 - 5.1 = _______ .8 .768<br />
5) 59.9233 + 86.21 + 99.396 = _______ 245.53<br />
6) 7.7 + 26.756 = _______ 34.5<br />
7) 66.8 + 2.3 + 4.8516 = _______ 74.0<br />
8) 9.7419 + 43.545 = 53.287 _______<br />
9) 4.8976 + 48.4644 + 1.514 = _______ 54.876<br />
245.5293<br />
34.456<br />
73.951<br />
53.2869<br />
54.876<br />
10) 4.335 + 35.85 = _______ 40.19 40.185<br />
11) 9.448 - 1.7 = _______ 7.7 7.748<br />
12) 75.826 - 8.6555 = _______ 67.171 67.1705<br />
13) 57.2 + 23.814 = _______ 81.0<br />
14) 77.684 - 4.394 = _______ 73.290<br />
15) 26.4496 + 3.339 = _______ 29.789<br />
16) 9.6848 + 29.85 = 39.53 _______<br />
17) 63.11 + 2.5412 + 4.93 = 70.58 _______<br />
18) 11.2471 + 75.4 = _______ 86.6<br />
19) 73.745 - 8.755 = 64.990 _______<br />
20) 6.5238 + 1.7 + 27.79 = _______ 36.0<br />
81.014<br />
73.29<br />
29.7886<br />
39.5348<br />
70.5812<br />
86.6471<br />
64.99<br />
36.0138<br />
23
24<br />
The following rule applies for multiplication and division:<br />
The LEAST number of significant figures in any number of the problem determines the number of<br />
significant figures in the answer.<br />
This means you MUST know how to recognize significant figures in order to use this rule.<br />
Solve the Problems and Round Accordingly...<br />
1) 0.6 x 65.0 x 602 = __________<br />
20000<br />
2) 720 ÷ 7.7 = __________<br />
94<br />
3) 929 x 0.3 = __________<br />
300<br />
4) 300 ÷ 44.31 = __________<br />
7<br />
5) 608 ÷ 9.8 = __________<br />
62<br />
.005<br />
6) 0.06 x 0.079 = __________<br />
7) 0.008 x 72.91 x 7000 = __________<br />
8) 73.94 x 67 x 780 = __________<br />
3,800,000<br />
9) 0.62 x 0.097 x 40 = __________<br />
2.4<br />
10) 600 x 10 x 5030 = __________<br />
30,000,000<br />
11) 5200 ÷ 4.46 = __________<br />
1,100<br />
12) 0.0052 x 0.4 x 107 = __________<br />
.2<br />
13) 0.099 x 8.8 = __________<br />
.87<br />
14) 0.0095 x 5.2 = __________<br />
.049<br />
15) 8000 ÷ 4.62 = __________<br />
1,000<br />
16) 0.6 x 0.8 = __________<br />
.5<br />
4000<br />
17) 2.84 x 0.66 = __________<br />
1.9<br />
18) 0.5 x 0.09 = __________<br />
.05<br />
19) 8100 ÷ 34.84 = __________<br />
230<br />
20) 8.24 x 6.9 x 8100 = __________<br />
460,000
Dimensional Analysis<br />
This is a way to convert from one unit of a given substance to<br />
another unit using ratios or conversion units. What this video<br />
www.youtube.com/watch?v=aZ3J60GYo6U<br />
Let’ look at a couple of examples:<br />
1. Convert 2.6 qt to mL.<br />
First we need a ratio or conversion unit so that we can go from quarts to milliliters. 1.00 qt = 946 mL<br />
Next write down what you are starting with<br />
2.6 qt<br />
Then make you conversion tree<br />
2.6 qt<br />
Then fill in the units in your ratio so that you can cancel out the original unit and will be left with the<br />
unit you need for the answer. Cross out units, one at a time that are paired, and one on top one on<br />
the bottom.<br />
2.6 qt mL<br />
qt<br />
Now fill in the values from the ratio.<br />
2.6 qt 946 mL<br />
1.00 qt<br />
Now multiply all numbers on the top and multiply all numbers on the bottom and write them as a<br />
fraction.<br />
2.6 qt 946 mL = 2,459.6 mL<br />
1.00 qt 1.00<br />
Now divide the top number by the bottom number and write that number with the unit that was not<br />
crossed out.<br />
25
1qt=32 oz 1gal = 4qts 1.00 qt = 946 mL 1L = 1000mL<br />
2. Convert 8135.6 mL to quarts<br />
8135.6<br />
1qt<br />
=<br />
8135.6<br />
8.6qts<br />
8.6000<br />
1<br />
946mL<br />
946<br />
3. Convert 115.2 oz to mL<br />
115.2oz<br />
1qt<br />
946ml<br />
=<br />
108979.2<br />
3405.6ml<br />
1<br />
32oz<br />
1qt<br />
32<br />
3406ml<br />
4. Convert 2.3 g to Liters<br />
2.3g<br />
4qt<br />
946mL<br />
1L<br />
=<br />
8703.2<br />
8.7032<br />
8.7<br />
1<br />
1g<br />
1qt<br />
1000mL<br />
1000<br />
5. Convert 8.42 L to oz<br />
8.42L<br />
1<br />
1000mL<br />
1L<br />
1qt<br />
946mL<br />
32oz<br />
1qt<br />
=<br />
169440<br />
946<br />
284.8202959831<br />
284<br />
Go to http://science.widener.edu/svb/tutorial/ chose #7 “Converting Volume” and do 5 more in the<br />
space provided.<br />
1. Convert _________ 8.42L to _________ qts<br />
8.42L 1000mL<br />
1qt 8420<br />
=<br />
8..9006342495<br />
8.90<br />
1L<br />
946mL<br />
946<br />
2. Convert _________ 5.39L to _________ oz<br />
5.39L 1000mL 1qt<br />
1L<br />
946mL<br />
=<br />
32oz 172480<br />
1qt<br />
946<br />
183.3255813953<br />
182<br />
3. Convert _________ 1.2G to _________ L<br />
1.2g 4qt<br />
946ml<br />
=<br />
1L 4540.8<br />
4.5408<br />
4.5<br />
1g<br />
1qt<br />
1000ml<br />
1000<br />
4. Convert _________ 1513.6mL to _________ oz<br />
1513.6mL 1qt<br />
32oz 48435.2<br />
946ml<br />
1qt<br />
5. Convert _________ 7.38L to _________ oz<br />
7.38L<br />
=<br />
=<br />
946<br />
1000 1qt 32 236160<br />
51.2<br />
51.200<br />
249.6405919662<br />
250<br />
1L 946ml 1qt 946<br />
26
Chapter 7<br />
Unit 3<br />
Ionic and Metallic Bonding<br />
The students will learn how ionic compounds form and how<br />
metallic bounding affects the properties of metals.<br />
Compare the magnitude and range of the four fundamental forces<br />
(gravitational, electromagnetic, weak nuclear, strong nuclear).<br />
Students will compare/contrast the characteristics of each fundamental force.<br />
gravity<br />
electromagnetic<br />
strong<br />
weak<br />
Distinguish between bonding forces holding compounds together and other<br />
attractive forces, including hydrogen bonding and van der Waals forces.<br />
Students will be able to compare/contrast traits of ionic and covalent bonds.<br />
Students will be able to compare/contrast basic attractive forces between<br />
molecules.<br />
<br />
Students will be able to predict the type of bond or attractive force between<br />
atoms or molecules.<br />
ionic bond<br />
covalent bond<br />
metallic bond<br />
polar covalent bond<br />
hydrogen bond<br />
van der Waals forces<br />
London dispersion forces<br />
Chapter 8<br />
Covalent Bonding<br />
The students will learn how molecular bonding is different<br />
than ionic bonding and electrons affect the shape of a<br />
molecule and its properties.<br />
Interpret formula representations of molecules and compounds in terms of<br />
composition and structure.<br />
Students will be able to interpret chemical formulas in terms of # of atoms.<br />
Students will be able to differentiate between ionic and molecular compounds.<br />
Students will be able to list various VSEPR shapes and identify examples of<br />
each.<br />
27
Students will be able to predict shapes of various compounds.<br />
Atom<br />
Electron<br />
Element<br />
Compound<br />
Molecule<br />
empirical formula<br />
Chapter 9<br />
Chemical Names and Formulas<br />
The students will learn how the periodic table helps them<br />
determine the names and formulas of ions and compounds.<br />
28
The Learning Goal for this assignment is:<br />
Distinguish between bonding forces holding compounds together<br />
and other attractive forces. incuding hydrogen bonding and van der<br />
vass forces.<br />
Introduction to Ionic Compounds<br />
Those molecules that consist of charged ions with opposite charges are called IONIC. These ionic<br />
compounds are generally solids with high melting points and conduct electrical current. Ionic<br />
compounds are generally formed from metal and a non-metal elements. See Ionic Bonding below.<br />
Ionic Compound Example<br />
For example, you are familiar with the fairly benign unspectacular behavior of common white<br />
crystalline table salt (NaCl). Salt consists of positive sodium ions (Na + ) & negative chloride ions (Cl - ).<br />
On the other hand the element sodium is a silvery gray metal composed of neutral atoms which react<br />
vigorously with water or air. Chlorine as an element is a neutral greenish-yellow, poisonous, diatomic<br />
gas (Cl2).<br />
The main principle to remember is that ions are completely different in physical and chemical<br />
properties from the neutral atoms of the elements.<br />
The notation of the + and - charges on ions is very important as it conveys a definite meaning.<br />
Whereas elements are neutral in charge, IONS have either a positive or negative charge depending<br />
upon whether there is an excess of protons (positive ion) or excess of electrons (negative ion).<br />
Formation of Positive Ions<br />
Metals usually have 1-4 electrons in the outer energy level. The electron arrangement of a rare gas is<br />
most easily achieved by losing the few electrons in the newly started energy level. The number of<br />
electrons lost must bring the electron number "down to" that of a prior rare gas.<br />
How will sodium complete its octet?<br />
First examine the electron arrangement of the atom. The atomic number is eleven, therefore, there<br />
are eleven electrons and eleven protons on the neutral sodium atom. Here is the Bohr diagram and<br />
Lewis symbol for sodium:<br />
29
This analysis shows that sodium has only one electron in its outer level. The nearest rare gas is neon<br />
with 8 electron in the outer energy level. Therefore, this electron is lost so that there are now eight<br />
electrons in the outer energy level, and the Bohr diagrams and Lewis symbols for sodium ion and<br />
neon are identical. The octet rule is satisfied.<br />
Ion Charge?<br />
What is the charge on sodium ion as a result of losing one electron? A comparison of the atom and<br />
the ion will yield this answer.<br />
Sodium Atom<br />
Sodium Ion<br />
11 p+ to revert to 11 p + Protons are identical in<br />
12 n an octet 12 n<br />
the atom and ion.<br />
11 e- lose 1 electron 10 e-<br />
Positive charge is<br />
caused by lack of<br />
0 charge + 1 charge<br />
electrons.<br />
Formation of Negative Ions<br />
How will fluorine complete its octet?<br />
First examine the electron arrangement of the atom. The atomic number is nine, therefore, there are<br />
nine electrons and nine protons on the neutral fluorine atom. Here is the Bohr diagram and Lewis<br />
symbol for fluorine:<br />
This analysis shows that fluorine already has seven electrons in its outer level. The nearest rare gas<br />
is neon with 8 electron in the outer energy level. Therefore only one additional electron is needed to<br />
complete the octet in the fluorine atom to make the fluoride ion. If the one electron is added, the Bohr<br />
diagrams and Lewis symbols for fluorine and neon are identical. The octet rule is satisfied.<br />
30
Ion Charge?<br />
What is the charge on fluorine as a result of adding one electron? A comparison of the atom and the<br />
ion will yield this answer.<br />
Fluorine Atom Fluoride Ion *<br />
9 p+ to complete 9 p + Protons are identical in<br />
10 n octet 10 n<br />
9 e- add 1 electron 10 e-<br />
0 charge - 1 charge<br />
the atom and ion.<br />
Negative charge is<br />
caused by excess<br />
electrons<br />
* The "ide" ending in the name signifies a simple negative ion.<br />
Summary Principle of Ionic Compounds<br />
An ionic compound is formed by the complete transfer of electrons from a metal to a nonmetal and<br />
the resulting ions have achieved an octet. The protons do not change. Metal atoms in Groups 1-3<br />
lose electrons to non-metal atoms with 5-7 electrons missing in the outer level. Non-metals gain 1-4<br />
electrons to complete an octet.<br />
Octet Rule<br />
Elemental atoms generally lose, gain, or share electrons with other atoms in order to achieve the<br />
same electron structure as the nearest rare gas with eight electrons in the outer level.<br />
The proper application of the Octet Rule provides valuable assistance in predicting and explaining<br />
various aspects of chemical formulas.<br />
Introduction to Ionic Bonding<br />
Ionic bonding is best treated using a simple<br />
electrostatic model. The electrostatic model<br />
is simply an application of the charge<br />
principles that opposite charges attract and<br />
similar charges repel. An ionic compound<br />
results from the interaction of a positive and<br />
negative ion, such as sodium and chloride in<br />
common salt.<br />
The IONIC BOND results as a balance<br />
between the force of attraction between<br />
opposite plus and minus charges of the ions<br />
and the force of repulsion between similar<br />
negative charges in the electron clouds. In<br />
crystalline compounds this net balance of<br />
forces is called the LATTICE ENERGY.<br />
Lattice energy is the energy released in the<br />
formation of an ionic compound.<br />
DEFINITION: The formation of an IONIC<br />
BOND is the result of the transfer of one or<br />
more electrons from a metal onto a nonmetal.<br />
31
Metals, with only a few electrons in the outer energy level, tend to lose electrons most readily. The<br />
energy required to remove an electron from a neutral atom is called the IONIZATION POTENTIAL.<br />
Energy + Metal Atom ---> Metal (+) ion + e-<br />
Non-metals, which lack only one or two electrons in the outer energy level have little tendency to lose<br />
electrons - the ionization potential would be very high. Instead non-metals have a tendency to gain<br />
electrons. The ELECTRON AFFINITY is the energy given off by an atom when it gains electrons.<br />
Non-metal Atom + e- --- Non-metal (-) ion + energy<br />
The energy required to produce positive ions (ionization potential) is roughly balanced by the energy<br />
given off to produce negative ions (electron affinity). The energy released by the net force of<br />
attraction by the ions provides the overall stabilizing energy of the compound.<br />
https://www.youtube.com/watch?v=zpaHPXVR8WU<br />
Writing Ionic Compounds<br />
An easy technique for creating a neutral combination of two charged ions is called the criss-cross<br />
technique. When writing a formula for an ionic compound the charges from each ion are simply<br />
switched to become the subscript values written to designate the number of atoms present in a<br />
compound. See the example below.<br />
Naming Binary Ionic Compounds<br />
Naming Ionic Compounds<br />
Learning to name ionic compounds is both easy and hard depending on the complexity of the<br />
compound. Before we start, though, I just wanted to review a few terms. Remember that positively<br />
charged ions are called cations. Negatively charged ions are called anions. An ionic compound is a<br />
compound held together by ionic bonds. A simple binary compound is just what it seems - a simple<br />
compound with two elements in it.<br />
Binary compounds are easy to name. The cation is always named first and gets its name from the<br />
name of the element. For example, K + is called a potassium ion. An anion also takes its name from its<br />
element, but it adds the suffix -ide to it. So, Cl - is called a chloride ion; O 2- is an oxide ion.<br />
Take the binary compound NaCl. The Na + is a sodium cation. The Cl - is a chlorine anion, which gets<br />
the suffix -ide added to it. When you put them together, it becomes sodium chloride.<br />
32
Here are some examples for you:<br />
Zn 2+ is zinc. S 2- is sulfide. Put them together for zinc sulfide (ZnS).<br />
Here's another:<br />
K2O is potassium oxide<br />
Naming Ionic Compounds Containing Transition Metals<br />
A transition metal is a metal that can use the inner shell before using the outer shell to bond. These<br />
are the elements in the middle of the periodic table - things like zinc, iron and copper. Naming<br />
polyatomic ionic compounds that have transition metals in them is also fairly easy. It follows the same<br />
naming rules as the simple binary compounds, but with an extra rule added in. So, you still name the<br />
cation first, followed by the anion with the suffix -ide added to the end of it.<br />
The new rule is that transition metals form more than one ion, so this has to be accounted for in the<br />
naming. We do this by using Roman numerals to denote which ion it is. The Roman numeral will<br />
equal the charge on the ion. For instance, Fe 2+ is iron (II). Fe 3+ is iron (III).<br />
When compounds are formed with these metals, the different ions still have to be accounted for. If I<br />
told you the compound was iron chloride, that wouldn't give you the full story. You wouldn't know if it<br />
was iron (II) or iron (III), which means you don't know how many chlorine atoms are in the compound<br />
to bond with the iron, since two chlorines would be needed for iron (II) and three for iron (III). If I<br />
instead told you that the compound was iron (II) chloride, you would know that it was Fe 2+ in there,<br />
which means you have two chlorine atoms bonding with it. The formula would be FeCl2. If I said it<br />
was iron (III) chloride, the formula would be FeCl3.<br />
Naming Polyatomic Ionic Compounds<br />
A polyatomic ionic compound is a compound made up of a polyatomic ion, which is two or more<br />
atoms bonded together, and a metal. Naming polyatomic ions is harder, but doable. First, name the<br />
cation, which is just the name of the element. Next, name the anion. This gets trickier.<br />
Notes Section:<br />
33
1<br />
Name of<br />
Cation<br />
Calcium ion<br />
Name of<br />
Anion<br />
Chloride ion<br />
Formula<br />
of Cation<br />
Formula of<br />
Anion<br />
Formula of<br />
Compound<br />
Name of Compound<br />
2<br />
3<br />
Iron(III) ion<br />
Phosphide<br />
ion<br />
Na 1+ S 2-<br />
4<br />
Al 3+ Br 1-<br />
5<br />
Lithium Sulfide<br />
6<br />
Platinum (IV) Oxide<br />
7 Magnesium<br />
ion<br />
8<br />
Carbonate<br />
ion<br />
Ca 2+ NO3 1-<br />
9<br />
HgS<br />
10<br />
Th3(PO4)2<br />
Write the formulas for these ionic compounds<br />
11. Chromium (IV) oxide<br />
12. Chromium (III) oxide<br />
13. Aluminum oxide<br />
14. Nickel (II) bromide<br />
15. Silver (I) sulfide<br />
16. Magnesium chloride<br />
17. Nickel (II) sulfate<br />
18. Iron (III) phosphate<br />
19. Potassium dichromate<br />
20. Lead (IV) hydroxide<br />
34
The Learning Goal for this assignment is:<br />
Introduction to Covalent Bonding:<br />
Bonding between non-metals consists of two electrons shared between two atoms. Using the Wave<br />
Theory, the covalent bond involves an overlap of the electron clouds from each atom. The electrons<br />
are concentrated in the region between the two atoms. In covalent bonding, the two electrons shared<br />
by the atoms are attracted to the nucleus of both atoms. Neither atom completely loses or gains<br />
electrons as in ionic bonding.<br />
There are two types of covalent bonding:<br />
1. Non-polar bonding with an equal sharing of electrons.<br />
The students will learn how molecular bonding is different<br />
than ionic bonding and electrons affect the shape of a<br />
