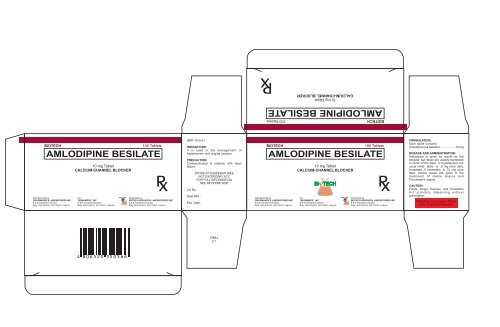
Jerly_Portfolio_Biotech Medicine Box
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
P x<br />
BIOTECH<br />
100 Tablets<br />
AMLODIPINE BESILATE<br />
10 mg Tablet<br />
CALCIUM-CHANNEL BLOCKER<br />
BIOTECH<br />
AMLODIPINE BESILATE<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES INC.<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
10 mg Tablet<br />
CALCIUM-CHANNEL BLOCKER<br />
For:<br />
TERRAMEDIC, INC.<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
DRP-1615-01<br />
100 Tablets INDICATIONS:<br />
BIOTECH<br />
100 Tablets<br />
It is used in the management of<br />
hypertension and angina pectoris.<br />
Distributed by:<br />
BIOTECH RESEARCH LABORATORIES INC.<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
PRECAUTION:<br />
Contraindicated to patients with heart<br />
failure.<br />
Lot No.:<br />
STORE AT TEMPERATURES<br />
NOT EXCEEDING 30°C<br />
FOR FULL INFORMATION<br />
SEE REVERSE SIDE<br />
Date Mfd.:<br />
Exp. Date:<br />
AMLODIPINE BESILATE<br />
10 mg Tablet<br />
CALCIUM-CHANNEL BLOCKER<br />
P x P x<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES INC.<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
For:<br />
TERRAMEDIC, INC.<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
Distributed by:<br />
BIOTECH RESEARCH LABORATORIES INC.<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
FORMULATION:<br />
Each tablet contains:<br />
Amlodipine (as besilate) .................. 10 mg<br />
DOSAGE AND ADMINISTRATION:<br />
Amlodipine is given by mouth as the<br />
besilate, but doses are usually expressed<br />
in terms of the base. In hypertension the<br />
usual initial dose is 5 mg once daily,<br />
increased, if necessary, to 10 mg once<br />
daily. Similar doses are given in the<br />
treatment of stable angina and<br />
Prinzmetal’s angina.<br />
CAUTION:<br />
Foods, Drugs, Devices, and Cosmetics<br />
Act prohibits dispensing without<br />
prescription.<br />
Retail Price not to exceed P38.50<br />
under Drug Price Regulation<br />
DMLI<br />
2-1<br />
4 806520 350566
DR-XY9723<br />
INDICATIONS:<br />
For the treatment of respiratory tract,<br />
genitourinary tract, skin and soft tissue<br />
infection, gastrointestinal infection.<br />
PRECAUTION:<br />
Contraindicated to patients with a history<br />
of penicillin allergy.<br />
CAUTION:<br />
Foods, Drugs, Devices and Cosmetics Act<br />
prohibits dispensing without prescription.<br />
STORE AT TEMPERATURES NOT<br />
EXCEEDING 30ºC<br />
SHAKE WELL BEFORE USE<br />
Lot No.:<br />
Mfg. Date:<br />
Exp. Date:<br />
BIOTECH<br />
BIOMOX<br />
100 mg/mL<br />
POWDER FOR SUSPENSION<br />
(ORAL DROPS)<br />
ANTIBACTERIAL<br />
10 mL<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES, INC.<br />
E&E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
For:<br />
BIOTECH RESEARCH LABORATORIES, INC.<br />
E&E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
FORMULATION:<br />
Each mL reconstituted suspension<br />
contains:<br />
Amoxicillin (as Trihydrate) ........ 100 mg<br />
DIRECTION FOR RECONSTITUTION:<br />
To make up 10 mL, add 8 mL of distilled<br />
water and shake well until all the powder<br />
are evenly suspended. The reconstituted<br />
suspension is stable for one week (7 days)<br />
at temperatures not exceeding 30ºC and<br />
two weeks (14 days) under refrigeration (2-<br />
