polymery 2010 - Institute of Macromolecular Chemistry - Akademie ...
polymery 2010 - Institute of Macromolecular Chemistry - Akademie ...
polymery 2010 - Institute of Macromolecular Chemistry - Akademie ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
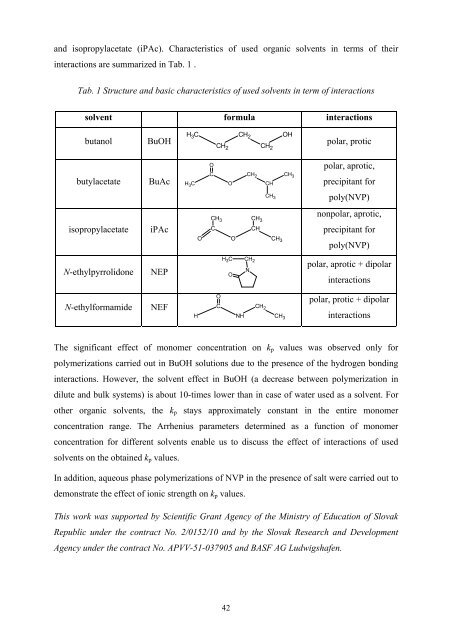
and isopropylacetate (iPAc). Characteristics <strong>of</strong> used organic solvents in terms <strong>of</strong> their<br />
interactions are summarized in Tab. 1 .<br />
Tab. 1 Structure and basic characteristics <strong>of</strong> used solvents in term <strong>of</strong> interactions<br />
solvent formula interactions<br />
butanol BuOH<br />
C<br />
H 3<br />
butylacetate BuAc C<br />
H 3<br />
isopropylacetate iPAc<br />
O<br />
O<br />
C<br />
C<br />
CH 2<br />
CH 3<br />
O<br />
42<br />
O<br />
CH 2<br />
N-ethylpyrrolidone NEP N<br />
N-ethylformamide NEF<br />
H<br />
O<br />
C<br />
C<br />
H 3<br />
O<br />
NH<br />
CH 2<br />
CH 2<br />
CH 3<br />
CH<br />
CH 2<br />
CH 2<br />
CH<br />
CH 3<br />
CH 3<br />
CH 3<br />
OH<br />
CH 3<br />
polar, protic<br />
polar, aprotic,<br />
precipitant for<br />
poly(NVP)<br />
nonpolar, aprotic,<br />
precipitant for<br />
poly(NVP)<br />
polar, aprotic + dipolar<br />
interactions<br />
polar, protic + dipolar<br />
interactions<br />
The significant effect <strong>of</strong> monomer concentration on kp values was observed only for<br />
polymerizations carried out in BuOH solutions due to the presence <strong>of</strong> the hydrogen bonding<br />
interactions. However, the solvent effect in BuOH (a decrease between polymerization in<br />
dilute and bulk systems) is about 10-times lower than in case <strong>of</strong> water used as a solvent. For<br />
other organic solvents, the kp stays approximately constant in the entire monomer<br />
concentration range. The Arrhenius parameters determined as a function <strong>of</strong> monomer<br />
concentration for different solvents enable us to discuss the effect <strong>of</strong> interactions <strong>of</strong> used<br />
solvents on the obtained kp values.<br />
In addition, aqueous phase polymerizations <strong>of</strong> NVP in the presence <strong>of</strong> salt were carried out to<br />
demonstrate the effect <strong>of</strong> ionic strength on kp values.<br />
This work was supported by Scientific Grant Agency <strong>of</strong> the Ministry <strong>of</strong> Education <strong>of</strong> Slovak<br />
Republic under the contract No. 2/0152/10 and by the Slovak Research and Development<br />
Agency under the contract No. APVV-51-037905 and BASF AG Ludwigshafen.