molecule and its properties.<br />
2. Polar bonding with an unequal sharing of electrons. The number of shared electrons depends on<br />
the number of electrons needed to complete the octet.<br />
NON-POLAR BONDING results when two identical non-metals equally share electrons between<br />
them. One well known exception to the identical atom rule is the combination of carbon and hydrogen<br />
in all organic compounds.<br />
Hydrogen<br />
The simplest non-polar covalent molecule is hydrogen. Each hydrogen<br />
atom has one electron and needs two to complete its first energy level.<br />
Since both hydrogen atoms are identical, neither atom will be able to<br />
dominate in the control of the electrons. The electrons are therefore<br />
shared equally. The hydrogen covalent bond can be represented in a<br />
variety of ways as shown here:<br />
The "octet" for hydrogen is only 2 electrons since the nearest rare gas is<br />
He. The diatomic molecule is formed because individual hydrogen atoms<br />
containing only a single electron are unstable. Since both atoms are<br />
identical a complete transfer of electrons as in ionic bonding is<br />
impossible.<br />
Instead the two hydrogen atoms SHARE both electrons equally.<br />
Oxygen<br />
Molecules of oxygen, present in about 20% concentration in air are<br />
also covalent molecules. See the graphic on the left of the Lewis Dot<br />
Structure.<br />
There are 6 electrons in the outer shell, therefore, 2 electrons are<br />
needed to complete the octet. The two oxygen atoms share a total of<br />
four electrons in two separate bonds, called double bonds.<br />
The two oxygen atoms equally share the four electrons.<br />
35
POLAR BONDING results when two different non-metals unequally share electrons between them.<br />
One well known exception to the identical atom rule is the combination of carbon and hydrogen in all<br />
organic compounds.<br />
The non-metal closer to fluorine in the Periodic Table has a greater tendency to keep its own electron<br />
and also draw away the other atom's electron. It is NOT completely successful. As a result, only<br />
partial charges are established. One atom becomes partially positive since it has lost control of its<br />
electron some of the time. The other atom becomes partially negative since it gains electron some of<br />
the time.<br />
Hydrogen Chloride<br />
Hydrogen Chloride forms a polar covalent molecule. The graphic<br />
on the left shows that chlorine has 7 electrons in the outer shell.<br />
Hydrogen has one electron in its outer energy shell. Since 8<br />
electrons are needed for an octet, they share the electrons.<br />
However, chlorine gets an unequal share of the two electrons,<br />
although the electrons are still shared (not transferred as in ionic<br />
bonding), the sharing is unequal. The electrons spends more of the<br />
time closer to chlorine. As a result, the chlorine acquires a "partial"<br />
negative charge. At the same time, since hydrogen loses the<br />
electron most - but not all of the time, it acquires a "partial" charge.<br />
The partial charge is denoted with a small Greek symbol for delta.<br />
Water<br />
Water, the most universal compound on all of the earth, has the property of<br />
being a polar molecule. As a result of this property, the physical and<br />
chemical properties of the compound are fairly unique.<br />
Dihydrogen Oxide or water forms a polar covalent molecule. The graphic on<br />
the left shows that oxygen has 6 electrons in the outer shell. Hydrogen has<br />
one electron in its outer energy shell. Since 8 electrons are needed for an<br />
octet, they share the electrons.<br />
Notes Section:<br />
Bonds between non-metals is dependant on electrons between both of the elements. not all elements will<br />
bond fully like with flourine will take part of others electrons.<br />
36
C 2 H 6 O Ethanol CH 3 CH 2 O<br />
Step 1<br />
Find valence e- for all atoms. Add them together.<br />
C: 4 x 2 = 8<br />
H: 1 x 6 = 6<br />
O: 6<br />
Total = 20<br />
Step 2<br />
Find octet e- for each atom and add them together.<br />
C: 8 x 2 = 16<br />
H: 2 x 6 = 12<br />
O: 8<br />
Total = 36<br />
Step 3<br />
Subtract Step 1 total from Step 2.<br />
Gives you bonding e-.<br />
36 – 20 = 16e-<br />
Step 4<br />
Find number of bonds by diving the number in step 3 by 2<br />
(because each bond is made of 2 e-)<br />
16e- / 2 = 8 bond pairs<br />
These can be single, double or triple bonds.<br />
Step 5<br />
Determine which is the central atom<br />
Find the one that is the least electronegative.<br />
Use the periodic table and find the one farthest<br />
away from Fluorine or<br />
The one that only has 1 atom.<br />
37
Step 6<br />
Put the atoms in the structure that you think it will<br />
have and bond them together.<br />
Put Single bonds between atoms.<br />
Step 7<br />
Find the number of nonbonding (lone pairs) e-.<br />
Subtract step 3 number from step 1.<br />
20 – 16 = 4e- = 2 lone pairs<br />
Step 8<br />
Complete the Octet Rule by adding the lone<br />
pairs.<br />
Add any left over bonds to make double or triple<br />
bonds.<br />
Then, if needed, use any lone pairs to make<br />
double or triple bonds so that all atoms meet<br />
the Octet Rule.<br />
See Step 4 for total number of bonds.<br />
Step 9<br />
Find the formal charges for the atoms in the compound.<br />
Arrange atoms so that all formal charges<br />
are as close to 0 as possible.<br />
Some central atoms do not meet the octet rule.<br />
Boron can sometimes have only 6 electrons and<br />
some elements in Periods 3—7 may exceed the<br />
octet rule.<br />
38
Name Formula Charge<br />
Dichromate Cr₂O₇ 2-<br />
Sulfate SO₄ 2-<br />
Hydrogen Carbonate HCO₃ 1-<br />
Hypochlorite ClO 1-<br />
Phosphate PO₄ 3-<br />
Nitrite NO₂ 1-<br />
Chlorite ClO₂ 1-<br />
Dihydrogen phosphate H₂PO₄ 1-<br />
Chromate CrO₄ 2-<br />
Carbonate CO₃ 2-<br />
Hydroxide OH 1-<br />
Hydrogen phosphate HPO₄ 2-<br />
Ammonium NH₄ 1+<br />
Acetate C₂H₃O₂ 1-<br />
Perchlorate ClO₄ 1-<br />
Permanganate MnO₄ 1-<br />
Chlorate ClO₃ 1-<br />
Hydrogen Sulfate HSO₄ 1-<br />
Phosphite PO₃ 3-<br />
Sulfite SO₃ 2-<br />
Silicate SiO₃ 2-<br />
Nitrate NO₃ 1-<br />
Hydrogen Sulfite HSO₃ 1-<br />
Oxalate C₂O₄ 2-<br />
Cyanide CN 1-<br />
Hydronium H₃O 1+<br />
Thiosulfate S₂O₃ 2-<br />
39
Orbitals Equation Lone Pairs Angle<br />
Name<br />
sp AX2 NONE 180<br />
LINEAR<br />
SP2 AX3 NONE 120 TRILINEAR PLANER<br />
SP2 AX2E 1 116<br />
BENT<br />
SP3 AX4 NONE 109.5<br />
TETRAHEDREAL<br />
SP3 AX3E 1 107<br />
TRIG PER<br />
SP3 AX2E2 2 104.5<br />
BENT<br />
SP3D AX5 NONE 100/90<br />
TRIG BI-PER<br />
SP3D AX3E2 2 90<br />
T-SHAPED<br />
SP3D2 AX6 NONE 90<br />
OCTOHEDREAL<br />
SP3D2 AX4E2 2 90<br />
SQUARE PLANAR<br />
40
Name<br />
Date<br />
Example:<br />
Xenon Hexafluoride<br />
Chemical Name<br />
XeF 6<br />
Chemical Formula<br />
Octahedral<br />
MUSTANG LABORATORIES<br />
EXPERIMENT 6 – Molecular Geometry<br />
In this lab you will use the website https://phet.colorado.edu/sims/html/moleculeshapes/latest/molecule-shapes_en.html.<br />
You will construct the following 10 molecular<br />
compounds using the rules of VSEPR. Use the snip it tool to get pictures of the model<br />
from the side and from the top showing its form. Place these pictures in the appropriate<br />
boxes. You can either do the diagram by hand and take a picture or you can construct<br />
it in this program. Then you will fill in the rest of the form with the relevant information.<br />
I have given you an example.<br />
H 3 O + O 3 SF 6<br />
PF 5 CO 2 OF 2<br />
COCl 2 XeF 4 ClF 3<br />
POF 3<br />
When you have finished save this as a pdf and put it in the drop box in Angel.<br />
sp 3 d 2<br />
Molecular Geometry<br />
None<br />
Side View<br />
Orbitals<br />
Lone Pairs<br />
Molecular Drawing<br />
With Angles<br />
Top View<br />
41
Carbon Dioxide<br />
Chemical Name<br />
CO 2<br />
Chemical Formula<br />
Linear<br />
sp<br />
Molecular Geometry<br />
none<br />
Side View<br />
Orbitals<br />
Lone Pairs<br />
Molecular Drawing<br />
With Angles<br />
Top View<br />
Phosgene<br />
Chemical Name<br />
COCL 2<br />
Chemical Formula<br />
Trigonal Planar<br />
sp 3<br />
Molecular Geometry<br />
none<br />
Side View<br />
Orbitals<br />
Lone Pairs<br />
Molecular Drawing<br />
With Angles<br />
Top View<br />
42
Oxygen difluoride<br />
Chemical Name<br />
OF 2<br />
Chemical Formula<br />
Bent<br />
Molecular Geometry<br />
sp 2<br />
Side View<br />
Orbitals<br />
Lone Pairs<br />
Molecular Drawing<br />
With Angles<br />
Top View<br />
Phosphoryl fluoride<br />
Chemical Name<br />
POF 3<br />
Chemical Formula<br />
Tetrahedral<br />
sp 3<br />
Molecular Geometry<br />
None<br />
Side View<br />
Orbitals<br />
Lone Pairs<br />
Molecular Drawing<br />
With Angles<br />
Top View<br />
43
Hydronium<br />
Chemical Name<br />
H 3 O +<br />
Chemical Formula<br />
Trigonal Pyramidal<br />
Molecular Geometry<br />
Sp 2 1<br />
Side View<br />
Orbitals<br />
Lone Pairs<br />
Molecular Drawing<br />
With Angles<br />
Top View<br />
Trioxide<br />
Chemical Name<br />
O 3<br />
Chemical Formula<br />
Bent<br />
Molecular Geometry<br />
sp 1<br />
Side View<br />
Orbitals<br />
Lone Pairs<br />
Molecular Drawing<br />
With Angles<br />
Top View<br />
44
PhosphorousPentaFlouride<br />
Chemical Name<br />
PF 5<br />
Chemical Formula<br />
Trigonal Bipyramidal<br />
sp 3 d<br />
Molecular Geometry<br />
none<br />
Side View<br />
Orbitals<br />
Lone Pairs<br />
Molecular Drawing<br />
With Angles<br />
Top View<br />
Chemical Name<br />
ClF 3<br />
Chemical Formula<br />
T-Shaped<br />
Molecular Geometry<br />
sp2 22<br />
Side View<br />
Orbitals<br />
Lone Pairs<br />
Molecular Drawing<br />
With Angles<br />
Top View<br />
45
Chemical Name<br />
SF 6<br />
Chemical Formula<br />
Octahedral<br />
Molecular Geometry<br />
Side View<br />
Orbitals<br />
Lone Pairs<br />
Molecular Drawing<br />
With Angles<br />
Top View<br />
Xenon TretraFlouride<br />
Chemical Name<br />
XeF4<br />
Chemical Formula<br />
Square Planar<br />
Molecular Geometry<br />
sp 3 2<br />
Side View<br />
Orbitals<br />
Lone Pairs<br />
Molecular Drawing<br />
With Angles<br />
Top View<br />
46
Finding Bond Angles, Shapes, and Hybridizations<br />
Sometimes people have a hard time with the whole VSEPR thing. In this helpdesk section we'll<br />
discuss what VSEPR means, what it's all about, and how you can use a great big flow chart to figure<br />
out the bond angles, shapes, and hybridizations of various covalent compounds.<br />
This whole thing assumes, by the way, that you know how to draw Lewis structures. If you don't, click<br />
here.<br />
What is VSEPR?<br />
VSEPR stands for Valence Shell Electron Pair Repulsion. It's a complicated acronym, but it means<br />
something that's not difficult to understand. Basically, the idea is that covalent bonds and lone pair<br />
electrons like to stay as far apart from each other as possible under all conditions. This is because<br />
covalent bonds consist of electrons, and electrons don't like to hang around next to each other much<br />
because they have the same charge.<br />
This VSEPR thing explains why molecules have their shapes. If carbon has four atoms stuck to it (as<br />
in methane), these four atoms want to get as far away from each other as they can. This isn't<br />
because the atoms necessarily hate each other, it's because the electrons in the bonds hate each<br />
other. That's the idea behind VSEPR.<br />
What is hybridization (AP Chemistry)?<br />
Now, one problem with the whole VSEPR thing is that if you have four things stuck to carbon, for<br />
example, there are no orbitals that want to get 109.5 degrees apart from each other (109.5 degrees<br />
corresponds to the geometric maximum distance the atoms can get apart). After all, s-orbitals go in a<br />
complete sphere (360 degrees) and p-orbitals are 90 degrees apart.<br />
What happens instead of using s- or p- orbitals is that when covalent bonds are formed, the s- and p-<br />
orbitals mix to form something called hybrid orbitals. "Hybrid" just means "mixture of two different<br />
things", and that's exactly what a hybrid orbital is. When three p-orbitals with 90 degree angles<br />
combine with one s-orbital with 360 degrees, they average to form four sp3 orbitals with 109.5 degree<br />
bond angles. Depending on the numbers of s- and p-orbitals that mix, you can get a bunch of<br />
different bond angles.<br />
Common shapes you should know<br />
There are a whole bunch of common shapes you need to know to accurately think of covalent<br />
molecules. Here they are:<br />
Tetrahedral: Tetrahedral molecules look like pyramids with four faces. Each point on the pyramid<br />
corresponds to an atom that's attached to the central atom. Bond angles are 109.5 degrees.<br />
Trigonal pyramidal: It's like a tetrahedral molecule, except flatter. It looks kind of like a squished<br />
pyramid because one of the atoms in the pyramid is replaced with a lone pair. Bond angles are 107.5<br />
degrees (it's less than tetrahedral molecules because the lone pair shoves the other atoms closer to<br />
each other).<br />
Trigonal planar: It looks like the hood ornament of a Mercedes automobile, or like a peace sign with<br />
that bottom-most line gone. The bond angles are 120 degrees.<br />
47
Bent: They look, well, bent. Bond angles can be either 116 degrees for molecules with one lone pair<br />
or 104.5 degrees for molecules with two lone pairs.<br />
Linear: The atoms in the molecule are in a straight line. This can be either because there are only<br />
two atoms in the molecule (in which case there is no bond angle, as there need to be three atoms to<br />
get a bond angle) or because the three atoms are lined up in a straight line (corresponding to a 180<br />
degree bond angle).<br />
There are other types, but we won't worry about them until college or AP Chemistry.<br />
Using a flow chart to figure out what shape, and bond angle an atom has<br />
Take a look at this flow chart. I'll explain how to use it to find all the stuff above at the end.<br />
Complicated, huh? Here's how to use it:<br />
1. Draw the Lewis structure for the molecule. This vital if you're going to get the answer right.<br />
2. Count the number of "things" on the atom you're interested in. Let's say that you're looking at<br />
methane, CH4. If you want to find the bond angles, shape, and hybridization for carbon, count<br />
the number of things that are stuck to it.<br />
Now, the vague term "things" refers to atoms and lone pairs. IT DOES NOT REFER TO THE<br />
NUMBER OF BONDS! When you look at methane, there are four atoms stuck to it, so you'd go down<br />
the line that says "four" toward the green boxes on this chart.<br />
People get confused with multiple bonds. Take carbon dioxide, for example. There are four bonds<br />
(carbon is double-bonded to each oxygen) but only two oxygen atoms bonded to carbon. In this<br />
48
case, we count two things stuck to carbon, because we only count the atoms, NOT the number of<br />
bonds.<br />
Likewise, with ammonia there are four things. Three of the things on nitrogen are hydrogen atoms<br />
and the fourth is a lone pair. For the purposes of VSEPR, lone pairs count exactly the same as<br />
atoms, because they consist of negative charge, too.<br />
3. Count the number of lone pairs that are on the atom you're interested in. IMPORTANT: This<br />
does NOT mean to count the number of lone pairs on all of the atoms in the molecule. Lone<br />
pairs on other atoms aren't important - what's important is only what's directly stuck to the atom<br />
you're interested in.<br />
We mentioned above that methane has four things stuck to it. Since all four things are hydrogen<br />
atoms, we moved toward the green boxes on the flow chart. When we get to our second question,<br />
we find that there are no lone pairs on carbon, so our answer is zero. When we go down the line that<br />
says "zero" from that box, we find that methane is sp3 hybridized, with a 109.5 degree bond angle<br />
and tetrahedral shape.<br />
And, hey, that's what we were looking for!<br />
Some sample problems:<br />
What are the shapes, bond angles, and hybridizations of the following molecules? Use the flow chart<br />
and instructions above to figure it out.<br />
1. carbon tetrabromide<br />
2. phosphorus trichloride<br />
3. oxygen
4. the chlorine atom in hydrochloric acid (HCl)<br />
5. boron trichloride<br />
6. CH2O<br />
7. sulfur difluoride<br />
8. either carbon atom in C2H2<br />
1. sp 3 , tetrahedral, 109.5 degrees.<br />
2. sp 3 , trigonal pyramidal, 107.5 degrees.<br />
3. sp 2 , linear, no bond angle<br />
4. sp 3 , linear, no bond angle<br />
5. sp 2 , trigonal planar, 120 degrees<br />
6. sp 2 , trigonal planar, 120 degrees<br />
7. sp 3 , bent, 104.5 degrees<br />
8. sp, linear, 180 degrees<br />
50
Unit 4<br />
Chapter 22 Hydrocarbon Compounds<br />
The student will learn how Hydrocarbons are named and the<br />
general properties of Hydrocarbons.<br />
Describe how different natural resources are produced and how their rates<br />
of use and renewal limit availability.<br />
Students will explore local, national, and global renewable and nonrenewable<br />
resources.<br />
Students will explain the environmental costs of the use of renewable and<br />
nonrenewable resources.<br />
Students will explain the benefits of renewable and nonrenewable resources.<br />
Nuclear reactors<br />
Natural gas<br />
Petroleum<br />
Refining<br />
Coal<br />
51
Chapter 23 Functional Groups<br />
The student will learn what effects functional groups have on<br />
organic compounds and how chemical reactions are used in<br />
organic compounds.<br />
Describe the properties of the carbon atom that make the diversity of carbon<br />
compounds possible.<br />
Identify selected functional groups and relate how they contribute to<br />
properties of carbon compounds.<br />
Students will identify examples of important carbon based molecules.<br />
Students will create 2D or 3D models of carbon molecules and explain why this<br />
molecule is important to life.<br />
covalent bond<br />
single bond<br />
double bond<br />
triple bond<br />
monomer<br />
polymer<br />
52
https://www.bbc.co.uk/education/guides/zvvwxnb/revision<br />
53
54
Introduction to Organic Chemistry<br />
To understand life as we know it, we must first understand a little bit of organic chemistry. Organic<br />
molecules contain both carbon and hydrogen. Though many organic chemicals also contain other<br />
elements, it is the carbon-hydrogen bond that defines them as organic. Organic chemistry defines life.<br />
Just as there are millions of different types of living organisms on this planet, there are millions of<br />