8ºC).<br />
DOSAGE:<br />
Infants: 20-40 mg/kg body weight/day<br />
divided into three equal doses<br />
Below 1 month: 0.25mL<br />
1 - 6 months: 0.5 mL<br />
7 - 12 months: 0.75 mL<br />
1 - 3 years old: 1 mL<br />
Every 8 hours or as prescribed by the<br />
physician.<br />
BIOTECH 10 mL<br />
BIOMOX<br />
100 mg/ mL<br />
POWDER FOR SUSPENSION<br />
(ORAL DROPS)<br />
ANTIBACTERIAL<br />
BIOTECH<br />
10 mL<br />
BIOMOX<br />
100 mg/mL<br />
POWDER FOR SUSPENSION<br />
(ORAL DROPS)<br />
ANTIBACTERIAL<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES, INC.<br />
E&E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
For:<br />
BIOTECH RESEARCH LABORATORIES, INC.<br />
E&E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
DR-XY9723<br />
INDICATIONS:<br />
For the treatment of<br />
r e s p i r a t o r y t r a c t ,<br />
genitourinary tract,<br />
skin and soft tissue<br />
i n f e c t i o n ,<br />
g a s t r o i n t e s t i n a l<br />
infection.<br />
PRECAUTION:<br />
Contraindicated to<br />
patients with a history<br />
of penicillin allergy.<br />
CAUTION:<br />
Foods, Drugs, Devices<br />
and Cosmetics Act<br />
prohibits dispensing<br />
without prescription.<br />
STORE AT<br />
TEMPERATURES<br />
NOT EXCEEDING<br />
30ºC<br />
FOR FULL<br />
INFORMATION<br />
SEE REVERSE SIDE<br />
SHAKE WELL<br />
BEFORE USE<br />
Lot No.:<br />
Mfg. Date:<br />
BIOTECH<br />
BIOMOX<br />
100 mg/mL<br />
POWDER FOR SUSPENSION<br />
(ORAL DROPS)<br />
ANTIBACTERIAL<br />
P x<br />
10 mL<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES, INC.<br />
E&E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
For:<br />
BIOTECH RESEARCH LABORATORIES, INC.<br />
E&E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
FORMULATION:<br />
Each mL reconstituted<br />
suspension contains:<br />
A m o x i c i l l i n ( a s<br />
Trihydrate) ..... 100 mg<br />
DIRECTION FOR<br />
RECONSTITUTION:<br />
To make up 10 mL, add<br />
8 mL of distilled water<br />
and shake well until all<br />
the powder are evenly<br />
s u s p e n d e d . T h e<br />
r e c o n s t i t u t e d<br />
suspension is stable<br />
for one week (7 days)<br />
at temperatures not<br />
exceeding 30ºC and<br />
two weeks (14 days)<br />
under refrigeration (2-<br />
8ºC).<br />
DOSAGE:<br />
Infants: 20-40 mg/kg<br />
b o d y w e i g h t / d a y<br />
divided into three<br />
equal doses<br />
Below 1 month:0.25mL<br />
1 - 6 months: 0.5 mL<br />
7 - 12 months: 0.75 mL<br />
1 - 3 years old: 1 mL<br />
Every 8 hours or as<br />
prescribed by the<br />
physician.<br />
Exp. Date:
50mL<br />
Clarithromycin<br />
Klaryz<br />
125 mg / 5mL<br />
Granules for Suspension<br />
ANTIBACTERIAL<br />
DRP - 714 - 01<br />
Direction for Reconstitution:<br />
Slowly add about 26.5 mL of<br />
distilled water. Put cap on and<br />
shake vigorously. This makes<br />
50 mL of suspension. The<br />
reconstituted suspension is stable<br />
for 14 days at temperatures not<br />
exceeding 30ºC.<br />
PRECAUTION:<br />
The liver excretes Clarithromycin.<br />
Therefore, caution should be<br />
exercised in administering the<br />
antibiotic to patients with impaired<br />
hepatic function and to patients<br />
with moderate to severe renal<br />
failure.<br />
CAUTION:<br />
Foods, Drugs, Devices and<br />
C o s m e t i c s A c t p r o h i b i t s<br />
dispensing without prescription.<br />
