Modelling and simulation of ice/snow melting
Modelling and simulation of ice/snow melting
Modelling and simulation of ice/snow melting
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Melting point depression<br />
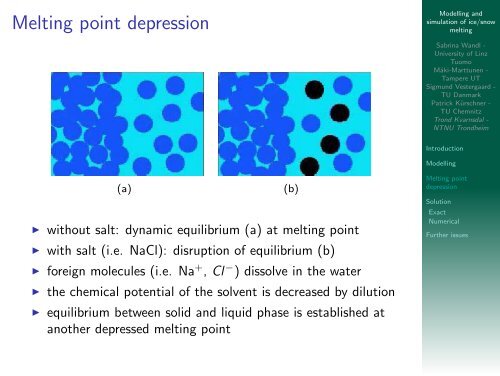
(a) (b)<br />
◮ without salt: dynamic equilibrium (a) at <strong>melting</strong> point<br />
◮ with salt (i.e. NaCl): disruption <strong>of</strong> equilibrium (b)<br />
◮ foreign molecules (i.e. Na + , Cl − ) dissolve in the water<br />
◮ the chemical potential <strong>of</strong> the solvent is decreased by dilution<br />
◮ equilibrium between solid <strong>and</strong> liquid phase is established at<br />
another depressed <strong>melting</strong> point<br />
<strong>Modelling</strong> <strong>and</strong><br />
<strong>simulation</strong> <strong>of</strong> <strong>ice</strong>/<strong>snow</strong><br />
<strong>melting</strong><br />
Sabrina W<strong>and</strong>l -<br />
University <strong>of</strong> Linz<br />
Tuomo<br />
Mäki-Marttunen -<br />
Tampere UT<br />
Sigmund Vestergaard -<br />
TU Danmark<br />
Patrick Kürschner -<br />
TU Chemnitz<br />
Trond Kvarnsdal -<br />
NTNU Trondheim<br />
Introduction<br />
<strong>Modelling</strong><br />
Melting point<br />
depression<br />
Solution<br />
Exact<br />
Numerical<br />
Further issues