on air - Messer
on air - Messer
on air - Messer
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
10 : Cover story<br />
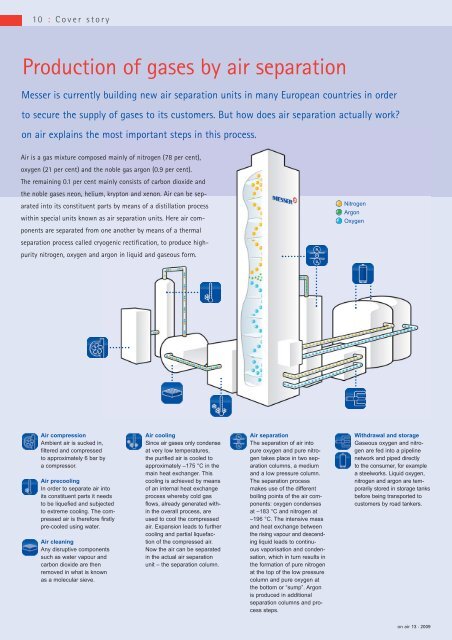
Producti<strong>on</strong> of gases by <strong>air</strong> separati<strong>on</strong><br />
<strong>Messer</strong> is currently building new <strong>air</strong> separati<strong>on</strong> units in many European countries in order<br />
to secure the supply of gases to its customers. But how does <strong>air</strong> separati<strong>on</strong> actually work?<br />
<strong>on</strong> <strong>air</strong> explains the most important steps in this process.<br />
Air is a gas mixture composed mainly of nitrogen (78 per cent),<br />
oxygen (21 per cent) and the noble gas arg<strong>on</strong> (0.9 per cent).<br />
The remaining 0.1 per cent mainly c<strong>on</strong>sists of carb<strong>on</strong> dioxide and<br />
the noble gases ne<strong>on</strong>, helium, krypt<strong>on</strong> and xen<strong>on</strong>. Air can be sep -<br />
arated into its c<strong>on</strong>stituent parts by means of a distillati<strong>on</strong> process<br />
within special units known as <strong>air</strong> separati<strong>on</strong> units. Here <strong>air</strong> com -<br />
p<strong>on</strong>ents are separated from <strong>on</strong>e another by means of a thermal<br />
sep arati<strong>on</strong> process called cryogenic rectificati<strong>on</strong>, to produce highpurity<br />
nitrogen, oxygen and arg<strong>on</strong> in liquid and gaseous form.<br />
Air compressi<strong>on</strong><br />
Ambient <strong>air</strong> is sucked in,<br />
filtered and compressed<br />
to approximately 6 bar by<br />
a compressor.<br />
Air precooling<br />
In order to separate <strong>air</strong> into<br />
its c<strong>on</strong>stituent parts it needs<br />
to be liquefied and subjected<br />
to extreme cooling. The compressed<br />
<strong>air</strong> is therefore firstly<br />
pre-cooled using water.<br />
Air cleaning<br />
Any disruptive comp<strong>on</strong>ents<br />
such as water vapour and<br />
carb<strong>on</strong> dioxide are then<br />
removed in what is known<br />
as a molecular sieve.<br />
Air cooling<br />
Since <strong>air</strong> gases <strong>on</strong>ly c<strong>on</strong>dense<br />
at very low temperatures,<br />
the purified <strong>air</strong> is cooled to<br />
approximately –175 °C in the<br />
main heat exchanger. This<br />
cooling is achieved by means<br />
of an internal heat exchange<br />
process whereby cold gas<br />
flows, already generated with -<br />
in the overall process, are<br />
used to cool the compressed<br />
<strong>air</strong>. Expansi<strong>on</strong> leads to further<br />
cooling and partial liquefac -<br />
ti<strong>on</strong> of the compressed <strong>air</strong>.<br />
Now the <strong>air</strong> can be separated<br />
in the actual <strong>air</strong> separati<strong>on</strong><br />
unit – the separati<strong>on</strong> column.<br />
Air separati<strong>on</strong><br />
The separati<strong>on</strong> of <strong>air</strong> into<br />
pure oxygen and pure nitrogen<br />
takes place in two sep -<br />
arati<strong>on</strong> columns, a medium<br />
and a low pressure column.<br />
The separati<strong>on</strong> process<br />
makes use of the different<br />
boiling points of the <strong>air</strong> comp<strong>on</strong>ents:<br />
oxygen c<strong>on</strong>denses<br />
at –183 °C and nitrogen at<br />
–196 °C. The intensive mass<br />
and heat exchange between<br />
the rising vapour and de scend -<br />
ing liquid leads to c<strong>on</strong>tinuous<br />
vaporisati<strong>on</strong> and c<strong>on</strong>densati<strong>on</strong>,<br />
which in turn results in<br />
the formati<strong>on</strong> of pure nitrogen<br />
at the top of the low pressure<br />
column and pure oxygen at<br />
the bottom or “sump”. Arg<strong>on</strong><br />
is produced in additi<strong>on</strong>al<br />
separati<strong>on</strong> columns and process<br />
steps.<br />
Nitrogen<br />
Arg<strong>on</strong><br />
Oxygen<br />
Withdrawal and storage<br />
Gaseous oxygen and nitrogen<br />
are fed into a pipeline<br />
network and piped directly<br />
to the c<strong>on</strong>sumer, for example<br />
a steelworks. Liquid oxygen,<br />
nitrogen and arg<strong>on</strong> are tem -<br />
po rarily stored in storage tanks<br />
before being transported to<br />
customers by road tankers.<br />
<strong>on</strong> <strong>air</strong> 13 · 2009