D o n o t r e f r i g e r a t e t h e<br />
r e c o n s t i t u t e d s u s p e n s i o n ;<br />
S t o r e a t t e m p e r a t u r e s<br />
50mL<br />
Clarithromycin<br />
Klaryz<br />
125 mg / 5mL<br />
Granules for Suspension<br />
ANTIBACTERIAL<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES INC.<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
Distributed by:<br />
FORMULATION:<br />
Each 5 mL (1 teaspoonful)<br />
suspension contains:<br />
Clarithromycin..........125mg<br />
INDICATIONS:<br />
For the treatment of infections<br />
due to susceptible organisms<br />
such as upper and lower<br />
respiratory infections, otitis<br />
media, skin structure infections<br />
a n d o t h e r m y c o b a c t e r i a l<br />
infections.<br />
DOSAGE:<br />
7.5 mg/kg twice a day up to a<br />
maximum dose of 500 mg twice<br />
a day or as prescribed by the<br />
physician.<br />
CONTRAINDICATION:<br />
CLARITHROMYCIN Granules for<br />
Suspension is contraindicated<br />
i n p a t i e n t s w i t h k n o w n<br />
hypersensitivity to macrolide<br />
antibiotic drugs.<br />
SHAKE WELL BEFORE USE<br />
KEEP TIGHTLY CLOSED<br />
FOR FULL INFORMATION<br />
SEE REVERSE SIDE<br />
Lot No.:<br />
50mL<br />
Clarithromycin<br />
Klaryz<br />
125 mg / 5mL<br />
Granules for Suspension<br />
ANTIBACTERIAL<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES INC.<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
Distributed by:<br />
Mfg. Date:<br />
4 800369 002129<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
Exp. Date:<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
DMLI<br />
6-1
10 Capsules<br />
AZITHROMYCIN<br />
AZTROCIN<br />
250 mg Capsule<br />
ANTIBACTERIAL<br />
Manufactured by:<br />
Drugmaker’s Laboratories,Inc.<br />
E & E Industrial Complex<br />
Brgy. San Antonio, San Pedro, Laguna<br />
For:<br />
E & E Industrial Complex<br />
Brgy. San Antonio, San Pedro, Laguna<br />
DMLI<br />
8-1<br />
DR-XY35341<br />
FORMULATION:<br />
Each capsule contains:<br />
Azithromycin (as dihydrate) ............................................................................................................ 250 mg<br />
INDICATIONS:<br />
For the treatment of the upper and lower respiratory tract infections, skin and soft tissue infections and<br />
uncomplicated genital infections.<br />
DOSAGE:<br />
500 mg as a single dose daily for 3 days. Alternatively, an initial dose of 500 mg may be followed<br />
by 250 mg daily for a further 4 days.<br />
PRECAUTION:<br />
Azithromycin should not be used in patients with hepatic impairment.<br />
ADVERSE EFFECTS:<br />
Gastrointestinal disturbances such as abdominal discomfort and cramps, nausea, vomiting, and diarrhea<br />
are fairly common after oral administration.<br />
CAUTION:<br />
Foods, Drugs, Devices and Cosmetics Act prohibits dispensing without prescription.<br />
STORE AT TEMPERATURES NOT EXCEEDING 30ºC<br />
FOR FULL INFORMATION SEE PACKAGE INSERT<br />
Lot No.:<br />
Mfg. Date:<br />
Exp. Date:<br />
4 8 0 0 3 6 9 0 0 2 0 7 5
VONWELT 100 Capsules<br />
FERROUS<br />
SULFATE<br />
325 mg Tablet<br />
ANTI-ANEMIC<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES, INC.<br />
San Pedro, Laguna<br />
For:<br />
PX<br />
INDICATION:<br />
For the prevention and treatment<br />
of anemia due to iron<br />
deficiency.<br />
PRECAUTIONS:<br />
Ferrous sulfate should not be<br />