different organic molecules, each with different chemical and physical properties. There are organic<br />
chemicals that make up your hair, your skin, your fingernails, and so on. The diversity of organic<br />
chemicals is due to the versatility of the carbon atom. Why is carbon such a special element? Let's<br />
look at its chemistry in a little more detail.<br />
The uniqueness of carbon<br />
Carbon (C) appears in the second row of the periodic table and has four bonding electrons in its<br />
valence shell (see our Periodic Table module for more information). Similar to other non-metals,<br />
carbon needs eight electrons to satisfy its valence shell. Carbon therefore forms four bonds with other<br />
atoms (each bond consisting of one of carbon's electrons and one of the bonding atom's electrons).<br />
Every valence electron participates in bonding; thus, a carbon atom's bonds will be distributed evenly<br />
over the atom's surface. These bonds form a tetrahedron (a pyramid with a spike at the top), as<br />
illustrated below:<br />
Carbon forms 4 bonds<br />
Organic chemicals get their diversity from the many different ways carbon can bond to other atoms.<br />
The simplest organic chemicals, called hydrocarbons, contain only carbon and hydrogen atoms; the<br />
simplest hydrocarbon (called methane) contains a single carbon atom bonded to four hydrogen<br />
atoms:<br />
Methane - a carbon atom bonded to 4 hydrogen atoms<br />
But carbon can bond to other carbon atoms in addition to hydrogen, as illustrated in the molecule<br />
ethane below:<br />
Ethane - a carbon-carbon bond<br />
In fact, the uniqueness of carbon comes from the fact that it can bond to itself in many different ways.<br />
Carbon atoms can form long chains:<br />
55
anched chains:<br />
Hexane - a 6-carbon chain<br />
rings:<br />
Isohexane - a branched-carbon chain<br />
Cyclohexane - a ringed hydrocarbon<br />
There appears to be almost no limit to the number of different structures that carbon can form. To add to the<br />
complexity of organic chemistry, neighboring carbon atoms can form double and triple bonds in addition to<br />
single carbon-carbon bonds:<br />
Single bonding Double bonding Triple bonding<br />
Keep in mind that each carbon atom forms four bonds. As the number of bonds between any two<br />
carbon atoms increases, the number of hydrogen atoms in the molecule decreases (as can be seen<br />
in the figures above).<br />
Simple hydrocarbons<br />
The simplest hydrocarbons are those that contain only carbon and hydrogen. These simple<br />
hydrocarbons come in three varieties depending on the type of carbon-carbon bonds that occur in the<br />
molecule.<br />
Alkanes<br />
Alkanes are the first class of simple hydrocarbons and contain only carbon-carbon single bonds. The<br />
alkanes are named by combining a prefix that describes the number of carbon atoms in the molecule<br />
with the root ending "ane". The names and prefixes for the first ten alkanes are given in the following<br />
table.<br />
56
Carbon Prefix Alkane Name Chemical Structural Formula<br />
atoms<br />
Formula<br />
1 Meth Methane CH4 CH4<br />
2 Eth Ethane C2H6 CH3CH3<br />
3 Prop Propane C3H8 CH3CH2CH3<br />
4 But Butane C4H10 CH3CH2CH2CH3<br />
5 Pent Pentane C5H12 CH3CH2CH2CH2CH3<br />
6 Hex Hexane C6H14 …<br />
7 Hept Heptane C7H16<br />
8 Oct Octane C8H18<br />
9 Non Nonane C9H20<br />
10 Dec Decane C10H22<br />
The chemical formula for any alkane is given by the expression CnH2n+2. The structural formula,<br />
shown for the first five alkanes in the table, shows each carbon atom and the elements that are<br />
attached to it. This structural formula is important when we begin to discuss more complex<br />
hydrocarbons. The simple alkanes share many properties in common. All enter into combustion<br />
reactions with oxygen to produce carbon dioxide and water vapor. In other words, many alkanes are<br />
flammable. This makes them good fuels. For example, methane is the main component of natural<br />
gas, and butane is common lighter fluid.<br />
CH4 + 2O2 → CO2 + H2O<br />
The chemical reaction between a fuel (for example wood) and an oxidation agent.<br />
Alkenes<br />
The second class of simple hydrocarbons, the alkenes, consists of molecules that contain at least<br />
one double-bonded carbon pair. Alkenes follow the same naming convention used for alkanes. A<br />
prefix (to describe the number of carbon atoms) is combined with the ending "ene" to denote an<br />
alkene. Ethene, for example is the two-carbon molecule that contains one double bond. The chemical<br />
formula for the simple alkenes follows the expression CnH2n. Because one of the carbon pairs is<br />
double bonded, simple alkenes have two fewer hydrogen atoms than alkanes.<br />
Ethene<br />
Alkynes<br />
Alkynes are the third class of simple hydrocarbons and are molecules that contain at least one triplebonded<br />
carbon pair. Like the alkanes and alkenes, alkynes are named by combining a prefix with the<br />
ending "yne" to denote the triple bond. The chemical formula for the simple alkynes follows the<br />
expression CnH2n-2.<br />
Ethyne<br />
57
Isomers<br />
Because carbon can bond in so many different ways, a single molecule can have different bonding<br />
configurations. Consider the two molecules illustrated here:<br />
C6H14<br />
CH3CH2CH2CH2CH2CH3<br />
C6H14<br />
CH3<br />
I<br />
CH2CH2CH2CH2CH3<br />
Both molecules have identical chemical formulas (shown in the left column); however, their structural<br />
formulas (and thus some chemical properties) are different. These two molecules are called isomers.<br />
Isomers are molecules that have the same chemical formula but different structural formulas.<br />
Functional groups<br />
In addition to carbon and hydrogen, hydrocarbons can also contain other elements. In fact, many<br />
common groups of atoms can occur within organic molecules, these groups of atoms are called<br />
functional groups. One good example is the hydroxyl functional group. The hydroxyl group consists of<br />
a single oxygen atom bound to a single hydrogen atom (OH - ). The group of hydrocarbons that contain<br />
a hydroxyl functional group is called alcohols. The alcohols are named in a similar fashion to the<br />
simple hydrocarbons, a prefix is attached to a root ending (in this case "anol") that designates the<br />
alcohol. The existence of the functional group completely changes the chemical properties of the<br />
molecule. Ethane, the two-carbon alkane, is a gas at room temperature; ethanol, the two-carbon<br />
alcohol, is a liquid.<br />
Ethanol<br />
Ethanol, common drinking alcohol, is the active ingredient in "alcoholic" beverages such as beer and<br />
wine.<br />
Summary<br />
The chemical basis of all living organisms is linked to the way that carbon bonds with other atoms.<br />
This introduction to organic chemistry explains the many ways that carbon and hydrogen form bonds.<br />
Basic hydrocarbon nomenclature is described, including alkanes, alkenes, alkynes, and isomers.<br />
Functional groups of atoms within organic molecules are discussed.<br />
58
Unit 5<br />
Chapter 10 Chemical Quantities<br />
The student will learn why the mole is important and how the<br />
molecular formula of a compound can be determined<br />
experimentally.<br />
Chapter 11 Chemical Reactions<br />
The students will learn how chemical reactions obey the law of<br />
conservation of mass and how they can predict the products<br />
of a chemical reaction.<br />
Characterize types of chemical reactions, for example: redox, acid-base,<br />
synthesis, and single and double replacement reactions.<br />
Students will be able to identify the type of chemical reaction that occurs.<br />
Students will be able to compare/contrast reactants and products of various<br />
types of chemical reactions.<br />
Students will be able to predict the product of various reactants.<br />
Students will be able to write balanced chemical equations for each type of<br />
reaction.<br />
Decomposition<br />
Combustion<br />
Redox<br />
Acid-Base<br />
Synthesis<br />
single-replacement<br />
double-replacement<br />
Differentiate between chemical and nuclear reactions.<br />
Students will compare/contrast chemical and nuclear reactions.<br />
fission<br />
fusion<br />
59
Chapter 12 Stoichiometry<br />
The students will learn how balanced chemical equations are<br />
used in stoichiometric calculations and how to calculate<br />
amounts of reactants and products in a chemical equation.<br />
Apply the mole concept and the law of conservation of mass to calculate<br />
quantities of chemicals participating in reactions.<br />
Students will be able to use a balanced equation to determine mole ratios.<br />
Students will be able to apply law of conservation of mass to chemical equations.<br />
Students will be able to calculate empirical and molecular formulas.<br />
Students will be able to calculate the % composition of a compound.<br />
Students will be able to calculate theoretical yield.<br />
Students will be able to calculate % error.<br />
Students will be able to calculate molar mass.<br />
Students will be able to perform stoichiometric calculations, including limiting<br />
reagents.<br />
mole<br />
Avogadro’ s number<br />
molar mass<br />
gram formula mass<br />
60
Learning Goal for this section:<br />
al equations are used in stoichiometric calculations and how to calculate amounts<br />
hemical equation<br />
The Mole: Its History and Use<br />
Simply put, the mole represents a number. Just as the term dozen refers to the number twelve, the<br />
mole represents the number 6.02 x 10 23 . (If you're confused by the form of this number refer to our<br />
The Metric System module).<br />
Now that's a big number! While a dozen eggs will make a nice omelet, a mole of eggs will fill all of the<br />
oceans on earth more than 30 million times over. Think about it: It would take 10 billion chickens<br />
laying 10 eggs per day more than 10 billion years to lay a mole of eggs. So why would we ever use<br />
such a big number? Certainly the local donut store is not going to "supersize" your dozen by giving<br />
you a mole of jelly-filled treats.<br />
The mole is used when we're talking about numbers of atoms and molecules. Atoms and molecules<br />
are very tiny things. A drop of water the size of the period at the end of this sentence would contain<br />
10 trillion water molecules. Instead of talking about trillions and quadrillions of molecules (and more),<br />
it's much simpler to use the mole.<br />
History of the mole<br />
The number of objects in one mole, that is, 6.02 x 10 23 , is commonly referred to as Avogadro's<br />
number. Amedeo Avogadro was an Italian physics professor who proposed in 1811 that equal<br />
volumes of different gases at the same temperature contain equal numbers of molecules. About fifty<br />
years later, an Italian scientist named Stanislao Cannizzaro used Avogadro's hypothesis to develop a<br />
set of atomic weights for the known elements by comparing the masses of equal volumes of gas.<br />
Building on this work, an Austrian high school teacher named Johann Josef Loschmidt calculated the<br />
size of a molecule of air in 1865, and thus developed an estimate for the number of molecules in a<br />
given volume of air. While these early estimates have since been refined, they led to the concept of<br />
the mole - that is, the theory that in a defined mass of an element (its atomic weight) there is a<br />
precise number of atoms: Avogadro's number.<br />
Molar mass<br />
A sample of any element with a mass equal to that element's atomic weight (in grams) will contain<br />
precisely one mole of atoms (6.02 x 10 23 atoms). For example, helium has an atomic weight of 4.00<br />
amu. Therefore, 4.00 grams of helium will contain one mole of helium atoms. You can also work with<br />
fractions (or multiples) of moles:<br />
Mole/weight relationship<br />
examples using helium<br />
moles<br />
helium<br />
# helium<br />
atoms<br />
grams<br />
helium<br />
¼ 1.505 X 10 23 1g<br />
½ 3.01 X 10 23 2g<br />
1 6.02 X 10 23 4g<br />
2 1.204 X 10 24 8g<br />
10 6.02 X 10 24 40g<br />
61
Other atomic weights are listed on the periodic table (see our Periodic Table module). For each<br />
element listed, measuring out a quantity of the element equal to its atomic weight in grams will yield<br />
6.02 x 10 23 atoms of that element.<br />
The atomic weight of an element identifies both the mass of one mole of that element and the total<br />
number of protons and neutrons in an atom of that element. How can that be? Let's look at hydrogen.<br />
One mole of hydrogen atoms will weigh 1.01 grams.<br />
A hydrogen atom<br />
with its single electron<br />
Each hydrogen atom consists of one proton surrounded by one electron. But remember, the electron<br />
weighs so little that it does not contribute much to an atom's weight. Ignoring the weight of hydrogen's<br />
electrons, we can say that one mole of protons (H nuclei) weighs approximately one gram. Since<br />
protons and neutrons have about the same mass, a mole of either of these particles will weigh about<br />
one gram. For example, in one mole of helium, there are two moles of protons and two moles of<br />
neutrons - four grams of particles.<br />
Molecular weight<br />
If you stand on a scale with a friend, the scale will register the combined weight of both you and your<br />
friend. When atoms form molecules, the atoms bond together, and the molecule's weight is the<br />
combined weight of all of its parts.<br />
For example, every water molecule (H2O) has two atoms of hydrogen and one atom of oxygen. One<br />
mole of water molecules will contain two moles of hydrogen and one mole of oxygen.<br />
2 moles<br />
hydrogen<br />
Mole/weight relationships<br />
of water and its parts<br />
1 mole<br />
oxygen<br />
1 mole<br />
water<br />
+ =<br />
A bottle filled with exactly 18.02 g water will contain 6.02 x 10 23 water molecules. The concept of<br />
fractions and multiples described above also applies to molecules: 9.01 g of water would contain 1/2<br />
mole, or 3.01 x 10 23 molecules. You can calculate the molecular weight of any compound simply by<br />
summing the weights of atoms that make up that compound.<br />
62
Example: Converting between mass and moles<br />
Knowing a substance’s molar mass is useful, because the molar mass acts as a conversion factor<br />
between the mass of a sample and the number of moles in that sample (Equation 1). For converting<br />
between the number of moles in a sample and the number of molecules in the sample, Avogadro’s<br />
number acts as the conversion factor, as shown in Equation 2 below.<br />
Equation 1<br />
Sample mass (g) = Moles in sample (mol)<br />
Sample's molar mass (g/mol)<br />
Equation 2<br />
Moles in sample (mol) x Avogadro's number (number/mol) = Number of sample molecules<br />
To understand how molar mass and Avogadro’s number act as conversion factors, we can turn to an<br />
example using a popular drink: How many CO2 molecules are in a standard bottle of carbonated<br />
soda?<br />
Thanks to molar mass and Avogadro’s number, figuring this out doesn’t require counting each<br />
individual CO2 molecule! Instead, we can start by determining the mass of CO2 in this sample. In an<br />
experiment, a scientist compared the mass of a standard 16-ounce (454 milliliters) bottle of soda<br />
before it was opened, and then after it had been shaken and left open so that the CO2 fizzed out of<br />
the liquid. The difference between the masses was 2.2 grams—the sample mass of CO2 (for this<br />
example, we’re going to assume that all the CO2 has fizzled out). Before we can calculate the number<br />
of CO2 molecules in 2.2 grams, we first have to calculate the number of moles in 2.2 grams of CO2<br />
using molar mass as the conversion factor (see Equation 1 above):<br />
Equation 3<br />
Now that we’ve figured out that there are 0.050 moles in 2.2 grams of CO2, we can use Avogadro’s<br />
number to calculate the number of CO2 molecules (see Equation 2 above):<br />
Equation 4<br />
While scientists today commonly use the concept of the mole to interconvert number of particles and<br />
mass of elements and compounds, the concept started with 19th-century chemists who were puzzling<br />
out the nature of atoms, gas particles, and those particles’ relationship with gas volume.<br />
63
Find Molecular Mass 1- 10<br />
1. NaBr<br />
94g<br />
2. PbSO4<br />
303g<br />
3. Zn(C2H3O2)2<br />
183g<br />
4. Na3PO4<br />
141g<br />
5. (NH4)2CO3<br />
86g<br />
6. Glucose<br />
7. Iron(II) Phosphate<br />
8. Ammonium Sulfide<br />
9. Calcium Hydroxide<br />
10. Silver(I) Fluoride<br />
11. How many moles is 51g of NaBr?<br />
.54 mol<br />
12. How many moles is 60g of PbSO4?<br />
.20mol<br />
13. How many moles is 150g of Zn(C2H3O2)2?<br />
.82mol<br />
14. How many moles is 578g of Na3PO4?<br />
4.1mol<br />
15. How many moles is 500g of (NH4)2CO3?<br />
5.8mol<br />
1. 103 g/mol 6. 180 g/mol 11. .50 mol<br />
2. 303 g/mol 7. 358 g/mol 12. .20 mol<br />
3. 74 g/mol 8. 68 g/mol 13. 2.0 mol<br />
4. 164 g/mol 9. 183 g/mol 14. 3.5 mol<br />
5. 96 g/mol 10. 127 g/mol 15. 5.2 mol<br />
64
The Learning Goal for this assignment is:<br />
How to Balance Chemical Equations<br />
A chemical equation is a theoretical or written representation of what happens during a chemical<br />
reaction. The law of conservation of mass states that no atoms can be created or destroyed in a<br />
chemical reaction, so the number of atoms that are present in the reactants has to balance the<br />
number of atoms that are present in the products. Follow this guide to learn how to balance chemical<br />
equations.<br />
Step 1<br />
Write down your given equation. For this example, we will use:<br />
C3H8 + O2 --> H2O + CO2<br />
Step 2<br />
Write down the number of atoms that you have on each side of the equation. Look at the subscripts<br />
next to each atom to find the number of atoms in the equation.<br />
Left side: 3 carbon, 8 hydrogen and 2 oxygen<br />
Right side: 1 carbon, 2 hydrogen and 3 oxygen<br />
65
Step 3<br />
Always leave hydrogen and oxygen for last. This means that you will need to balance the carbon<br />
atoms first.<br />
Step 4<br />
66<br />
Add a coefficient to the single carbon atom on the right of the equation to balance it with the 3 carbon<br />
atoms on the left of the equation.<br />
C3H8 + O2 --> H2O + 3CO2<br />
The coefficient 3 in front of carbon on the right side indicates 3 carbon atoms just as the subscript 3<br />
on the left side indicates 3 carbon atoms.<br />
In a chemical equation, you can change coefficients, but you should never alter the subscripts.