given to patient receiving blood<br />
transfusions or to patient with<br />
anemias not produced by iron<br />
deficiency unless iron deficiency<br />
is also present. Care should be<br />
taken in patients with iron-storage<br />
or iron-absorption diseases such<br />
a s h a e m o c h r o m a t o s i s ,<br />
haemoglobinopathies, or existing<br />
gastrointestinal diseases such as<br />
inflammatory bowel diseases,<br />
i n t e s t i n a l s t r u c t u r e s a n d<br />
diverticulae.<br />
STORE AT TEMPERATURES<br />
NOT EXCEEDING 30ºC<br />
Magdalena, Laguna<br />
VONWELT<br />
325 mg Tablet<br />
ANTI-ANEMIC<br />
100 Capsules<br />
FERROUS<br />
SULFATE<br />
DMLI<br />
2-1<br />
VONWELT<br />
100 Capsules<br />
FERROUS<br />
SULFATE<br />
325 mg Tablet<br />
ANTI-ANEMIC<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES, INC.<br />
San Pedro, Laguna<br />
For:<br />
Magdalena, Laguna<br />
P X<br />
DR-XY12745<br />
FORMULATION:<br />
Each tablet contains:<br />
F e r r o u s S u l f a t e ( a s<br />
dried)............................. 325 mg<br />
(Equivalent to 65 mg Elemental<br />
Iron)<br />
DOSAGE:<br />
Adult: One tablet daily or as<br />
prescribed by the physician.<br />
CAUTION:<br />
Foods, Drugs, Devices and<br />
C o s m e t i c s A c t p r o h i b i t s<br />
dispensing without prescription.<br />
FOR FULL INFORMATION<br />
SEE REVERSE SIDE<br />
Lot No.:<br />
Mfg. Date:<br />
Exp. Date:<br />
VONWELT<br />
325 mg Tablet<br />
ANTI-ANEMIC<br />
100 Capsules<br />
FERROUS<br />
SULFATE
R /<br />
100 Tablets<br />
CLOPIDOGREL<br />
BISULFATE<br />
CLOVAZ<br />
75 mg Film-Coated Tablet<br />
Antiplatelet<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES, INC.<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
Distributed by:<br />
E & E Industrial Complex<br />
Brgy. San Antonio, San Pedro, Laguna<br />
CLOPIDOGREL<br />
BISULFATE<br />
CLOVAZ<br />
75 mg Film-Coated Tablet<br />
Antiplatelet<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES, INC.<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
Distributed by:<br />
E & E Industrial Complex<br />
Brgy. San Antonio, San Pedro, Laguna<br />
100 Tablets<br />
R/<br />
DRP-2267-01<br />
INDICATION:<br />
It is given prophylactically as an alternative<br />
to Aspirin in patients at risk of<br />
thromboembolic disorders such as<br />
myocardial infarction, peripheral arterial<br />
disease, and stroke.<br />
PRECAUTION:<br />
Clopidogrel is limited to patients at risk of<br />
increased bleeding from trauma, surgery, or<br />
other pathological conditions; ulcer; renal<br />
and hepatic impairment; history of bleeding<br />
or haemostatic disorders and pregnancy.<br />
Lot No.:<br />
Mfg. Date:<br />
Exp. Date:<br />
CLOPIDOGREL<br />
BISULFATE<br />
CLOVAZ<br />
75 mg Film-Coated Tablet<br />
Antiplatelet<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES, INC.<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
Distributed by:<br />
E & E Industrial Complex<br />
Brgy. San Antonio, San Pedro, Laguna<br />
100 Tablets<br />
R/<br />
FORMULATION:<br />
Each Film-Coated Tablet contains:<br />
Clopidogrel bisulfate............75 mg<br />
DOSAGE AND ADMINISTRATION:<br />
It is given by mouth as the bisulfate,<br />
but doses are expressed in terms of<br />
the base. The dose is 75 mg once<br />
daily.<br />
CAUTION:<br />