Step 5<br />
Balance the hydrogen atoms next. You have 8 on the left side, so you'll need 8 on the right side.<br />
C3H8 + O2 --> 4H2O + 3CO2<br />
On the right side, we added a 4 as the coefficient because the subscript showed that we already<br />
had 2 hydrogen atoms.<br />
When you multiply the coefficient 4 times the subscript 2, you end up with 8.<br />
Step 6<br />
Finish by balancing the oxygen atoms.<br />
Because we've added coefficients to the molecules on the right side of the equation, the number of<br />
oxygen atoms has changed. We now have 4 oxygen atoms in the water molecule and 6 oxygen<br />
atoms in the carbon dioxide molecule. That makes a total of 10 oxygen atoms.<br />
Add a coefficient of 5 to the oxygen molecule on the left side of the equation. You now have 10<br />
oxygen molecules on each side.<br />
C3H8 + 5O2 --> 4H2O + 3CO2.<br />
The carbon, hydrogen and oxygen atoms are balanced. Your equation is complete.<br />
67
1) ___ NaNO3 + ___ PbO ___ Pb(NO3)2 + ___ Na2O<br />
2) ___ AgI + ___ Fe2(CO3)3 ___ FeI3 + ___ Ag2CO3<br />
3) ___ C2H4O2 + ___ O2 ___ CO2 + ___ H2O<br />
4) ___ ZnSO4 + ___ Li2CO3 ___ ZnCO3 + ___ Li2SO4<br />
5) ___ V2O5 + ___ CaS ___ CaO + ___ V2S5<br />
68
6) ___ Mn(NO2)2 + ___ BeCl2 ___ Be(NO2)2 + ___ MnCl2<br />
7) ___ AgBr + ___ GaPO4 ___ Ag3PO4 + ___ GaBr3<br />
8) ___ H2SO4 + ___ B(OH)3 __ B2(SO4)3 + ___ H2O<br />
9) ___ S8 + ___ O2 ___ SO2<br />
10) ___ Fe + ___ AgNO3 ___ Fe(NO3)2 + ___ Ag<br />
69
1) 2 NaNO3 + PbO Pb(NO3)2 + Na2O<br />
2) 6 AgI + Fe2(CO3)3 2 FeI3 + 3 Ag2CO3<br />
3) C2H4O2 + 2 O2 2 CO2 + 2 H2O<br />
4) ZnSO4 + Li2CO3 ZnCO3 + Li2SO4<br />
5) V2O5 + 5 CaS 5 CaO + V2S5<br />
6) Mn(NO2)2 + BeCl2 Be(NO2)2 + MnCl2<br />
7) 3 AgBr + GaPO4 Ag3PO4 + GaBr3<br />
8) 3 H2SO4 + 2 B(OH)3 B2(SO4)3 + 6 H2O<br />
9) S8 + 8 O2 8 SO2<br />
10) Fe + 2 AgNO3 Fe(NO3)2 + 2 Ag<br />
Additional Notes:<br />
70
The Learning Goal for this assignment is:<br />
we will learn how chemical reactions obey the law of conservation of mass and how<br />
we can predict the products of a chemical reaction<br />
Categories of Reactions<br />
All chemical reactions can be placed into one of six categories. Here they are, in no<br />
particular order:<br />
1) Synthesis: A synthesis reaction is when two or more simple compounds combine to form a<br />
more complicated one. These reactions come in the general form of:<br />
One example of a synthesis reaction is the combination of iron and sulfur to form iron (II) sulfide:<br />
8 Fe + S8 ---> 8 FeS<br />
If two elements or very simple molecules combine with each other, it’s probably a synthesis reaction.<br />
The products will probably be predictable using the octet rule to find charges.<br />
2) Decomposition: A decomposition reaction is the opposite of a synthesis reaction - a<br />
complex molecule breaks down to make simpler ones. These reactions come in the general form:<br />
One example of a decomposition reaction is the electrolysis of water to make oxygen and hydrogen<br />
gas:<br />
2 H2O ---> 2 H2 + O2<br />
If one compound has an arrow coming off of it, it’s probably a decomposition reaction. The products<br />
will either be a couple of very simple molecules, or some elements, or both.<br />
3) Single displacement: This is when one element trades places with another element in a<br />
compound. These reactions come in the general form of:<br />
A+BC->AC+B or BA+C<br />
One example of a single displacement reaction is when magnesium replaces hydrogen in water to<br />
make magnesium hydroxide and hydrogen gas:<br />
Mg + 2 H2O ---> Mg(OH)2 + H2<br />
If a pure element reacts with another compound (usually, but not always, ionic), it’s probably a single<br />
displacement reaction. The products will be the compounds formed when the pure element switches<br />
places with another element in the other compound.<br />
Important note: these reactions will only occur if the pure element on the reactant side of the equation<br />
is higher on the activity series than the element it replaces.<br />
71
4) Double displacement: This is when the anions and cations of two different molecules<br />
switch places, forming two entirely different compounds. These reactions are in the general form:<br />
AB+CD->AD+CB<br />
One example of a double displacement reaction is the reaction of lead (II) nitrate with potassium<br />
iodide to form lead (II) iodide and potassium nitrate:<br />
Pb(NO3)2 + 2 KI ---> PbI2 + 2 KNO3<br />
If two ionic compounds combine, it’s probably a double displacement reaction. Switch the cations<br />
and balance out the charges to figure out what will be made.<br />
Important note: These reactions will only occur if both reactants are soluble in water and only one<br />
product is soluble in water.<br />
5) Acid-base: This is a special kind of double displacement reaction that takes place when an<br />
acid and base react with each other. The H + ion in the acid reacts with the OH - ion in the base,<br />
causing the formation of water. Generally, the product of this reaction is some ionic salt and water:<br />
HA+B OH-> BA+H2O<br />
One example of an acid-base reaction is the reaction of hydrobromic acid (HBr) with sodium<br />
hydroxide:<br />
HBr + NaOH ---> NaBr + H2O<br />
If an acid and a base combine, it’s an acid-base reaction. The products will be an ionic compound<br />
and water.<br />
6) Combustion: A combustion reaction is when oxygen combines with another compound to<br />
form water and carbon dioxide. These reactions are exothermic, meaning they produce heat. An<br />
example of this kind of reaction is the burning of napthalene:<br />
Org+O2->CO2+H2O<br />
Organic<br />
C10H8 + 12 O2 ---> 10 CO2 + 4 H2O<br />
If something that has carbon and hydrogen reacts with oxygen, it’s probably a combustion reaction.<br />
The products will be CO2 and H2O.<br />
Follow this series of questions. When you can answer "yes" to a question, then<br />
stop!<br />
1) Does your reaction have two (or more) chemicals combining to form one chemical? If yes, then it's<br />
a synthesis reaction<br />
2) Does your reaction have one large molecule falling apart to make several small ones? If yes, then<br />
it's a decomposition reaction<br />
3) Does your reaction have any molecules that contain only one element? If yes, then it's a single<br />
displacement reaction<br />
4) Does your reaction have water as one of the products? If yes, then it's an acid-base reaction<br />
5) Does your reaction have oxygen as one of it's reactants and carbon dioxide and water as<br />
products? If yes, then it's a combustion reaction<br />
6) If you haven't answered "yes" to any of the questions above, then you've got a double<br />
displacement reaction.<br />
72
List what type the following reactions are:<br />
1) NaOH + KNO3 --> NaNO3 + KOH<br />
double displacement<br />
2) CH4 + 2 O2 --> CO2 + 2 H2O<br />
3) 2 Fe + 6 NaBr --> 2 FeBr3 + 6 Na<br />
Combustion<br />
single displacement<br />
4) CaSO4 + Mg(OH)2 --> Ca(OH)2 + MgSO4<br />
double displacement<br />
5) NH4OH + HBr --> H2O + NH4Br<br />
acid base<br />
6) Pb + O2 --> PbO2<br />
synthesis<br />
7) Na2CO3 --> Na2O + CO2<br />
decomposition<br />
73
Determine the Type of Reaction for each equation.<br />
Then predict the products of each of the following chemical reactions. If a reaction will not occur,<br />
explain why not.<br />
Then Balance the equation.<br />
1) __Ag2SO4 + __NaNO3 →<br />
2) __NaI + __CaSO4 →<br />
3) __HNO3 + __Ca(OH)2 →<br />
4) __CaCO3 →<br />
5) __AlCl3 + __(NH4)PO4 →<br />
6) __Pb + __Fe(NO3)3 →<br />
7) __C3H6 + __O2 →<br />
8) __Na + __CaSO4 →<br />
74
Stoichiometry<br />
Cristopher Crisostomo<br />
Pd.5<br />
Chemistry<br />
Molecular mass: the sum of the atomic weights of each constituent element multiplied by the<br />
number of atoms of that element in the molecular formula<br />
Steps:<br />
Find Molecular mass<br />
Find the conversion units from grams to moles and to moles to moles<br />
Convert one molecule to the other for example from O2 to CO2 by using the conversion units<br />
Multiply the totals and then divide<br />
Mole To Mole<br />
Mass to Mass<br />
106
Gas StoiChiometry<br />
30.0 g of chlorine gas bubbled through liquid sulfur to produce liquid disulfur dichloride.How<br />
much product is produced in grams<br />
_Cl2+2S=_S2Cl2<br />
1Mol of Cl2 = 1Mol of S2Cl2<br />
71 g Cl2=1Mol of Cl2<br />
135 g S2Cl2 = 1Mol of S2Cl2<br />
Limiting Reagents<br />
Is like a regular mole to mole or mass to mass just that you have to do it twice to see which<br />
reactant runs out first<br />
Percentile yields<br />
Percent yield = Actual/Theoretical<br />
Actual yield = Theoretical*percentage<br />
107
Unit 6<br />
Chapter 13 States of Matter<br />
The students will learn what are the factors that determine and<br />
characteristics that distinguish gases liquids and solids and<br />
how substances change from one state to another.<br />
Differentiate among the four states of matter.<br />
Students will measure the physical characteristics of matter such as temperature<br />
and density.<br />
Students will compare and contrast the physical characteristics of the 4 states of<br />
matter.<br />
solid<br />
liquid<br />
gas<br />
plasma<br />
Relate temperature to the average molecular kinetic energy.<br />
Students will be able to compare and contrast the motion of particles of a sample<br />
at various temperatures.<br />
Kinetic energy<br />
Kinetic theory<br />
Temperature<br />
Describe phase transitions in terms of kinetic molecular theory.<br />
Students will be able to identify and describe phase changes.<br />
Students will be able to compare and contrast the change in particle motion<br />
for phase changes.<br />
Students will be able to interpret heating/cooling curves and phase diagrams.<br />
melting point<br />
freezing point<br />
boiling point<br />
condensation<br />
sublimation<br />
phase diagram<br />
kinetic molecular theory<br />
108
Chapter 14 The Behavior of Gases<br />
The students will learn how gases respond to changes in<br />
pressure, volume, and temperature and why the ideal gas law<br />
is useful even though ideal gases do not exist.<br />
Interpret the behavior of ideal gases in terms of kinetic molecular theory.<br />
Students will be able to describe the behavior of an ideal gas.<br />
Students will participate in activities to apply the Ideal Gas Law and its<br />
component laws to predict gas behavior.<br />
Students will be able to perform temperature/pressure conversions.<br />
Compressibility<br />
Boyle’s Law<br />
Charles’s Law<br />
Gay-Lussac’s Law<br />
Combine Gas Law<br />
Ideal Gas Law<br />
Partial pressure<br />
Dalton’s Law of partial pressure<br />
Diffusion<br />
Effusion<br />
Graham’s Law of effusion<br />
Chapter 15 Water and Aqueous Systems<br />
The students will learn how the interactions between water<br />
molecules account for the unique properties of water and how<br />
aqueous solutions form.<br />
Discuss the special properties of water that contribute to Earth's suitability<br />
as an environment for life: cohesive behavior, ability to moderate<br />
temperature, expansion upon freezing, and versatility as a solvent.<br />
Students will be able to prepare a solution of known molarity<br />
Students will participate in activities to calculate molarity<br />
Surface tension<br />
Surfactant<br />
Solvent<br />
Aqueous solution<br />
Solute<br />
109
Name:<br />
Name:<br />
Grade:<br />
States of Matter Project<br />
You and you lab partner are going to create a study aid in the form of a game for the<br />
information in Chapter 13 States of Matter.<br />
First, each of you, independently from each other, will summarize the chapter on 3<br />
pages of a pdf which will be submitted in Angel by the end of class on Monday Feb 26.<br />
Second, you and you lab partner will be given a game platform which you will use for<br />
your questions and answers, either Jeopardy or Kahoot.<br />
Third, you will fill in the information at the bottom of this page with your username,<br />
passwords and/or websites so that you do not forget this and I have a copy in case<br />
anything gets misplaced. This page will be submitted into Angel as a Word Document<br />
on Monday February 26 during class.<br />
Fourth, you will use your notes to generate the questions and answers.<br />
Finally you will give me access to your game by putting the website or Game Number<br />
on this page adding this page to your 3 pages of notes and resubmitting it in Angel as<br />
a pdf by the end of the class on Wednesday Feb 28.<br />
This page is due by the end of class on Monday February 26.<br />
This Project is due by the end of class on Wednesday February 28.<br />
Jeopardy (https://jeopardylabs.com)<br />
Password:<br />
Edit Link:<br />
Play Link:<br />
Kahoot (https://getkahoot.com)<br />
Username: Dreadnought213<br />
Email:<br />
Password: Mustang2134<br />
110
Chapter 13 notes<br />
Differentiate among the four states of matter<br />
The Nature of Gases<br />
Key Q’s<br />
What are the three assumptions of the kinetic theory as it applies to gases?<br />
Single particles of gas are considered to be small, hard spheres of an insignificant volume.<br />
The movement of the particles in a gas is random, constant, and rapid<br />
All collisions between particles in gases are perfectly elastic<br />
How does kinetic theory explain gas pressure?<br />
Gas pressure is the result of billions of moving particles in a gas simultaneously colliding with an object<br />
What is the relationship between the template in kelvins and the average kinetic energy of particles?<br />
The Kelvin of a substance is directly proportional to the average kinetic energy of the particles of the substance<br />
Definitions<br />
Kinetic energy Energy because of motion<br />
Kinetic Theory All matter consists of particles that are constantly moving<br />
Gas pressure Force exerted by a gas per unit surface area of an object<br />
Vacuum Empty space with no particles and no pressure<br />
Atmospheric pressure Collisions of molecules and atoms in air with objects<br />
Barometer Device to measure Atmospheric pressure<br />
Pascal(Pa) SI unit of pressure<br />
Standard atmosphere(atm) Pressure required to support 760mm of mercury in a mercury barometer<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
Moving bodies exert a force when they collide with other bodies<br />
Like a liquid a gas will always fill the container no matter the size or shape<br />
Uncontained gas can spread through space limitlessly because particles travel straight until they collide<br />
with something like another particle.<br />
An elastic collision is when particles collide transferring kinetic energy without losing any<br />
If no particles are present then no collisions can occur so consequently no pressure<br />
Air exerts pressure on earth since gravity holds it in the atmosphere<br />
Higher altitudes results in lower atmospheric pressure<br />
Particles in a collection of anything varies in kinetic energy<br />
In a wide collection of particles most of the particles have a kinetic energy somewhere in the middle of<br />
the range<br />
Particles of all substances, regardless of state, have the same kinetic energy in varying temperatures<br />
111
The Nature of Liquids<br />
Key Q’s<br />
What factors determine the physical properties of a liquid<br />
The interplay between the disruptive motions of particles in a liquid and the attractions among the particles<br />
determines the physical properties of liquids<br />
What is the relationship between evaporation and kinetic energy<br />
During evaporation, only those molecules with a certain minimum kinetic energy can escape from the surface of<br />
the liquid<br />
When can a dynamic equilibrium exist between a liquid and its vapor<br />
In a system at constant vapor pressure, a dynamic equilibrium exists between the vapor and the liquid. The<br />
system is in equilibrium because the rate of evaporation of liquid equals the rate of condensation of vapor<br />
Under what conditions does boiling occur<br />
When a liquid is heated to a temperature at which the particles throughout the liquid have enough kinetic<br />
energy to vaporize, the liquid begins to boil.<br />
Definitions<br />
Vaporization Conversion of a liquid into a gas or vapor<br />
Evaporation Vaporization of but on the surface of a liquid that is not boiling<br />
Vapor pressure Measure of the force exerted by a gas above a liquid<br />
Boiling point Temperature at which the vapor pressure is equal to the external pressure on the liquid<br />
Normal boiling point The boiling point of a liquid at a pressure of 101.3kPa<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
According to Kinetic theory liquids and gases both have kinetic energy<br />
The ability of gases and liquids to flow allows them to change into the shape of a container<br />
Particles in liquids are attracted to each other rather than gases that aren’t<br />
Liquids are denser than gases and have a definite volume because of the attractaction<br />
Increasing pressure on a liquid has no effect on the volume<br />
Liquids and solids are known as condensed states of matter because pressure doesn’t affect volume<br />
During evaporation most molecules don’t have enough kinetic energy to escape into the state of a gas<br />
Some of the particles that evaporate and become a gas collide with something and return back into the<br />
liquid they evaporated from<br />
Heating a liquid leads to faster evaporation because heat increases kinetic energy which allows them to<br />
overcome the attractive forces<br />
Since in evaporation the the particles with the most kinetic energy escape first and since the particles<br />
take their heat with them evaporation is technically a cooling process<br />
Overtime the number of particles turning into vapor increases and some return to being liquid<br />
112
The Nature of Solids<br />
Key Q’s<br />
How are the structure and properties of solids related<br />
The general properties of solids reflect the orderly arrangement of their particles and the fixed locations of their<br />
particles<br />
What determines the shape of a crystal<br />
The shape of a crystal reflects the arrangement of the particles within the solid<br />
Definitions<br />
Melting point The temperature at which a solid changes into a liquid<br />
Freezing point The temperature at which a liquid changes into a solid<br />
Crystal Particles arranged in an orderly, repeating, three dimensional patterns<br />
Unit cell Smallest group of particles that within a crystal that retains the geometric shape of the crystal<br />
Allotropes Two or more different molecular forms of the same element in the same physical state<br />
Amorphous solid Solid that lacks internal structure<br />
Glass Transparent fusion product of inorganic substances that have cooled to a rigid state without crystallizing<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
The particles in liquids are capable of movement but the particles of solids aren’t<br />
Most solids are packed tightly together and are difficult to compress<br />
When you heat solids the organization of the particles in solids break down<br />
At the melting point the kinetic energy of the solids is able to overcome the attractions that hold them<br />
together<br />
When the freezing temperature and melting temperature then the liquid and solid phases are in<br />
equilibrium<br />
Ionic solids have higher melting points because of the strong forces holding them together compared to<br />
molecular solids that have that have relatively low melting points<br />
The angles at which the faces of a crystal intersect are always the same for a given substance and are<br />
characteristics of that specific substance<br />
Crystals are classified in seven groups that differ in terms of angles between faces and the number of<br />
edges of equal length on each face<br />
A lattice is a repeating array of any of the fourteen kinds of unit cells<br />
Each crystal system can be composed of one to four kinds of cell units<br />
Solids can come in multiple forms such as carbon<br />
Allotropes are composed of the same atoms of the same element but different properties because of their<br />
different structures<br />
few elements have allotropes: phosphorus,sulfur,oxygen,boron,carbon,and antimony have allotropes<br />
When a crystalline solid is shattered the fragments have the same surface angles as the original<br />
113
114<br />
The Learning Goal for this is:<br />
will learn how gases respond to changes in pressure, volume, and temperature<br />
and why the ideal gas law is useful even though ideal gases do not exist<br />
Gas Laws<br />
Introduction to Gases<br />
Of the three common states of matter, the gaseous state was most easily described by early<br />
scientists. As early as 1662, Robert Boyle showed how the volume of a gas, any gas, changed as the<br />
pressure applied to it was changed. Soon thereafter, the effects of temperature and the quantity of<br />
gas on the volume were discovered. The result of all these studies was a set of fundamental<br />
mathematical equations known as the gas laws that applied equally well to any gas, whether pure<br />
oxygen, nitrogen, or a mixture of the two. Through careful studies of gases reacting with one another,<br />
Amedeo Avogadro later concluded that equal volumes of different gases must contain the same<br />
number of molecules. For example, 10.0 L of oxygen contained the same number of oxygen<br />
molecules as there were nitrogen molecules in 10.0 L of nitrogen.<br />
As time passed, it became clear that one mole of any gas contained the same number of molecules,<br />
6.02 × 10 23 molecules to be exact, a number known today as Avogadro's number. One mole of<br />
anything is Avogadro's number of that thing—atoms, molecules, people, or dollars—and that would<br />
be a lot of dollars!<br />
Boyle's Law<br />
Pressure is the amount of force exerted on one unit of area. The example of an ocean diver should<br />
make the concept clearer: The greater the depth the diver reaches, the greater the pressure due to<br />
the weight of the overlying water. Pressure is not unique to liquids but can be transmitted by gases<br />
and solids, too. At the surface of the Earth, the weight of the overlying air exerts a pressure equal to<br />
that generated by a column of mercury 760 mm high. The two most common units of pressure in<br />
chemical studies are atmosphere and millimeters of mercury.<br />
1 atm = 760 mm Hg = 760 torr.<br />
However, the standard international unit for pressure is the Pascal.<br />
1 atm = 101325 Pa<br />
1 atm = 101.3 kPa<br />
The English scientist Robert Boyle performed a series of experiments involving pressure and, in<br />
1662, arrived at a general law—that the volume of a gas varies inversely with pressure.<br />
P1V1 = P2V2<br />
This formulation has become established as Boyle's law. Of course, the relationship is valid only if the<br />
temperature remains constant.<br />
As an example of the use of this law, consider an elastic balloon holding 5 L of air at the normal<br />
atmospheric pressure of 760 mm Hg. If an approaching storm causes the pressure to fall to 735 mm<br />
Hg, the balloon expands. The product of the initial pressure and volume is equal to the product of the<br />
final pressure and volume while the temperature is constant.
It is important that you realize that pressure and volume vary inversely; therefore, an increase in<br />
either one necessitates a proportional decrease in the other.<br />
Convert a pressure of 611 mm Hg to atmospheres.<br />
1. If a gas at 1.13 atm pressure occupies 732 milliliters, what pressure is needed to reduce the<br />
volume to 500 milliliters?<br />
Charles' Law<br />
In 1787, the French inventor Jacques Charles, while investigating the inflation of his man‐carrying<br />
hydrogen balloon, discovered that the volume of a gas varied directly with temperature. This relation<br />
can be written as<br />
V1 = V2<br />
and is called Charles' law. For this law to be valid, the pressure must be held constant, and the<br />
temperature must be expressed on the absolute temperature or Kelvin scale.<br />
T1<br />
Because the volume of a gas decreases with falling temperature, scientists realized that a natural<br />
zero‐point for temperature could be defined as the temperature at which the volume of a gas<br />
theoretically becomes zero. At a temperature of absolute zero, the volume of an ideal gas would be<br />
zero. The absolute temperature scale was devised by the English physicist Kelvin, so temperatures<br />
on this scale are called Kelvin (K) temperatures. The relationship of the Kelvin scale to the common<br />
Celsius scale must be memorized by every chemistry student:<br />
T2<br />
K = °C + 273<br />
Therefore, at normal pressure, water freezes at 273 K (0°C), which is called the freezing point, and<br />
boils at 373 K (100°C). Room temperature is approximately 293 K (20°C). Both temperature scales<br />
are used in tables of chemical values, and many simple errors arise from not noticing which scale is<br />
presented.<br />
Use Charles' law to calculate the final volume of a gas that occupies 400 ml at 20°C and is<br />
subsequently heated to 300°C. Begin by converting both temperatures to the absolute scale:<br />
T1 = 20°C = 293.15 K<br />
T2 = 300°C = 573.15 K<br />
115
Then substitute them into the constant ratio of Charles' law:<br />
When using Charles' law, remember that volume and Kelvin temperature vary directly; therefore, an<br />
increase in either requires a proportional increase in the other.<br />
2. A gas occupying 660 ml at a laboratory temperature of 20°C was refrigerated until it shrank to 125<br />
ml. What is the temperature in degrees Celsius of the chilled gas?<br />
Gay-Lussac's Law<br />
This relationship between temperature and pressure is known as Gay-Lussac's law. It states that if<br />
the volume of a container is held constant as the temperature of a gas increases, the pressure inside<br />
the container will also increase. As with the other gas laws, this one can be represented in the form of<br />
an equation:<br />
P1 = P2<br />
T2<br />
Recall that we use 1s and 2s to indicate the quantities before (1s) and after (2s) a change has taken<br />
place. Also, note that the units for pressure do not matter, as long as they are the same throughout<br />
the entire equation. The units for temperature must be Kelvins or the equation will not work. This is<br />
because the Kelvin scale is an absolute scale - it doesn't go negative. Finally, this equation only<br />
works for an ideal gas. Most gases that surround you and me behave very much like ideal gases, so<br />
we can use this equation as an approximation for the gases we encounter.<br />
T2<br />
116
3. The pressure of nitrogen gas in a light bulb is 60 kPa at 25°C. Calculate the pressure of the gas<br />
when the temperature inside the bulb rises to 167°C after the bulb is lighted up?<br />
Combined gas law<br />
The combined gas law makes use of the relationships shared by pressure, volume, and temperature:<br />
the variables found in other gas laws, such as Boyle's law, Charles' law and Gay-Lussac's law. Let's<br />
review the basic principles of these three laws.<br />
Imagine you are a diver, and you begin your dive with lungs full of air. As<br />
you go deeper under water, the pressure you experience in your lungs<br />
increases. When this happens, the air inside your lungs gets squished, so<br />
the volume decreases. This is an example of Boyle's law in action, which<br />
states that the higher the pressure (P), the lower the volume (V), as<br />
shown in this image. Here, k is any constant number.<br />
Have you ever tried putting a balloon in the refrigerator and notice that it<br />
shrinks? As the temperature of the refrigerated balloon decreases, the<br />
volume of the gas inside the balloon also decreases. When you take the<br />
balloon out of the refrigerator, it reverts to its original size, so the opposite is<br />
also true; when the temperature increases, the volume also increases. The<br />
shrinking balloon serves as a demonstration of Charles' law, which states<br />
that the higher the temperature (T), the higher the volume (V).<br />
Imagine yourself driving down a road, which can cause the<br />
temperature to increase within your tires. As a result, the air inside the<br />
tires expands, and the pressure increases. This is an example of Gay-<br />
Lussac's law, which shows the relationship between pressure (P) and<br />
temperature (T) when the volume remains constant; as the<br />
temperature increases, the pressure also increases.<br />
When we put Boyle's law, Charles' law, and Gay-Lussac's law together, we come up with the<br />
combined gas law, which shows that:<br />
<br />
<br />
<br />
Pressure is inversely proportional to volume, or higher volume equals lower pressure.<br />
Pressure is directly proportional to temperature, or higher temperature equals higher pressure.<br />
Volume is directly proportional to temperature, or higher temperature equals higher volume.<br />
Let's take a look at the formula for the combined gas law. Here, PV / T = k shows how pressure,<br />
volume and temperature relate to each other, where k is a constant number.<br />
The formula for the combined gas law can be adjusted to compare two sets of conditions in one<br />
substance. In the equation, the figures for pressure (P), volume (V), and temperature (T) with<br />
subscripts of one represent the initial condition, and those with the subscripts of two represent the<br />
final condition.<br />
117
P1V1 = P2V2<br />
T1<br />
It is important to note that the temperature should always be in Kelvin, so if the given units are in<br />
Celsius, then those should be converted to Kelvin by adding 273.<br />
450 mL of a gas occupies a container that has a temperature of 28°C and a pressure of 788 mmHg.<br />
What is the temperature if the volume is reduced to 50 mL at 760 mmHg? As the unit of<br />
measurement is in Celsius, remember to convert it to Kelvin.<br />
T2<br />
4. A closed gas system initially has pressure and temperature of 1160torr and 542K with the volume<br />
unknown. If the same closed system has values of 912torr, 9720mL and 686K, what was the initial<br />
volume in L?<br />
Ideal Gas Equation<br />
The relations known as Boyle's law, Charles' law, and Avogadro's law can be combined into an<br />
exceedingly useful formula called the Ideal Gas Equation,<br />
where R denotes the gas constant:<br />
PV = nRT<br />
The temperature is, as always in gas equations, measured in Kelvin.<br />
118
This formula is strictly valid only for ideal gases—those in which the molecules are far enough apart<br />
so that intermolecular forces can be neglected. At high pressures, such forces cause significant<br />
departure from the Ideal Gas Equation, and more complicated equations have been devised to treat<br />
such cases. The Ideal Gas Equation, however, gives useful results for most gases at pressures less<br />
than 100 atmospheres.<br />
This is the value stated in the carbon dioxide reaction; you were asked to memorize that 1 mole of<br />
any gas occupies 22.4 liters at STP.<br />
You should be able to use the Ideal Gas Equation to determine any one of the four quantities—<br />
pressure, volume, moles, or temperature—if you are given values for the other three.<br />
One important application is to deduce the molecular mass and formula for a gas. Assume that you<br />
know that the hydrocarbon propylene is, by mass, 85.6% carbon and 14.4% hydrogen. Then the<br />
atomic ratios of the compound are<br />
Therefore, the propylene molecule is some integral multiple of CH2: It can be CH2 or C2H4 or C3H6 or<br />
a yet larger molecule. Measuring the volume of 10 grams of propylene at STP yields 5.322 liters,<br />
which you can use to calculate its molecular mass.<br />
Because the atomic masses of 1 CH2 unit added together is 14.03, the molecule contains three such<br />
units. Consequently, the molecular formula for propylene is C3H6.<br />
5. What is the volume occupied by 1 kilogram of carbon monoxide at 700°C and 0.1 atm?<br />
6. The ozone molecule contains only oxygen atoms. Determine the molecular formula of ozone given<br />
that 2.3 grams occupies 1,073 milliliters at standard temperature and pressure.<br />
1*1.073=2.3*.1*.0821<br />
119
The students will learn how the interactions<br />
between water molecules account for the unique<br />
The Learning Goal for this assignment is: properties of water and how aqueous solutions<br />
form.<br />
Take note over the following chapter. Use the Headings provided to organize your notes. Define and number all highlighted vocabulary (total 22<br />
) as well<br />
as summarize and take notes over the sections. You may add pictures where needed. The pictures should be an appropriate size. Use Arial 12 for all<br />
text. This document should be 2 pages and should be saved as a pdf before you submit it into Angel.<br />
Chapter 15 Water and Aqueous Systems<br />
Pages 488 - 507<br />
15.1 Water and Its Properties<br />
Water in the Liquid State<br />
Water is essential for all life on earth being present deep underground and in the atmosphere. Water<br />
is simple being made of 3 atoms 2 hydrogen and one oxygen. This simple structure allows it to be<br />
highly polar, one side (the hydrogens) being positive and the other (the oxygen) negative. water is<br />
capable of high surface pressure, low vapor pressure, and a high boiling point because to the<br />
hydrogen bonding between the negative end of one molecule to the positive end of another. attractive<br />
forces between water are balanced everywhere except the surface. at the surface the molecules<br />
experience unbalanced attractive forces surface particles to be pulled in wards which reduces surface<br />
tension 1 ( surface area of liquid). All liquids have surface tension but water has the highest causing it<br />
to have a spherical shape compared to other liquids that flatten because of gravity. The tension that<br />
water has can be reduced by adding a surfactant2(substance that interferes with hydrogen bondings).<br />
Adding a surfactant to water will cause the tension decrease by interfering with the hydrogen bonding<br />
between the molecules. The hydrogen bonding between water molecules are also responsible for low<br />
vapor pressure since the bonds have tom be broken to be able to evaporate. The hydrogen bonding<br />
in water increases the amount of heat needed to break these bonds.<br />
Water in the Solid State<br />
Water in solid state has a lower density than liquid water, on the contrary most liquids increase in<br />
density as molecules pack together causing it to sink in it’s own liquid water’s density is the highest at<br />
4 degrees celsius at 1.0000 yet below that the density decreases again. Because of hydrogen<br />
bonding water molecules are more spaced out and arranged when solid compared to liquid. Ice<br />
capable of becoming an insulator for water that is underneath it keeping it warmer that 0 degrees<br />
celsius. more energy is required to turn a liquid to<br />
15.2 Homogeneous Aqueous Systems<br />
Solutions<br />
Water that is out in nature and even tap water are aqueous solutions 3 (water that contains dissolved<br />
substances) and never chemically pure because it dissolves so many things it comes in contact with.<br />
In solutions solvents4(the dissolving medium in the solvent) are used to dissolve solutes5(dissolved<br />
particles in a solution). Solutions are homogeneous and stable. some things dissolve better than<br />
others; in water ionic compounds and polar covalent compounds are the most ready to dissolve but<br />
nonpolar covalent will not dissolve in water. water being a liquid has kinetic energy and is always<br />
moving and begins a process called solvation 6 (the process by which positive and negative ionic<br />
solids become surrounded by solvent molecules)when there are solid ionic compounds are present.<br />
some compound have bonds stronger than the attractions of water and are nearly insoluble. polar<br />
solvents dissolve polar and ionic compounds while nonpolar solvents dissolve nonpolar solutes.<br />
Electrolytes and Nonelectrolytes<br />
water being a aqueous solvent is used to find if a substance is an electrolyte 7 ( a compound that<br />
conducts an electric current when in an aqueous solution or molten state)or a<br />
nonelectrolyte 8 (Compound that does not conduct electricity in neither a aqueous solution nor a molten<br />
state) there are two electrolytes strong electrolytes 9 (solutioremove rn which solute mostly exists as<br />
120
ions)and weak electrolytes 10 (solution that has few or no ions)that can be determined by placing<br />
soluthold es in water. Electrolytes are essential for living because they are capable of delivering<br />
electrical impulses.<br />
Hydrates<br />
there is water in crystals that is called water of hydration 11 ( Water contained within a crystal) a crystal<br />
that contains water of hydration is called a hydrate12. whenever a crystal is heated to 100 degrees<br />
celsius or above they begin to lose water but since the forces that hold the water molecules in<br />
hydrates aren’t strong they lose and regain water easily. substances that do not contain water are<br />
known as anhydrous 13 . although some anhydrous substances can regain water if it comes in contact<br />
with it like copper(II) sulfate will turn blue with water. sometimes a hydrate can have a higher vapor<br />
pressure than water it will lose its water of hydration or effloresce14. Hydrated ionic compounds that<br />
have low vapor pressure can remove water from moist air these hydrates are called hygroscopic 15 .<br />
Certain substances can also remove water from the air to create a dry atmosphere they are called<br />
desiccant 16 . some compounds are so sufficient that they can dissolve completely and cause solutions<br />
they are called deliquisent 17 .<br />
15.3 Heterogeneous Aqueous Systems<br />
Suspensions<br />
a suspension 18 is a mixture of particles that will settle after a moment. they are different than a<br />
solution because the size of the particle are 1000 times larger than that of a solution. for example clay<br />
and water don’t mix so if you shake them together then the water will become cloudy with the clay<br />
particles but will not stay that way since the clay will eventually settle.<br />
Colloids<br />
One of the things we eat as snacks is a colloid 19 , a colloid is a heterogeneous mixture that has<br />
particles ranging in sizes from 1 nm to 1000 nm. they are different from suspensions because of size,<br />
colloids are smaller than suspensions but bigger than solutions. another key difference is the tyndall<br />
effect20, in which visible light is scattered by colloidal particles. both suspensions and colloids share<br />
this while solutions do not as light does not pass through. colloids also scintillate because of the<br />
radical movement of particles, this movement is called brownian motion 21 . The final colloid is<br />
Emulsion 22 , colloidal dispersion of a liquid in a liquid. for example oils wont become solutions with<br />
water but will form a colloidal dispersion if soap is added to the water<br />
121
123<br />
Unit 7<br />
Chapter 16 Solutions<br />
The students will learn what properties are used to describe<br />
the nature of solutions and how to quantify the concentration<br />
of a solution.<br />
Chapter 17 Thermochemistry<br />
The student will learn how energy is converted in a chemical<br />
or physical process and how to determine the amount of<br />
energy is absorbed or released in that process.<br />
Differentiate among the various forms of energy and recognize that they can<br />
be transformed from one form to others.<br />
Students will participate in activities to investigate and describe the<br />
transformation of energy from one form to another (i.e. batteries, food, fuels,<br />
etc.)<br />
Explore the Law of Conservation of Energy by differentiating among open,<br />
closed, and isolated systems and explain that the total energy in an isolated<br />
system is a conserved quantity.<br />
Students will be able to calculate various energy changes:<br />
o q = mc∆t<br />
o ∆Hfus<br />
o ∆Hmelt<br />
Thermochemistry<br />
Heat<br />
System<br />
Surrounding<br />
Law of conservation of energy<br />
Bond Making is exothermic<br />
Bond Breaking is endothermic<br />
Heat capacity<br />
Specific heat<br />
Calorimetry<br />
Enthalpy<br />
Thermochemical equation<br />
Molar heat of (fusion, solidification,<br />
vaporization, condensation, solution)<br />
Distinguish between endothermic and exothermic chemical processes.<br />
Students will be able to recognize exothermic and endothermic reactions through<br />
experimentation.<br />
Students will participate in activities (Pasco) to create exothermic and<br />
endothermic graphs.<br />
Endothermic<br />
Exothermic
124<br />
Create and interpret potential energy diagrams, for example: chemical<br />
reactions, orbits around a central body, motion of a pendulum<br />
Students will participate in activities (Pasco) to create exothermic and<br />
endothermic graphs.<br />
Students will be able to interpret exothermic and endothermic reaction graphs.<br />
Potential energy diagram<br />
Thermochemical equations<br />
Chapter 18 Reaction Rates and Equilibrium<br />
The student will learn how the rate of a chemical reaction can<br />
be controlled, what the role of energy is and why some<br />
reactions occur naturally and others do not.<br />
Explain how various factors, such as concentration, temperature, and<br />
presence of a catalyst affect the rate of a chemical reaction.<br />
Students will be able to describe how each factor may affect the rate of a<br />
chemical reaction.<br />
Students will be able to compare the relative effect of each factor on the rate of a<br />
chemical reaction.<br />
Rate<br />
Collision theory<br />
Activation energy<br />
Catalyst<br />
Activated complex<br />
Inhibitor<br />
Explain the concept of dynamic equilibrium in terms of reversible processes<br />
occurring at the same rates.<br />
Students will be able to describe a system in dynamic equilibrium.<br />
Students will be able to describe how factors may affect the equilibrium of a<br />
reaction.<br />
Reversible reaction<br />
Chemical equilibrium<br />
Le Chatelier principle<br />
Explain entropy’s role in determining the efficiency of processes that convert<br />
energy to work.<br />
Students will be able to describe the change in entropy of a reaction.<br />
Students will be able to determine if a reaction is spontaneous<br />
Entropy<br />
Law of disorder<br />
Spontaneous/nonspontaneous reaction
The Learning Goal for this assignment is:<br />
The students will learn what properties are used to describe the nature of solutions and how to quantify<br />
the concentration of a solution.<br />
Properties of Solutions<br />
Solution Composition<br />
Solute - substance which is dissolved (if liquid-liquid, the one you have less of is considered the<br />
solute)<br />
Solvent - substance which is doing the dissolving.<br />
Molarity (M) - the number of moles of solute per liter of solution.<br />
Mass percent (weight percent) - percent by mass of solute in the solution.<br />
Molality - the number of moles of solute per kilogram of solvent.<br />
Energies of Solution Formation<br />
Three steps in creating a liquid solution<br />
Step 1 - break up the solute into individual components (expanding the solute).<br />
Step 2 - overcoming intermolecular forces in the solvent to make room for the solute<br />
(expanding the solvent).<br />
Step 3 - allowing the solute and solvent to interact to form the solution.<br />
Enthalpy - a thermodynamic quantity equivalent to the total heat content of a system. It is equal to the<br />
internal energy of the system plus the product of pressure and volume.<br />
Enthalpy (heat) of solution - sum of the energies it takes to dissolve a substance.<br />
Enthalpy (heat) of hydration - represents the enthalpy change associated with the dispersal of a<br />
gaseous solute in water.<br />
Processes which require large amounts of energy tend not to occur.<br />
Factors Affecting Solubility<br />
Since it is the molecular structure that determines polarity, there should be a definite connection<br />
between structure and solubility.<br />
Like dissolves like - the rule says that most typically, polar solutes can dissolve in polar solvents and<br />
non-polar solutes can dissolve in non-polar solvents. But, polar solutes rarely dissolve in non-polar<br />
solvents and vice versa.<br />
125
Structure effects: determines what kind of substance will be able to dissolve the sample.<br />
hydrophobic - water-fearing<br />
hydrophilic - water-loving<br />
While pressure has little effect on the solubilities of solids or liquids, it does significantly increase the<br />
solubility of a gas.<br />
Pressure effects: gas solubility increases with increased pressure.<br />
Henry's Law: the amount of a gas dissolved in a solution is directly proportional to the pressure of the<br />
gas above the solution.<br />
P = kC<br />
P represents the partial pressure of the gas, C represents the concentration of the dissolved gas, k is<br />
a constant of the particular solution.<br />
Solubility and Pressure are Directly Related<br />
Dilution is the process of decreasing the concentration of a solute in solution, usually simply by mixing<br />
with more solvent. To dilute a solution means to add more solvent without the addition of more solute.<br />
The resulting solution is thoroughly mixed so as to ensure that all parts of the solution are identical.<br />
Temperature effects - the solubility of most solids increases with temperature.<br />
The Vapor Pressures of Solutions<br />
Volatile - quantified by the tendency of a substance to vaporize. Volatility is directly related to a<br />
substance's vapor pressure. At a given temperature, a substance with higher vapor pressure<br />
vaporizes more readily than a substance with a lower vapor pressure.<br />
Nonvolatile - does not readily form a vapor.<br />
Nonvolatile solutes lowers the vapor pressure of a solvent. The nonvolatile solute decreases the<br />
number of solvent molecules per unit volume. Thus it lowers the number of solvent molecules at the<br />
surface, and it should proportionately lower the escaping tendency of the solvent molecules.<br />
Boiling-Point elevation and Freezing-Point Depression<br />
Colligative properties - depend on the number, and not the identity of the solute particles in an ideal<br />
solution. ex. - freezing-point depression and boiling-point elevation.<br />
Boiling-point elevation - a nonvolatile solute elevates the boiling point of the solvent.<br />
Freezing-point depression - the water in the solution has a lower vapor pressure than that of pure ice.<br />
http://slideplayer.com/slide/10775304/<br />
126
The Learning Goal for this assignment is:<br />
The System and the Surroundings in Chemistry<br />
Thermochemistry<br />
The system is the part of the universe we wish to focus our attention on. In the world of chemistry, the<br />
system is the chemical reaction. For example:<br />
2H2 + O2 ---> 2H2O<br />
The system consists of those molecules which are reacting.<br />
The surroundings are everything else; the rest of the universe. For example, say the above reaction is<br />
happening in gas phase; then the walls of the container are part of the surroundings.<br />
There are two important issues:<br />
1. a great majority of our studies will focus on the change in the amount of energy, not the<br />
absolute amount of energy in the system or the surroundings.<br />
2. regarding the direction of energy flow, we have a "sign convention."<br />
Two possibilities exist concerning the flow of energy between system and surroundings:<br />
1. The system can have energy added to it, which increases its amount and lessens the energy<br />
amount in the surroundings.<br />
2. The system can have energy removed from it, thereby lowering its amount and increasing the<br />
amount in the surroundings.<br />
We will signify an increase in energy with a positive sign and a loss of energy with a negative sign.<br />
Also, we will take the point-of-view from the system. Consequently:<br />
1. When energy (heat or work) flow out of the system, the system decreases in its amount. This<br />
is assigned a negative sign and is called exothermic.<br />
2. When energy (heat or work) flows into the system, the system increases its energy amount.<br />
This is assigned a positive sign and is called endothermic.<br />
We do not discuss chemical reactions from the surrounding's point-of-view. Only from the system's.<br />
Notes:<br />
endothermic reactions are When energy is added to a substance from the environment<br />
exothermic reactions are When energy is removed from a substance and released off into the<br />
environment
Specific Heat<br />
Here is the definition of specific heat:<br />
the amount of heat necessary for 1.00 gram of a substance to change 1.00 °C<br />
Note the two important factors:<br />
1. It's 1.00 gram of a substance<br />
2. and it changes 1.00 °C<br />
Keep in mind the fact that this is a very specific value. It is only for one gram going one degree. The<br />
specific heat is an important part of energy calculations since it tells you how much energy is needed<br />
to move each gram of the substance one degree.<br />
Every substance has its own specific heat and each phase has its own distinct value. In fact, the<br />
specific heat value of a substance changes from degree to degree, but we will ignore that.<br />
The units are often Joules per gram-degree Celsius (J/g*°C). Sometimes the unit J/kg K is also used.<br />
This last unit is technically the most correct unit to use, but since the first one is quite common, you<br />
will need to know both.<br />
I will ignore calorie-based units almost entirely.<br />
Here are the specific heat values for water:<br />
Phase J g¯1 °C¯1 J kg¯1<br />
K¯1<br />
Gas 2.02 2.02 x 10 3<br />
Liquid 4.184 4.184 x 10 3<br />
Solid 2.06 2.06 x 10 3<br />
Notice that one set of values is simply 1000 times bigger than the other. That's to offset the influence<br />
of going from grams to kilograms in the denominator of the unit.<br />
Notice that the change from Celsius to Kelvin does not affect the value. That is because the specific<br />
heat is measured on the basis of one degree. In both scales (Celsius and Kelvin) the jump from one<br />
degree to the next are the same "distance." Sometimes a student will think that 273 must be involved<br />
somewhere. Not in this case.<br />
Specific heat values can be looked up in reference books. Typically, in the classroom, you will not be<br />
asked to memorize any specific heat values. However, you may be asked to memorize the values for<br />
the three phases of water.<br />
As you go about the Internet, you will find different values cited for specific heats of a given<br />
substance. For example, I have seen 4.186 and 4.187 used in place of 4.184 for liquid water. None of<br />
the values are wrong, it's just that specific heat values literally change from degree to degree. What<br />
happens is that an author will settle on one particular value and use it. Often, the one particular value<br />
used is what the author used as a student.<br />
Hence, 4.184.<br />
128
The Time-Temperature Graph<br />
We are going to heat a container that has 72.0 grams of ice (no liquid water yet!) in it. To make the<br />
illustration simple, please consider that 100% of the heat applied goes into the water. There is no loss<br />
of heat into heating the container and no heat is lost to the air.<br />
Let us suppose the ice starts at -10.0 °C and that the pressure is always one atmosphere. We will<br />
end the example with steam at 120.0 °C.<br />
There are five major steps to discuss in turn before this problem is completely solved. Here they are:<br />
1. the ice rises in temperature from -10.0 to 0.00 °C.<br />
2. the ice melts at 0.00 °C.<br />
3. the liquid water then rises in temperature from zero to 100.0 °C.<br />
4. the liquid water then boils at 100.0 °C.<br />
5. the steam then rises in temperature from 100.0 to 120.0 °C<br />
Each one of these steps will have a calculation associated with it. WARNING: many homework and<br />
test questions can be written which use less than the five steps. For example, suppose the water in<br />
the problem above started at 10.0 °C. Then, only steps 3, 4, and 5 would be required for solution.<br />
To the right is the type of graph which is typically used to<br />
show this process over time.<br />
You can figure out that the five numbered sections on the<br />
graph relate to the five numbered parts of the list just above<br />
the graph.<br />
Also, note that numbers 2 and 4 are phases changes: solid<br />
to liquid in #2 and liquid to gas in #4.<br />
Q=mcΔT<br />
where ΔT is (Tf – Ti)<br />
Here are some symbols that will be used, A LOT!!<br />
Δt = the change in temperature from start to finish in degrees Celsius (°C)<br />
m = mass of substance in grams<br />
c = the specific heat. Its unit is Joules per gram X degree Celsius (J / g °C is one way to write<br />
the unit; J g¯1 °C¯1 is another)<br />
q = the amount of heat involved, measured in Joules or kilojoules (symbols = J and kJ)<br />
mol = moles of substance.<br />
ΔH is the symbol for the molar heat of fusion and ΔH is the symbol for the molar heat of<br />
vaporization.<br />
We will also require the molar mass of the substance. In this example it is water, so the molar mass is<br />
18.0 g/mol.<br />
Notes:<br />
specific heat is increasing or decreasing the temperature of a gram of a substance by one degree<br />
in celsius.