Foods, Drugs Devices and<br />
Cosmetics Act prohibits dispensing<br />
without prescription.<br />
STORE AT TEMPERATURES NOT<br />
EXCEEDING 30ºC<br />
4 800369 002297
DR-XY7704<br />
INDICATION:<br />
For general infections caused by Gram-positive<br />
a n d G r a m - N e g a t i v e m i c r o o r g a n i s m s .<br />
CONTRAINDICATION:<br />
Contraindicated to patients known to be sensitive<br />
to penicillin V therapy.<br />
CAUTION:<br />
Foods, Drugs, Devices and Cosmetics Act<br />
prohibits dispensing without prescription.<br />
WARNING<br />
This product contains FD&C yellow No.5<br />
(Tartrazine) which may cause allergic reaction<br />
(including bronchial asthma) in certain<br />
susceptible persons.<br />
STORE AT TEMPERATURES<br />
NOT EXCEEDING 30ºC<br />
SHAKE WELL BEFORE USE<br />
60 mL<br />
AMOXICILLIN<br />
TRIHYDRATE<br />
VONWELT<br />
CYCAMIL<br />
250 mg / 5 mL<br />
Powder for Suspension<br />
ANTIBACTERIAL<br />
P x<br />
DRUGMAKER’S LABORATORIES, INC.<br />
Manufactured by:<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
For:<br />
VONWELT, INC.<br />
Brgy. Sabang, Magdalena, Laguna<br />
FORMULATION:<br />
Per 5 mL powder for suspension contains:<br />
Amoxicillin (as Trihydrate) .................. 250 mg<br />
DOSAGE:<br />
Children up to 2 years of age 125 mg or 2.5 mL<br />
( ½ t e a s p o o n ) e v e r y 6 - 8 h o u r s .<br />
3 - 10 years of age 250 mg or 5.0 mL (1<br />
t e a s p o o n f u l ) e v e r y 6 - 8 h o u r s .<br />
Adults: 250 mg to 500 mg every 6 - 8 hours.<br />
(Dosage based on weight: 20 - 40 mg / Kg / BW<br />
/ day divided in 3 equal doses given by the<br />
physician.<br />
DIRECTION FOR RECONSTITUTION:<br />
To make up to 60 mL suspension, add 40 mL of<br />
water, invert bottle and shake well until powder<br />
is uniformly dispersed. The suspension may be<br />
kept at room temperature for one week and two<br />
weeks in a refrigerator.<br />
Lot No.:<br />
Date Mfd.:<br />
Exp. Date:<br />
VONWELT 60 mL<br />
AMOXICILLIN<br />
TRIHYDRATE<br />
CYCAMIL<br />
250 mg / 5 mL<br />
Powder for Suspension<br />
ANTIBACTERIAL<br />
DR-XY7704<br />
INDICATION:<br />
For general infections caused by<br />
Gram-positive and Gram-Negative<br />
microorganisms.<br />
CONTRAINDICATION:<br />
Contraindicated to patients known to<br />
be sensitive to penicillin V therapy.<br />
CAUTION:<br />
Foods, Drugs, Devices and Cosmetics<br />
Act prohibits dispensing without<br />
prescription.<br />
WARNING<br />
This product contains FD&C yellow<br />
No.5 (Tartrazine) which may cause<br />
allergic reaction (including bronchial<br />
asthma) in certain susceptible<br />
persons.<br />
STORE AT TEMPERATURES<br />
NOT EXCEEDING 30ºC<br />
FOR FULL INFORMATION<br />
SEE REVERSE SIDE<br />
VONWELT 60 mL<br />
AMOXICILLIN<br />
TRIHYDRATE<br />
CYCAMIL<br />
250 mg / 5 mL<br />
Powder for Suspension<br />
ANTIBACTERIAL<br />
P X<br />
FORMULATION:<br />
Per 5 mL powder for suspension<br />
contains:<br />
Amoxicillin (as Trihydrate) ... 250 mg<br />
DOSAGE:<br />
Children up to 2 years of age 125 mg<br />
or 2.5 mL (½ teaspoon) every 6 - 8<br />
hours.<br />
3 - 10 years of age 250 mg or 5.0 mL<br />
(1 teaspoonful) every 6 - 8 hours.<br />
Adults: 250 mg to 500 mg every 6 - 8<br />
hours.<br />
(Dosage based on weight: 20 - 40<br />
mg / Kg / BW / day divided in 3 equal<br />
doses given by the physician.<br />