Step One: solid ice rises in temperature<br />
As we apply heat, the ice will rise in temperature until it<br />
arrives at its normal melting point of zero Celsius.<br />
Once it arrives at zero, the Δt equals 10.0 °C.<br />
Here is an important point: THE ICE HAS NOT MELTED<br />
YET.<br />
At the end of this step we have SOLID ice at zero<br />
degrees. It has not melted yet. That's an important point.<br />
Each gram of water requires a constant amount of energy<br />
to go up each degree Celsius. This amount of energy is<br />
called specific heat and has the symbol c.<br />
72.0 grams of ice (no liquid water yet!) has changed 10.0 °C. We need to calculate the energy<br />
needed to do this.<br />
This summarizes the information needed:<br />
Δt = 10 °C<br />
The mass = 72.0 g<br />
c = 2.06 Joules per gram-degree Celsius<br />
The calculation needed, using words & symbols is:<br />
q = (mass) (Δt) (c)<br />
Why is this equation the way it is?<br />
Think about one gram going one degree. The ice needs 2.06 J for that. Now go the second degree.<br />
Another 2.06 J. Go the third degree and use another 2.06 J. So one gram going 10 degrees needs<br />
2.06 x 10 = 20.6 J. Now we have 72 grams, so gram #2 also needs 20.6, gram #3 needs 20.6 and so<br />
on until 72 grams.<br />
With the numbers in place, we have:<br />
q = (72.0 g) (10 °C) (2.06 J/g °C)<br />
So we calculate and get 1483.2 J. We won't bother to round off right now since there are four more<br />
calculations to go. Maybe you can see that we will have to do five calculations and then sum them all<br />
up.<br />
One warning before going on: three of the calculations will yield J as the unit on the answer and two<br />
will give kJ. When you add the five values together, you MUST have them all be the same unit.<br />
In the context of this problem, kJ is the preferred unit. You might want to think about what 1483.2 J is<br />
in kJ.<br />
Notes:<br />
all of the values must be in the same unit<br />
We use the equation q = mc/\T<br />
there is no need to round the final answer
Step Two: solid ice melts<br />
Now, we continue to add energy and the ice begins to<br />
melt.<br />
However, the temperature DOES NOT CHANGE. It<br />
remains at zero during the time the ice melts.<br />
Each mole of water will require a constant amount of<br />
energy to melt. That amount is named the molar heat of<br />
fusion and its symbol is ΔHf. The molar heat of fusion is<br />
the energy required to melt one mole of a substance at its<br />
normal melting point. One mole of solid water, one mole<br />
of solid benzene, one mole of solid lead. It does not<br />
matter. Each substance has its own value.<br />
During this time, the energy is being used to overcome water molecules' attraction for each other,<br />
destroying the three-dimensional structure of the ice.<br />
The unit for this is kJ/mol. Sometimes you see older references that use kcal/mol. The conversion<br />
between calories and Joules is 4.184 J = 1.000 cal.<br />
Sometimes you also see this number expressed "per gram" rather than "per mole." For example,<br />
water's molar heat of fusion is 6.02 kJ/mol. Expressed per gram, it is 334.16 J/g.<br />
Typically, the term "heat of fusion" is used with the "per gram" value.<br />
72.0 grams of solid water is 0.0 °C. It is going to melt AND stay at zero degrees. This is an important<br />
point. While the ice melts, its temperature will remain the same. We need to calculate the energy<br />
needed to do this.<br />
This summarizes the information needed:<br />
ΔHf = 6.02 kJ/mol<br />
The mass = 72.0 g<br />
The molar mass of H2O = 18.0 gram/mol<br />
The calculation needed, using words & symbols is:<br />
q = (moles of water) (ΔHf)<br />
We can rewrite the moles of water portion and make the equation like this:<br />
q = (grams water / molar mass of water) (ΔHf)<br />
Why is this equation the way it is?<br />
Think about one mole of ice. That amount of ice (one mole or 18.0 grams) needs 6.02 kilojoules of<br />
energy to melt. Each mole of ice needs 6.02 kilojoules. So the (grams water / molar mass of water) in<br />
the above equation calculates the amount of moles.<br />
With the numbers in place, we have:<br />
q = (72.0 g / 18.0 g mol¯1 ) (6.02 kJ / mol)<br />
So we calculate and get 24.08 kJ. We won't bother to round off right now since there are three more<br />
calculations to go. We're doing the second step now. When all five are done, we'll sum them all up.<br />
131
Step Three: liquid water rises in temperature<br />
Once the ice is totally melted, the temperature can now<br />
begin to rise again.<br />
It continues to go up until it reaches its normal boiling<br />
point of 100.0 °C.<br />
Since the temperature went from zero to 100, the Δt is<br />
100.<br />
Here is an important point: THE LIQUID HAS NOT<br />
BOILED YET.<br />
At the end of this step we have liquid water at 100 degrees. It has not turned to steam yet.<br />
Each gram of water requires a constant amount of energy to go up each degree Celsius. This amount<br />
of energy is called specific heat and has the symbol c. There will be a different value needed,<br />
depending on the substance being in the solid, liquid or gas phase.<br />
72.0 grams of liquid water is 0.0 °C. It is going to warm up to 100.0 °C, but at that temperature, the<br />
water WILL NOT BOIL. We need to calculate the energy needed to do this.<br />
This summarizes the information needed:<br />
Δt = 100.0 °C (100.0 °C – 0.0 °C)<br />
The mass = 72.0 g<br />
c = 4.184 Joules per gram-degree Celsius<br />
The calculation needed, using words & symbols is:<br />
q = (mass) (Δt) (c)<br />
Why is this equation the way it is?<br />
Think about one gram going one degree. The liquid water needs 4.184 J for that. Now go the second<br />
degree. Another 4.184 J. Go the third degree and use another 4.184 J. So one gram going 100<br />
degrees needs 4.184 x 100 = 418.4 J. Now we have 72 grams, so gram #2 also needs 418.4, gram<br />
#3 needs 418.4 and so on until 72 grams.<br />
With the numbers in place, we have:<br />
q = (72.0 g) (100.0 °C) (4.184 J/g °C)<br />
So we calculate and get 30124.8 J. We won't bother to round off right now since there are two more<br />
calculations to go. We will have to do five calculations and then sum them all up.<br />
Notes:<br />
as water reaches 100 degrees Celsius then /\t is also 100
133<br />
Step Four: liquid water boils<br />
Now, we continue to add energy and the water begins to<br />
boil.<br />
However, the temperature DOES NOT CHANGE. It<br />
remains at 100 during the time the water boils.<br />
Each mole of water will require a constant amount of<br />
energy to boil. That amount is named the molar heat of<br />
vaporization and its symbol is ΔH. The molar heat of<br />
vaporization is the energy required to boil one mole of a<br />
substance at its normal boiling point. One mole of liquid water, one mole of liquid benzene, one mole<br />
of liquid lead. It does not matter. Each substance has its own value.<br />
During this time, the energy is being used to overcome water molecules' attraction for each other,<br />
allowing them to move from close together (liquid) to quite far apart (the gas state).<br />
The unit for this is kJ/mol. Sometimes you see older references that use kcal/mol. The conversion<br />
between calories and Joules is 4.184 J = 1.000 cal.<br />
Typically, the term "heat of vaporization" is used with the "per gram" value.<br />
72.0 grams of liquid water is at 100.0 °C. It is going to boil AND stay at 100 degrees. This is an<br />
important point. While the water boils, its temperature will remain the same. We need to calculate the<br />
energy needed to do this.<br />
This summarizes the information needed:<br />
ΔH = 40.7 kJ/mol<br />
The mass = 72.0 g<br />
The molar mass of H2O = 18.0 gram/mol<br />
The calculation needed, using words & symbols is:<br />
q = (moles of water) (ΔH)<br />
We can rewrite the moles of water portion and make the equation like this:<br />
q = (grams water / molar mass of water) (ΔH)<br />
Why is this equation the way it is?<br />
Think about one mole of liquid water. That amount of water (one mole or 18.0 grams) needs 40.7<br />
kilojoules of energy to boil. Each mole of liquid water needs 40.7 kilojoules to boil. So the (grams<br />
water / molar mass of water) in the above equation calculates the amount of moles.<br />
With the numbers in place, we have:<br />
q = (72.0 g / 18.0 g mol¯1 ) (40.7 kJ / mol)<br />
So we calculate and get 162.8 kJ. We won't bother to round off right now since there is one more<br />
calculation to go. We're doing the fourth step now. When all five are done, we'll sum them all up.
Step Five: steam rises in temperature<br />
Once the water is completely changed to steam, the<br />
temperature can now begin to rise again.<br />
It continues to go up until we stop adding energy. In this<br />
case, let the temperature rise to 120 °C.<br />
Since the temperature went from 100 °C to 120°C, the Δt<br />
is 20°C.<br />
Each gram of water requires a constant amount of energy<br />
to go up each degree Celsius. This amount of energy is called specific heat and has the symbol c.<br />
There will be a different value needed, depending on the substance being in the solid, liquid or gas<br />
phase.<br />
72.0 grams of steam is 100.0 °C. It is going to warm up to 120.0 °C. We need to calculate the energy<br />
needed to do this.<br />
This summarizes the information needed:<br />
Δt = 20 °C<br />
The mass = 72.0 g<br />
c = 2.02 Joules per gram-degree Celsius<br />
The calculation needed, using words & symbols is:<br />
q = (mass) (Δt) (c)<br />
Why is this equation the way it is?<br />
Think about one gram going one degree. The liquid water needs 2.02 J for that. Now go the second<br />
degree. Another 2.02 J. Go the third degree and use another 2.02 J. So one gram going 20 degress<br />
needs 2.02 x 20 = 44 J. Now we have 72 grams, so gram #2 also needs 44, gram #3 needs 44 and<br />
so on until 72 grams.<br />
I hope that helped.<br />
With the numbers in place, we have:<br />
q = (72.0 g) (20 °C) (2.02 J/g °C)<br />
So we calculate and get 2908.8 J. We won't bother to round off right now since we still need to sum<br />
up all five values.<br />
Notes:<br />
134
The following table summarizes the five steps and their results. Each step number is a link back to<br />
the explanation of the calculation.<br />
Converting to kJ gives us this:<br />
1.4832 kJ<br />
24.08 kJ<br />
30.1248 kJ<br />
162.8 kJ<br />
2.9088 kJ<br />
Step q 72.0 g of H2O<br />
1 1483.2 J Δt = 10 (solid)<br />
2 24.08 kJ melting<br />
3 30124.8 J Δt = 100 (liquid)<br />
4 162.8 kJ boiling<br />
5 2908.8 J Δt = 20 (gas)<br />
Summing up gives 221.3968 kJ and proper significant digits gives us 221.4 kJ for the answer.<br />
Notice how all units were converted to kJ before continuing on. Joules is a perfectly fine unit; it's just<br />
that 221,396.8 J is an awkward number to work with. Usually Joules is used for values under 1000,<br />
otherwise kJ is used.<br />
By the way, on other sites you may see kj used for kilojoules. I've also seen Kj used. Both of these<br />
are wrong symbols. kJ is the only correct symbol.<br />
Enthalpy<br />
When a process occurs at constant pressure, the heat evolved (either released or absorbed) is equal<br />
to the change in enthalpy. Enthalpy (H) is the sum of the internal energy (U) and the product of<br />
pressure and volume (PV) given by the equation:<br />
H=U+PV<br />
When a process occurs at constant pressure, the heat evolved (either released or absorbed) is equal<br />
to the change in enthalpy. Enthalpy is a state function which depends entirely on the state<br />
functions T, P and U. Enthalpy is usually expressed as the change in enthalpy (ΔH) for a process<br />
between initial and final states:<br />
ΔH=ΔU+ΔPVΔ<br />
If temperature and pressure remain constant through the process and the work is limited to pressurevolume<br />
work, then the enthalpy change is given by the equation:<br />
ΔH=ΔU+PΔV<br />
Also at constant pressure the heat flow (q) for the process is equal to the change in enthalpy defined<br />
by the equation:<br />
ΔH=q<br />
By looking at whether q is exothermic or endothermic we can determine a relationship between ΔH<br />
and q. If the reaction absorbs heat it is endothermic meaning the reaction consumes heat from the<br />
surroundings so q>0 (positive). Therefore, at constant temperature and pressure, by the equation<br />
above, if q is positive then ΔH is also positive. And the same goes for if the reaction releases heat,<br />
135
then it is exothermic, meaning the system gives off heat to its surroundings, so q
The Learning Goal for this assignment is:<br />
Thermochemistry Review Questions<br />
Particulate Nature of Matter and Changes of State<br />
Watch the following video and answer the questions.<br />
1. How are solid particles arranged?<br />
Solid particles are arranged very compactly and dont have much room to move.<br />
2. What is supplied to make a solid become a liquid?<br />
Energy is supplied to convert a solid to a liquid<br />
3. What does ice take from the environment when melting?<br />
it takes the surrounding energy from the environment<br />
4. What do molecules need to evaporate?<br />
they need to have enough energy to escape liquid form<br />
5. Do all substance need the same amount of energy to evaporate?<br />
no some substance require less like Ether can evaporate at room temperature<br />
6. Can gases change straight into solids?<br />
Yes if it is cold enough for the environment to aborb enough energy from the gas the gas become a solid<br />
1. Which of the following statements about the atoms and molecules in all three states of matter is<br />
NOT true.<br />
a. Particles of matter are in constant motion<br />
b. Space exists between particles<br />
c. Adding heat increases the size of the particles<br />
d. The particles of matter are too small to be seen with the unaided eye<br />
2. Which statement is correct?<br />
a. All particles in a given substance at a given temperature have equal kinetic energy<br />
b. Heat always flows from a hotter object to a cooler object<br />
c. Heat is a measure of average kinetic energy of a substance<br />
d. Temperature measures the total kinetic energy of a substance<br />
3. In what state does matter have the most kinetic energy?<br />
a. Solid<br />
b. Liquid<br />
c. Gas<br />
d. Plasma<br />
4. In what state does matter have the least kinetic energy?<br />
a. Solid<br />
b. Liquid<br />
c. Gas<br />
d. Plasma<br />
5. What is the best description of the relationship between states of matter and kinetic energy?<br />
a. There is no relationship<br />
b. As kinetic energy increases the attraction between molecules increases<br />
c. As the kinetic energy decreases the attraction between molecules decreases<br />
d. As the kinetic energy increases the attraction between molecules decreases<br />
137
Define the types of reactions. Give the names of the changes of States of Matter and describe the<br />
relative kinetic energy of the particles.<br />
Endothermic Reactions:<br />
Gas to Liquid<br />
Condensation<br />
Liquid to Solid<br />
freezing<br />
Gas to Solid<br />
deposition<br />
Exothermic Reactions:<br />
Solid to Liquid<br />
melting<br />
Liquid to Gas<br />
vaporization evaporation and boiling<br />
Solid to Gas<br />
sublimination<br />
138
Phase Diagram<br />
Information on a Phase Diagram<br />
1. In each colored region the substance is in a single phase<br />
2. The lines describes the equilibrium conditions for the phases on either side of the line<br />
3. The triple point is the conditions required for all three state of matter to exist in equilibrium<br />
139
1. If this compound was at room temperature (25°C), what phase would it most likely be in?<br />
gas<br />
2. At what temperature would all three phases of this substance exist?<br />
350 degrees<br />
3. If this substance is at 45 atm and 100°C what will happen if I raise the temperature to 400°C?<br />
it will subliminate<br />
4. If this substance is at 70 atm and 500°C and the pressure is decreased to 40 atm what phase<br />
change will occur?<br />
it will vaporize<br />
5. Why can’t this substance be boiled at a temperature of 200°C?<br />
because it's liquid phase is at 350 degrees at and it can't be a liquid at 200 degrees<br />
6. If I wanted to, could I drink this substance?<br />
50 atm at 350 degrees is required for it to be a liquid so it is not possible<br />
140
Heating Curve<br />
Information on a Phase Diagram<br />
1. In general the temperature goes up the longer the heating continues.<br />
Q=mc∆T<br />
2. The horizontal parts to the graph is a change of state.<br />
Q=mHf<br />
Q=mHv<br />
141
1. In what part of the curve would a substance have a definite shape and definite volume?<br />
in part 1 of the curve the substance would be frozen and have a definite shape<br />
2. In what part of the curve would substance a have a definite volume but no definite shape?<br />
in part 3 the substance would have a volume but no shape since liquids take up anyshape<br />
3. In what part of the curve would a substance have no definite shape or volume?<br />
in part 5 of the curve the substance would be a gas and have no definite shape or volume<br />
4. What part of the curve represents a mixed solid/liquid phases?<br />
Part 2<br />
5. What part of the curve represents a mixed liquid/vapor phases?<br />
Part 4<br />
6. What is the melting temperature of substance?<br />
the substances melts at 5 degrees<br />
7. What is the boiling temperature of substance?<br />
the boiling temperature of the substance at 55 degrees<br />
8. In what part of the curve would the molecules have the lowest kinetic energy?<br />
the substance would have the least in phase 1 since it would be a solid<br />
9. In what part of the curve would the molecules have the greatest kinetic energy?<br />
it would have the most in phase 5 since it would be a gas<br />
142
The Learning Goal for this assignment is:<br />
Thermochemistry Review Questions<br />
Particulate Nature of Matter and Changes of State<br />
Watch the following video and answer the questions.<br />
1. How are solid particles arranged?<br />
Solid particles are arranged very compactly and dont have much room to move.<br />
2. What is supplied to make a solid become a liquid?<br />
Energy is supplied to convert a solid to a liquid<br />
3. What does ice take from the environment when melting?<br />
it takes the surrounding energy from the environment<br />
4. What do molecules need to evaporate?<br />
they need to have enough energy to escape liquid form<br />
5. Do all substance need the same amount of energy to evaporate?<br />
no some substance require less like Ether can evaporate at room temperature<br />
6. Can gases change straight into solids?<br />
Yes if it is cold enough for the environment to aborb enough energy from the gas the gas become a solid<br />
1. Which of the following statements about the atoms and molecules in all three states of matter is<br />
NOT true.<br />
a. Particles of matter are in constant motion<br />
b. Space exists between particles<br />
c. Adding heat increases the size of the particles<br />
d. The particles of matter are too small to be seen with the unaided eye<br />
2. Which statement is correct?<br />
a. All particles in a given substance at a given temperature have equal kinetic energy<br />
b. Heat always flows from a hotter object to a cooler object<br />
c. Heat is a measure of average kinetic energy of a substance<br />
d. Temperature measures the total kinetic energy of a substance<br />
3. In what state does matter have the most kinetic energy?<br />
a. Solid<br />
b. Liquid<br />
c. Gas<br />
d. Plasma<br />
4. In what state does matter have the least kinetic energy?<br />
a. Solid<br />
b. Liquid<br />
c. Gas<br />
d. Plasma<br />
5. What is the best description of the relationship between states of matter and kinetic energy?<br />
a. There is no relationship<br />
b. As kinetic energy increases the attraction between molecules increases<br />
c. As the kinetic energy decreases the attraction between molecules decreases<br />
d. As the kinetic energy increases the attraction between molecules decreases<br />
143
Define the types of reactions. Give the names of the changes of States of Matter and describe the<br />
relative kinetic energy of the particles.<br />
Endothermic Reactions:<br />
Gas to Liquid<br />
Condensation<br />
Liquid to Solid<br />
freezing<br />
Gas to Solid<br />
deposition<br />
Exothermic Reactions:<br />
Solid to Liquid<br />
melting<br />
Liquid to Gas<br />
vaporization evaporation and boiling<br />
Solid to Gas<br />
sublimination<br />
144
Phase Diagram<br />
Information on a Phase Diagram<br />
1. In each colored region the substance is in a single phase<br />
2. The lines describes the equilibrium conditions for the phases on either side of the line<br />
3. The triple point is the conditions required for all three state of matter to exist in equilibrium<br />
145
1. If this compound was at room temperature (25°C), what phase would it most likely be in?<br />
gas<br />
2. At what temperature would all three phases of this substance exist?<br />
350 degrees<br />
3. If this substance is at 45 atm and 100°C what will happen if I raise the temperature to 400°C?<br />
it will subliminate<br />
4. If this substance is at 70 atm and 500°C and the pressure is decreased to 40 atm what phase<br />
change will occur?<br />
it will vaporize<br />
5. Why can’t this substance be boiled at a temperature of 200°C?<br />
because it's liquid phase is at 350 degrees at and it can't be a liquid at 200 degrees<br />
6. If I wanted to, could I drink this substance?<br />
50 atm at 350 degrees is required for it to be a liquid so it is not possible<br />
146
Heating Curve<br />
Information on a Phase Diagram<br />
1. In general the temperature goes up the longer the heating continues.<br />
Q=mc∆T<br />
2. The horizontal parts to the graph is a change of state.<br />
Q=mHf<br />
Q=mHv<br />
147
1. In what part of the curve would a substance have a definite shape and definite volume?<br />
in part 1 of the curve the substance would be frozen and have a definite shape<br />
2. In what part of the curve would substance a have a definite volume but no definite shape?<br />
in part 3 the substance would have a volume but no shape since liquids take up anyshape<br />
3. In what part of the curve would a substance have no definite shape or volume?<br />
in part 5 of the curve the substance would be a gas and have no definite shape or volume<br />
4. What part of the curve represents a mixed solid/liquid phases?<br />
Part 2<br />
5. What part of the curve represents a mixed liquid/vapor phases?<br />
Part 4<br />
6. What is the melting temperature of substance?<br />
the substances melts at 5 degrees<br />
7. What is the boiling temperature of substance?<br />
the boiling temperature of the substance at 55 degrees<br />
8. In what part of the curve would the molecules have the lowest kinetic energy?<br />
the substance would have the least in phase 1 since it would be a solid<br />
9. In what part of the curve would the molecules have the greatest kinetic energy?<br />
it would have the most in phase 5 since it would be a gas<br />
148
Unit 8<br />
Chapter 19 Acid and Bases<br />
The student will learn what are the different ways chemists<br />
define aids and bases, what the pH of a solution means and<br />
how chemist use acid-base reactions.<br />
Relate acidity and basicity to hydronium and hydroxyl ion concentration and<br />
pH.<br />
Students will be able to use a pH scale to identify substances as acids or bases.<br />
Students will be able to use various equipment (probeware, universal pH, etc.) to<br />
identify the pH of substances.<br />
Students will be able to calculate H3O+ and OH- concentration of various<br />
substances.<br />
pH scale<br />
Hydronium ion<br />
Arrhenius acid/base<br />
Lewis acid/base<br />
Bronsted-Lowry acid/base<br />
Strong acid/base<br />
Weak acid/base<br />
Neutralization reaction<br />
Titration<br />
Chapter 20 Oxidation-Reduction Reactions<br />
The student will learn what happens during oxidation and<br />
reduction and how to balance redox equations.<br />
Describe oxidation-reduction reactions in living and non-living systems.<br />
Students will be able to compare and contrast redox reactions.<br />
Students will be able to assign oxidation numbers to redox reactions.<br />
Students will be able to write half reactions<br />
Oxidation<br />
149
Reduction<br />
Oxidation reduction reaction<br />
Oxidation number<br />
Half reaction<br />
Electrochemical process<br />
Battery<br />
Cathode<br />
Anode<br />
Electrolysis<br />
150
The Learning Goal for this section is:<br />
Acids and Bases<br />
The Observable Properties of Acids and Bases<br />
The words acid and alkaline (an older word for base) are derived from direct sensory experience.<br />
Acid Property #1:<br />
The word acid comes from the Latin word acere, which means "sour." All acids taste sour. Well<br />
known from ancient times were vinegar, sour milk and lemon juice. Aspirin (scientific name:<br />
acetylsalicylic acid) tastes sour if you don't swallow it fast enough. Other languages derive their word<br />
for acid from the meaning of sour. So, in France, we have acide. In Germany, we have säure from<br />
saure and in Russia, kislota from kisly.<br />
Base Property #1:<br />
The word "base" has a more complex history (see below) and its name is not related to taste. All<br />
bases taste bitter. For example, mustard is a base. It tastes bitter. Many medicines, because they are<br />
bases, taste bitter. This is the reason cough syrups are advertised as having a "great grape taste."<br />
The taste is added in order to cover the bitterness of the active ingredient in cough syrup.<br />
Acid Property #2:<br />
Acids make a blue vegetable dye called litmus turn red.<br />
Base Property #2:<br />
Bases are substances which will restore the original blue color of litmus after having been reddened<br />
by an acid.<br />
Acid Property #3:<br />
Acids destroy the chemical properties of bases.<br />
Base Property #3:<br />
Bases destroy the chemical properties of acids.<br />
Neutralization is the name for this type of reaction.<br />
Acid Property #4:<br />
Acids conduct an electric current.<br />
Base Property #4:<br />
Bases conduct an electric current.<br />
Acids<br />
Turn blue litmus red<br />
taste sour<br />
acid corrode metal<br />
positively charged hydrogen ions (H)<br />
Bases<br />
turn red litmus blue<br />
taste bitter<br />
negatively hydroxide ions<br />
most hand soaps are bases<br />
This is a common property shared with salts. Acids, bases and salts are grouped together into a<br />
category called electrolytes, meaning that a water solution of the given substance will conduct an<br />
electric current.<br />
Non-electrolyte solutions cannot conduct a current. The most common example of this is sugar<br />
dissolved in water.<br />
151
So far, the properties have an obvious relationship: taste, color change, mutual destruction, and<br />
response to electric current. This last property is related, but in a less obvious way. The property<br />
below identifies a unique chemical reaction that acids and bases engage in.<br />
Acid Property #5:<br />
Upon chemically reacting with an active metal, acids will evolve hydrogen gas (H2). The key word, of<br />
course, is active. Some metals, like gold, silver or platinum, are rather unreactive and it takes rather<br />
extreme conditions to get these "unreactive" metals to react. Not so with the metals in this property.<br />
They include the alkali metals (Group I, Li to Rb), the alkaline earth metals (Group II, Be to Ra), as<br />
well as zinc and aluminum. Just bring the acid and the metal together at anything close to room<br />
temperature and you get a reaction. Here's a sample reaction:<br />
Zn + 2 HCl(aq) ---> ZnCl2 + H2<br />
Another common acid reaction some sources mention is that acids react with carbonates (and<br />
bicarbonates) to give carbon dioxide gas:<br />
HCl + NaCO3 ---> CO2 + H2O + NaCl<br />
Base Property #5:<br />
Bases feel slippery, sometimes people say soapy. This is because they dissolve the fatty acids and<br />
oils from your skin and this cuts down on the friction between your fingers as you rub them together.<br />
In essence, the base is making soap out of you. Yes, bases are involved in the production of soap! In<br />
the early years of soap making, the soaps were very harsh on the skin and clothes due to the high<br />
base content. Even today, people with very sensitive skin must sometimes use a non-soap-based<br />
product for bathing.<br />
It was not until more modern times that the chemical nature (as opposed to observable properties) of<br />
acids and bases began to be explored. That leads to this property that is not directly observable by<br />
the senses.<br />
Acid Property #6:<br />
Acids produce hydrogen ion (H + ) in solution. A more correct formula for what is produced is that of the<br />
hydronium ion, H3O + . Both formulas are used interchangeably.<br />
Acid base theories: Svante Arrhenius<br />
I. Introduction<br />
The basic idea is that certain substances remain ionized in solution all the time. Today, everyone<br />
accepts this without question, but it was the subject of much dissention and disagreement in 1884,<br />
when a twenty-five-year-old Arrhenius presented and defended his dissertation.<br />
II. The Acid Base Theory<br />
Acid - any substance which delivers hydrogen ion (H + ) to the solution.<br />
Base - any substance which delivers hydroxide ion (OH¯) to the solution.<br />
Here is a generic acid dissociating, according to Arrhenius:<br />
HA ---> H + + A¯<br />
152
This would be a generic base:<br />
XOH ---> X + + OH¯<br />
When acids and bases react according to this theory, they neutralize each other, forming water and a<br />
salt:<br />
HA + XOH ---> H2O + XA<br />
Keeping in mind that the acid, the base and the salt all ionize, we can write this:<br />
Finally, we can drop all spectator ions, to get this:<br />
H + + A¯ + X + + OH¯ ---> H2O + X + + A¯<br />
H + + OH¯ ---> H2O<br />
These ideas covered all of the known acids at the time (the usual suspects like hydrochloric acid,<br />
acetic acid, and so on) and most of the bases (sodium hydroxide, potassium hydroxide, calcium<br />
hydroxide and so on). HOWEVER, and it is a big however, the theory did not explain why ammonia<br />
(NH3) was a base. There are other problems with the theory also.<br />
III. Problems with Arrhenius' Theory<br />
1. The solvent has no role to play in Arrhenius' theory. An acid is expected to be an acid in any<br />
solvent. This was found to not be the case. For example, HCl is an acid in water, behaving in<br />
the manner Arrhenius expected. However, if HCl is dissolved in benzene, there is no<br />
dissociation, the HCl remaining as un-dissociated molecules. The nature of the solvent plays a<br />
critical role in acid-base properties of substances.<br />
2. All salts in Arrhenius' theory should produce solutions that are neither acidic or basic. This is<br />
not the case. If equal amounts of HCl and ammonia react, the solution is slightly acidic. If equal<br />
amounts of acetic acid and sodium hydroxide are reacted, the resulting solution is basic.<br />
Arrhenius had no explanation for this.<br />
3. The need for hydroxide as the base led Arrhenius to propose the formula NH4OH as the<br />
formula for ammonia in water. This led to the misconception that NH4OH is the actual base,<br />
not NH3.<br />
In fact, by 1896, several years before Arrhenius announced his theory, it had been recognized that<br />
characteristic base properties where just as evident in such solvents as aniline, where no hydroxide<br />
ions were possible.<br />
4. H + , a bare proton, does not exist for very long in water. The proton affinity of H2O is about 799<br />
kJ/mol. Consequently, this reaction:<br />
H2O + H + ---> H3O +<br />
happens to a very great degree. The "concentration" of free protons in water has been estimated to<br />
be 10¯130 M. A rather preposterous value, indeed.<br />
The Arrhenius theory of acids and bases will be fully supplanted by the theory proposed<br />
independently by Johannes Brønsted and Thomas Lowry in 1923.<br />
153
The acid base theory of Brønsted and Lowry<br />
I. Introduction<br />
In 1923, within several months of each other, Johannes Nicolaus Brønsted (Denmark) and Thomas<br />
Martin Lowry (England) published essentially the same theory about how acids and bases behave.<br />
Since they came to their conclusions independently of each other, both names have been used for<br />
the theory name.<br />
II. The Acid Base Theory<br />
Using the words of Brønsted:<br />
". . . acids and bases are substances that are capable of splitting off or taking up hydrogen ions,<br />
respectively."<br />
Or an acid-base reaction consists of the transfer of a proton from an acid to a base. KEEP THIS<br />
THOUGHT IN MIND!!<br />
Here is a more recent way to say the same thing:<br />
An acid is a substance from which a proton can be removed.<br />
A base is a substance that can remove a proton from an acid.<br />
Remember: proton, hydrogen ion and H + all mean the same thing<br />
Very common in the chemistry world is this definition set:<br />
An acid is a "proton donor."<br />
A base is a "proton acceptor."<br />
In an acid, the hydrogen ion is bonded to the rest of the molecule. It takes energy (sometimes a little,<br />
sometimes a lot) to break that bond. So the acid molecule does not "give" or "donate" the proton, it<br />
has it taken away. In the same sense, you do not donate your wallet to the pickpocket, you have it<br />
removed from you.<br />
The base is a molecule with a built-in "drive" to collect protons. As soon as the base approaches the<br />
acid, it will (if it is strong enough) rip the proton off the acid molecule and add it to itself.<br />
Now this is where all the fun stuff comes in that you get to learn. You see, some bases are stronger<br />
than others, meaning some have a large "desire" for protons, while other bases have a weaker drive.<br />
It's the same way with acids, some have very weak bonds and the proton is easy to pick off, while<br />
other acids have stronger bonds, making it harder to "get the proton."<br />
One important contribution coming from Lowry has to do with the state of the hydrogen ion in solution.<br />
In Brønsted's announcement of the theory, he used H + . Lowry, in his paper (actually a long letter to<br />
the editor) used the H3O + that is commonly used today.<br />
III. Sample Equations written in the Brønsted-Lowry Style<br />
A. Reactions that proceed to a large extent:<br />
154
HCl + H2O ⇌ H3O + + Cl¯<br />
HCl - this is an acid, because it has a proton available to be transferred.<br />
H2O - this is a base, since it gets the proton that the acid lost.<br />
Now, here comes an interesting idea:<br />
H3O + - this is an acid, because it can give a proton.<br />
Cl¯ - this is a base, since it has the capacity to receive a proton.<br />
Notice that each pair (HCl and Cl¯ as well as H2O and H3O + differ by one proton (symbol = H + ). These<br />
pairs are called conjugate pairs.<br />
HNO3 + H2O ⇌ H3O + + NO3¯<br />
The acids are HNO3 and H3O + and the bases are H2O and NO3¯.<br />
Remember that an acid-base reaction is a competition between two bases (think about it!) for a<br />
proton. If the stronger of the two acids and the stronger of the two bases are reactants (appear on the<br />
left side of the equation), the reaction is said to proceed to a large extent.<br />
Here are some more conjugate acid-base pairs to look for:<br />
H2O and OH¯<br />
HCO3¯ and CO3 2¯<br />
H2PO4¯ and HPO4 2¯<br />
HSO4¯ and SO4 2¯<br />
NH4 + and NH3<br />
CH3NH3 + and CH3NH2<br />
HC2H3O2 and C2H3O2¯<br />
B. Reactions that proceed to a small extent:<br />
If the weaker of the two acids and the weaker of the two bases are reactants (appear on the left side<br />
of the equation), the reaction is said to proceed to only a small extent:<br />
HC2H3O2 + H2O ⇌ H3O + + C2H3O2¯<br />
NH3 + H2O ⇌ NH4 + + OH¯<br />
Identify the conjugate acid base pairs in each reaction.<br />
HC 2H 3O 2 and C 2H 3O 2¯<br />
is one conjugate pair.<br />
H 2O and H 3O + is the other.<br />
NH 3 and NH 4<br />
+<br />
is one pair.<br />
H 2O and OH¯ is the other.<br />
Notice that H 2O in the first equation is acting as a base and in the second equation is acting as an acid.<br />
155
IV. Problems with the Theory<br />
This theory works very nicely in all protic solvents (water, ammonia, acetic acid, etc.), but fails to<br />
explain acid base behavior in aprotic solvents such as benzene and dioxane. That job will be left for a<br />
more general theory, such as the Lewis Theory of Acids and Bases.<br />
The Lewis theory of acids and bases<br />
I. Introduction<br />
Lewis gives his definition of an acid and a base:<br />
"We are inclined to think of substances as possessing acid or basic properties, without having a<br />
particular solvent in mind. It seems to me that with complete generality we may say that a basic<br />
substance is one which has a lone pair of electrons which may be used to complete the stable group<br />
of another atom, and that an acid is one which can employ a lone pair from another molecule in<br />
completing the stable group of one of its own atoms."<br />
"In other words, the basic substance furnishes a pair of electrons for a chemical bond, the acid<br />
substance accepts such a pair."<br />
It is important to make two points here:<br />
1. NO hydrogen ion need be involved.<br />
2. NO solvent need be involved.<br />
The Lewis theory of acids and bases is more general than the "one sided" nature of the Bronsted-<br />
Lowry theory. Keep in mind that Bronsted-Lowry, which defines an acid as a proton donor and a base<br />
as a proton acceptor, REQUIRES the presence of a solvent, specifically a protic solvent, of which<br />
water is the usual example. Since almost all chemistry is done in water, the fact that this limits the<br />
Bronsted-Lowry definition is of little practical consequence.<br />
The Lewis definitions of acid and base do not have the constraints that the Bronsted-Lowry theory<br />
does and, as we shall see, many more reactions were seen to be acid base in nature using the Lewis<br />
definition than when using the Bronsted-Lowry definitions.<br />
II. The Acid Base Theory<br />
The modern way to define a Lewis acid and base is a bit more concise than above:<br />
Acid: an electron acceptor.<br />
Base: an electron donor.<br />
A "Lewis acid" is any atom, ion, or molecule which can accept electrons and a "Lewis base" is any<br />
atom, ion, or molecule capable of donating electrons. However, a warning: many textbooks will say<br />
"electron pair" where I have only written "electron." The truth is that it sometimes is an electron pair<br />
and sometimes it is not.<br />
It turns out that it may be more accurate to say that "Lewis acids" are substances which are electrondeficient<br />
(or low electron density) and "Lewis bases" are substances which are electron-rich (or high<br />