DIRECTION FOR<br />
RECONSTITUTION:<br />
To make up to 60 mL suspension,<br />
add 40 mL of water, invert bottle and<br />
shake well until powder is uniformly<br />
dispersed. The suspension may be<br />
kept at room temperature for one<br />
week and two weeks in a<br />
refrigerator.<br />
VONWELT 60 mL<br />
AMOXICILLIN<br />
TRIHYDRATE<br />
CYCAMIL<br />
250 mg / 5 mL<br />
Powder for Suspension<br />
ANTIBACTERIAL<br />
P X<br />
SHAKE WELL BEFORE USE<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES, INC.<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
For:<br />
VONWELT, INC.<br />
Brgy. Sabang, Magdalena, Laguna<br />
Lot No.:<br />
Date Mfd.:<br />
Exp. Date:<br />
Manufactured by:<br />
DRUGMAKER’S LABORATORIES, INC.<br />
E & E Industrial Complex,<br />
Brgy. San Antonio, San Pedro, Laguna<br />
For:<br />
VONWELT, INC.<br />
Brgy. Sabang, Magdalena, Laguna
VONWELT<br />
Glutathione<br />
250 mg Capsule<br />
Dietary Supplement<br />
30 Capsules<br />
VONWELT<br />
Glutathione<br />
250 mg Capsule<br />
Dietary Supplement<br />
30 Capsules<br />
FR89702<br />
SPECIAL<br />
PRECAUTION:<br />
P r e g n a n c y a n d<br />
lactation.<br />
VONWELT<br />
Glutathione<br />
250 mg Capsule<br />
Dietary Supplement<br />
30 Capsules<br />
FORMULATION:<br />
E a c h c a p s u l e<br />
contains:<br />
G l u t a t h i o n e<br />
................. 250 mg<br />
A s c o r b i c A c i d<br />
................... 30 mg<br />
DOSAGE:<br />
1 - 2 capsules daily<br />
KEEP IN A COOL<br />
DRY PLACE<br />
AVOID EXPOSURE<br />
TO SUNLIGHT<br />
NO APPROVED THERAPEUTIC CLAIMS<br />
Manufactured by:<br />
Lot No.:<br />
Date Mfd.:<br />
NO APPROVED THERAPEUTIC CLAIMS<br />
Manufactured by:<br />
Magdalena, Laguna<br />
Expiry Date:<br />
Magdalena, Laguna<br />
DMLI<br />
6-1
Food Supplement<br />
Malunggay<br />
30 Capsules<br />
Food Supplement<br />
Malunggay<br />
30 Capsules<br />
FR No.: 82563<br />
Food Supplement<br />
Malunggay<br />
30 Capsules<br />
FORMULATION:<br />
E a c h c a p s u l e<br />
contains:<br />
Malunggay leaves<br />
(Moringa Oleifera)<br />
..................250 mg<br />
RECOMMENDED<br />
USE:<br />
One to two times<br />
daily with meal<br />
NO APPROVED THERAPEUTIC CLAIMS<br />
Manufactured by:<br />
Lot No.:<br />
NO APPROVED THERAPEUTIC CLAIMS<br />
Manufactured by:<br />
KEEP IN A COOL<br />
DRY PLACE<br />
Date Mfd.:<br />
Magdalena, Laguna<br />
Expiry Date:<br />
Magdalena, Laguna<br />
DMLI<br />
6-1
VONWELT<br />
Ampalaya<br />
350 mg Capsule<br />
Food Supplement<br />
30 Capsules<br />
VONWELT<br />
Ampalaya<br />
350 mg Capsule<br />
Food Supplement<br />
30 Capsules<br />
FR-38137<br />
DOSAGE:<br />
A d u l t : 2 - 3<br />
Capsules a day<br />
with meal.<br />
KEEP IN A COOL<br />
DRY PLACE<br />
VONWELT<br />
Ampalaya<br />
350 mg Capsule<br />
Food Supplement<br />
30 Capsules<br />
FORMULATION:<br />
N U T R I T I O N<br />
FACTS:<br />
A m o u n t p e r<br />
1,000 mg<br />
Calcium ...... 65 mg<br />
Iron ............ 88 mg<br />
Protein ....... 18.0%<br />
Fiber .......... 17.5%<br />
Cholesterol ........ 0<br />
Fat ..................... 0<br />
Calories ............. 0<br />
NO APPROVED THERAPEUTIC CLAIMS<br />
Manufactured by:<br />
Magdalena, Laguna<br />
Lot No.:<br />
Date Mfd.:<br />
Expiry Date:<br />
NO APPROVED THERAPEUTIC CLAIMS<br />
Manufactured by:<br />
Magdalena, Laguna<br />
DMLI<br />
6-1