electron density).<br />
156
Several categories of substances can be considered Lewis acids:<br />
1. positive ions<br />
2. having less than a full octet in the valence shell<br />
3. polar double bonds (one end)<br />
4. expandable valence shells<br />
Several categories of substances can be considered Lewis bases:<br />
1. negative ions<br />
2. one of more unshared pairs in the valence shell<br />
3. polar double bonds (the other end)<br />
4. the presence of a double bond<br />
Sören Sörenson and the pH scale<br />
I. Short Historical Introduction<br />
In the late 1880's, Svante Arrhenius proposed that acids were substances that delivered hydrogen ion<br />
to the solution. He has also pointed out that the law of mass action could be applied to ionic<br />
reactions, such as an acid dissociating into hydrogen ion and a negatively charged anion.<br />
This idea was followed up by Wilhelm Ostwald, who calculated the dissociation constants (the<br />
modern symbol is Ka) of many weak acids. Ostwald also showed that the size of the constant is a<br />
measure of an acid's strength.<br />
By 1894, the dissociation constant of water (today called Kw) was measured to the modern value of<br />
1 x 10¯14 .<br />
In 1904, H. Friedenthal recommended that the hydrogen ion concentration be used to characterize<br />
solutions. He also pointed out that alkaline (modern word = basic) solutions could also be<br />
characterized this way since the hydroxyl concentration was always 1 x 10¯14 ÷ the hydrogen ion<br />
concentration. Many consider this to be the real introduction of the pH scale.<br />
III. The Introduction of pH<br />
Sörenson defined pH as the negative logarithm of the hydrogen ion concentration.<br />
pH = - log [H + ]<br />
Remember that sometimes H3O + is written, so<br />
pH = - log [H3O + ]<br />
means the same thing.<br />
So let's try a simple problem: The [H + ] in a solution is measured to be 0.010 M. What is the pH?<br />
The solution is pretty straightforward. Plug the [H + ] into the pH definition:<br />
pH = - log 0.010<br />
An alternate way to write this is:<br />
pH = - log 10¯2<br />
Since the log of 10¯2 is -2, we have:<br />
pH = - (- 2)<br />
Which, of course, is 2.<br />
157
Let's discuss significant figures and pH.<br />
Another sample problem: Calculate the pH of a solution in which the [H3O + ] is 1.20 x 10¯3 M.<br />
For the solution, we have:<br />
pH = - log 1.20 x 10¯3<br />
This problem can be done very easily using your calculator. However, be warned about putting<br />
numbers into the calculator.<br />
So you enter (-), log, 1.20, X10 n , (-), 3, enter.<br />
The answer, to the proper number of significant digits is: 2.921.<br />
III. Significant Figures in pH<br />
Here is the example problem: Calculate the pH of a solution where the [H + ] is 0.00100 M. (This could<br />
also be a pOH problem. The point being made is the same.)<br />
OK, you say, that's pretty easy, the answer is 3. After all, 0.00100 is 10¯3 and the negative log of 10¯3<br />
is 3.<br />
You would be graded wrong!! Why? Because the pH is not written to reflect the number of significant<br />
figures in the concentration.<br />
Notice that there are three sig figs in 0.00100. (Hopefully you remember significant figures, since you<br />
probably studied them months ago before getting to acid base stuff. THEY ARE STILL IMPORTANT!)<br />
So, our pH value should also reflect three significant figures.<br />
However, there is a special rule to remember with pH (and pOH) values. The whole number portion<br />
DOES NOT COUNT when figuring out how many digits to write down.<br />
Let's phrase that another way: in a pH (and a pOH), the only place where significant figures are<br />
contained is in the decimal portion.<br />
So, the correct answer to the above problem is 3.000. Three sig figs and they are all in the decimal<br />
portion, NOT (I repeat NOT) in the whole number portion.<br />
Practice Problems<br />
Convert each hydrogen ion concentration into a pH. Identify each as an acidic pH or a basic pH.<br />
1. 0.0015<br />
2.82<br />
2. 5.0 x 10¯9<br />
3. 1.0<br />
4. 3.27 x 10¯4<br />
8.30<br />
0.00<br />
3.485<br />
5. 1.00 x 10¯12<br />
6. 0.00010<br />
12.000<br />
4.00<br />
158
1. 2.82<br />
2. 8.30<br />
3. 0.00<br />
4. 3.485<br />
5. 12.000<br />
6. 4.00<br />
Sörenson also just mentions the reverse direction. That is, suppose you know the pH and you want to<br />
get to the hydrogen ion concentration ([H + ])?<br />
Here is the equation for that:<br />
[H + ] = 10¯pH<br />
That's right, ten to the minus pH gets you back to the [H + ] (called the hydrogen ion concentration).<br />
This is actually pretty easy to do with the calculator. Here's the sample problem: calculate the [H + ]<br />
from a pH of 2.45.<br />
This problem can be done very easily using your calculator. However, be warned about putting<br />
numbers into the calculator.<br />
So you enter 2nd, 10 x , (-), 2.45, enter.<br />
The answer, to the proper number of significant digits is: .00355.<br />
The pH of an acidic pond is 5. What is the hydrogen ion concentration (moles per liter)?<br />
The answer is:<br />
pH = -log (hydrogen ion concentration)<br />
The answer was .00001. Thus, 5 = -log (.00001).<br />
We'll take the formula that you started with (pH = -log([H+])) and work to the answer (solve for [H+]).<br />
pH = - log ([H+]) Given.<br />
pH = log ([H+] (-1) ) Since logarithms are like exponents, when you multiply a log by<br />
something, you can just move it to the inside of log as an exponent.<br />
10 pH = 10 log ([H+] (-1)) Take each side to tenth power.<br />
10 pH = [H+] (-1) Since "log" is just another notation for "log base 10", when you<br />
raise a log to the tenth power, the log cancels out.<br />
[H+] = 10 (-pH)<br />
Take the reciprocal of both sides.<br />
That is the general form. To answer the specific question,<br />
5 = - log ([H+])<br />
5 = log ([H+] (-1) )<br />
10 5 = [H+] (-1)<br />
10 (-5) = [H+]<br />
[H+]<br />
= .00001 mol/L<br />
159
On your calculator you would input 10, ^, (-), 5 and you would get 0.00001.<br />
This is also the way to find the amount of OH + that are present in a base.<br />
To find the pH: -log(concentration)<br />
To find the concentration: 10 -pH<br />
Define these terms:<br />
pH scale<br />
Hydronium ion<br />
Arrhenius acid/base<br />
Lewis acid/base<br />
Bronsted-Lowry acid/base<br />
Strong acid/base<br />
Weak acid/base<br />
Neutralization reaction<br />
Acid base reaction, taking hydrogens adding hydroxides to get water<br />
Titration<br />
160
The Learning Goal for this assignment is:<br />
1. Oxidation Numbers<br />
Redox Reactions<br />
Oxidation and reduction<br />
Every atom, ion or polyatomic ion has a formal oxidation number associated with it. This value<br />
compares the number of protons in an atom (positive charge) and the number of electrons assigned<br />
to that atom (negative charge).<br />
In many cases, the oxidation number reflects the actual charge on the atom, but there are many<br />
cases where it does not. Think of oxidation numbers as a bookkeeping exercise simply to keep track<br />
of where electrons go.<br />
2. Reduction<br />
Reduction means what it says: the oxidation number is reduced in reduction.<br />
This is accomplished by adding electrons. The electrons, being negative, reduce the overall oxidation<br />
number of the atom receiving the electrons.<br />
3. Oxidation<br />
Oxidation is the reverse process: the oxidation number of an atom is increased during oxidation.<br />
This is done by removing electrons. The electrons, being negative, make the atom that lost them<br />
more positive.<br />
I use this mnemonic to help me remember which is which: LEO the lion says GER.<br />
LEO = Loss of Electrons is Oxidation<br />
GER = Gain of Electrons is Reduction<br />
Another well-known mnemonic is this: OIL RIG<br />
OIL = Oxidation Is Loss (of Electrons)<br />
RIG = Reduction Is Gain (of Electrons)<br />
Another way is to simply remember that reduction is to reduce the oxidation number. Therefore,<br />
oxidation must increase the value.<br />
4. Reduction-Oxidation Reactions<br />
There are many chemical reactions in which one substance gets reduced in oxidation number<br />
(reduction) while another participating substance gets increased in oxidation number (oxidation).<br />
Such a reaction is called called a REDOX reaction. The RED, of course, comes from REDuction and<br />
OX from OXidation. However, it is pronounced re-dox and not red-ox.<br />
Here is a simple example of a redox reaction:<br />
161
Ag+ + Cu ---> Ag + Cu2+ Ag+ -> Ag reduction Cu-> Cu2+<br />
I have deliberately not balanced it and I have also written it in net ionic form. I have found that kids<br />
studying redox get confused by net ionic form and how to change a full equation into net ionic form.<br />
Redox equations need to be balanced but, except for the simplest ones, it cannot be done by<br />
inspection (also called trial and error). I take that back, complex ones can be done by trial and error. It<br />
typically takes quite a bit of work, especially when compared to how long it takes when the proper<br />
technique is used.<br />
There is a technique used to balance redox reactions. It is called "balancing by half-reactions." The<br />
basic plan will be to split the full equation into two simpler parts (called half-reactions), balance them<br />
following several standard steps, then recombine the balanced half-reactions into the final answer.<br />
This is another technique called the "ion-electron method." I plan to ignore it.<br />
Notes:<br />
Oxidation agent is reducing the oxidation number. And a reduction agent does the opposite<br />
5. Some Definitions<br />
Oxidizing Agent - that substance which oxidizes somebody else. It is reduced in the process.<br />
Reducing Agent - that substance which reduces somebody else. It is oxidized in the process.<br />
It helps me to remember these definitions by the opposite nature of what happens. By that, I mean<br />
the oxidizing agent gets reduced and the reducing agent gets oxidized.<br />
6. Rules for Assigning Oxidation Numbers<br />
The Oxidation Number of an element corresponds to the number of electrons, e - , that an atom loses,<br />
gains, or appears to use when joining with other atoms in compounds. In determining the Oxidation<br />
Number of an atom, there are seven guidelines to follow:<br />
1. The Oxidation Number of an individual atom is 0. This includes diatomic elements such as<br />
O2 or others like P4 and S8<br />
2. The total Oxidation Number of all atoms in: a neutral species is 0 and in an ion is equal to the<br />
ion charge.<br />
3. Group 1 metals have an Oxidation Number of +1 and Group 2 an Oxidation Number of +2<br />
4. The Oxidation Number of fluorine is -1 in compounds<br />
5. Hydrogen generally has an Oxidation Number of +1 in compounds, except hydrides<br />
6. Oxygen generally has an Oxidation Number of -2 in compounds, except peroxides<br />
7. In binary metal compounds, Group 17 elements have an Oxidation Number of -1, Group 16 of -<br />
2, and Group 15 of -3.<br />
Note: The sum of the Oxidation Number s is equal to zero for neutral compounds and equal to the<br />
charge for polyatomic ion species.<br />
Now, some examples:<br />
1. What is the oxidation number of Cl in HCl?<br />
Since H = +1, the Cl must be -1 (minus one).<br />
162
2. What is the oxidation number of Na in Na2O?<br />
Since O = -2, the two Na must each be +1.<br />
3. What is the oxidation number of Cl in ClO¯?<br />
The O is -2, but since a -1 must be left over, then the Cl is +1.<br />
4. What is the oxidation number for each element in KMnO4?<br />
K = +1 because KCl exists. We know the Cl = -1 because HCl exists.<br />
O = -2 by definition<br />
Mn = +7. There are 4 oxygens for a total of -8, K is +1, so Mn must be the rest.<br />
5. What is the oxidation number of S in SO4 2¯<br />
O = -2. There are four oxygens for -8 total. Since -2 must be left over, the S must = +6.<br />
Please note that, if there is no charge indicated on a formula, the total charge is taken to be zero.<br />
Practice Problems<br />
Find oxidation numbers<br />
1. N in NO3¯<br />
2. C in CO3 2¯<br />
3. Cr in CrO4 2¯<br />
4. Cr in Cr2O7 2¯<br />
5. Fe in Fe2O3<br />
6. Pb in PbOH +<br />
7. V in VO2 +<br />
8. V in VO 2+<br />
9. Mn in MnO4¯<br />
5+<br />
4+<br />
6+<br />
6+<br />
3+<br />
2+<br />
5+<br />
4+<br />
7+<br />
10. Mn in MnO4 2¯<br />
6+<br />
Notes:<br />
The way to find the number is doing the first rule left to right if there is none on the left then start the one on the<br />
left<br />
163
7. Half Reactions<br />
A half-reaction is simply one which shows either reduction OR oxidation, but not both. Here is the<br />
example redox reaction used in a different file:<br />
Ag + + Cu → Ag + Cu 2+<br />
It has BOTH a reduction and an oxidation in it. That is why we call it a redox reaction, from REDuction<br />
and OXidation.<br />
What you must be able to do is look at a redox reaction and separate out the two half-reactions in it.<br />
To do that, identify the atoms which get reduced and get oxidized. Here are the two half-reactions<br />
from the above example:<br />
Ag+ → Ag<br />
Cu → Cu 2+<br />
The silver is being reduced, its oxidation number going from +1 to zero. The copper's oxidation<br />
number went from zero to +2, so it was oxidized in the reaction. In order to figure out the halfreactions,<br />
you MUST be able to calculate the oxidation number of an atom.<br />
Keep in mind that a half-reaction shows only one of the two behaviors we are studying. A single halfreaction<br />
will show ONLY reduction or ONLY oxidation, never both in the same equation.<br />
Also, notice that the reaction is read from left to right to determine if it is reduction or oxidation. If you<br />
read the reaction in the opposite direction (from right to left) it then becomes the other of our two<br />
choices (reduction or oxidation). For example, the silver half-reaction above is a reduction, but in the<br />
reverse direction it is an oxidation, going from zero on the right to +1 on the left.<br />
There will be times when you want to switch a half-reaction from one of the two types to the other. In<br />
that case, rewrite the entire equation and swap sides for everything involved. If I needed the silver<br />
half-reaction to be oxidation, I'd write Ag → Ag+ rather than just doing it mentally.<br />
The next step is that both half-reactions must be balanced. However, there is a twist. When you<br />
learned about balancing equation, you made equal the number of atoms of each element on each<br />
side of the arrow. That still applies, but there is one more thing: the total amount of charge on each<br />
side of the half-reaction MUST be the same.<br />
When you look at the two half-reactions above, you will see they are already balanced for atoms with<br />
one Ag on each side and one Cu on each side. So, all we need to do is balance the charge. To do<br />
this you add electrons to the more positive side. You add enough to make the total charge on each<br />
side become EQUAL.<br />
To the silver half-reaction, we add one electron:<br />
To the copper half-reaction, we add two electrons:<br />
Ag+ + e¯ ---> Ag<br />
Cu ---> Cu 2+ + 2e¯<br />
164
One point of concern: notice that each half-reaction wound up with a total charge of zero on each<br />
side. This is not always the case. You need to strive to get the total charge on each side EQUAL, not<br />
zero.<br />
One more point to make before wrapping this up. A half-reaction is a "fake" chemical reaction. It's just<br />
a bookkeeping exercise. Half-reactions NEVER occur alone. If a reduction half-reaction is actually<br />
happening (say in a beaker in front of you), then an oxidation reaction is also occurring. The two halfreactions<br />
can be in separate containers, but they do have to have some type of "chemical<br />
connection" between them.<br />
Half-Reaction Practice Problems<br />
Balance each half-reaction for atoms and charge:<br />
1) Cl2 → Cl¯<br />
2) Sn → Sn 2+<br />
2+<br />
0 1-<br />
3) Fe 2+ → Fe 3+<br />
4) I3¯ → I¯<br />
3+<br />
Reduction<br />
Oxidation<br />
Oxidation<br />
5) ICl2¯ → I¯ (I'm being mean on this one. Hint: the iodine is the only thing reduced or oxidized.)<br />
Separate each of these redox reactions into their two half-reactions (but do not balance):<br />
6) Sn + NO3¯ →SnO2 + NO2<br />
7) HClO + Co →Cl2 + Co 2+<br />
8) NO2 →NO3¯ + NO<br />
Here are the two half-reactions to be combined:<br />
Here is the rule to follow:<br />
Ag+ + e¯ → Ag<br />
Cu → Cu 2+ + 2e¯<br />
the total electrons MUST cancel when the two half-reactions are added.<br />
Another way to say it:<br />
the number of electrons in each half-reaction MUST be equal when the two half-reactions are<br />
added.<br />
What that means is that one (or both) equations must be multiplied through by a factor. The value of<br />
the factor is selected so as to make the number of electrons equal.<br />
In our example problem, the top reaction (the one with silver) must be multiplied by two, like this:<br />
2Ag+ + 2e¯ → 2Ag<br />
165
Notice that each separate substance is increased by the factor amount. Occasionally, a student will<br />
multiply ONLY the electrons by the factor. That is incorrect.<br />
When the two half-reactions are added, we get:<br />
2Ag+ + 2e¯ + Cu → 2Ag + Cu 2+ + 2e¯<br />
With two electrons on each side, they may be canceled, resulting in:<br />
2Ag+ + Cu → 2Ag + Cu 2+<br />
This is the correct answer. Notice that there are two silvers on each side and one copper. Notice also<br />
that the total charge on each side is +2. It is balanced for both atoms and charge. Sometimes, I am<br />
asked if the order matters, if the Cu could be first on the left-hand side. The answer is that the order<br />
does not matter. There happen to be certain styles about where particular substances are put in the<br />
final answer, but these are only styles. They do not affect the chemical correctness of the answer.<br />
Notes:<br />
Practice Problems<br />
Separate into half-reactions, balance them and then recombine.<br />
1) Sn + Cl2 ---> Sn 2+ + Cl¯<br />
2) Fe 2+ + I3¯ ---> Fe 3+ + I¯<br />
1. N in NO 3¯<br />
The O is -2 and three of them makes -6. Since -1 is left over, the N must be +5<br />
2. C in CO 3<br />
2¯<br />
The O is -2 and three of them makes -6. Since -2 is left over, the C must be +4<br />
3. Cr in CrO 4<br />
2¯<br />
The O is -2 and four of them makes -8. Since -2 is left over, the Cr must be +6<br />
4. Cr in Cr 2 O 7<br />
2¯<br />
The O is -2 and seven of them makes -14. Since -2 is left over, the two Cr must be +12<br />
What follows is an important point. Each Cr is +6. Each one. We do not speak of Cr 2 being +12. Each Cr atom is considered individually.<br />
5. Fe in Fe 2 O 3<br />
The O is -2 and three of them makes -6. Each Fe must then be +3<br />
6. +2 9. +7<br />
7. +5 10. +6<br />
8. +4<br />
1) Cl 2 + 2e¯ →2Cl¯ 5) ICl 2¯<br />
+ 2e¯ →I¯ + 2Cl¯<br />
2) Sn →Sn 2+ + 2e¯ 6) Sn →SnO 2 and NO 3¯<br />
→NO 2<br />
3) Fe 2+ →Fe 3+ + e¯ 7) HClO →Cl 2 and Co ---> Co 2+<br />
4) I 3¯ → 3I¯ + 2e¯ 8) NO 2 →NO 3¯<br />
and NO 2 →NO<br />
Note that in this last redox equation the NO 2 (actually just the N) is both being reduced AND being oxidized. In the first half-reaction, the N goes from +4<br />
to +5 (oxidation) and in the second, the N goes from +4 to +2 (reduction).<br />
By the way, we could flip the reaction so that NO 3¯<br />
and NO are reacting together to produce only one product, the NO 2 . In that case, the two halfreactions<br />
would be reversed.<br />
166