Targeted development of biosimilar pharmaceutical products
Targeted development of biosimilar pharmaceutical products
Targeted development of biosimilar pharmaceutical products
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
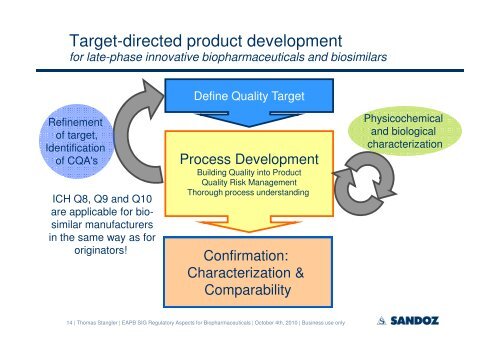
Target-directed product <strong>development</strong><br />
for late-phase innovative bio<strong>pharmaceutical</strong>s and <strong>biosimilar</strong>s<br />
Refinement<br />
<strong>of</strong> target,<br />
Identification<br />
<strong>of</strong> CQA's<br />
ICH Q8, Q9 and Q10<br />
are applicable for <strong>biosimilar</strong><br />
manufacturers<br />
in the same way as for<br />
originators!<br />
Define Quality Target<br />
Process Development<br />
Building Quality into Product<br />
Quality Risk Management<br />
Thorough process understanding<br />
Confirmation:<br />
Characterization &<br />
Comparability<br />
14 | Thomas Stangler | EAPB SIG Regulatory Aspects for Bio<strong>pharmaceutical</strong>s | October 4th, 2010 | Business use only<br />
Physicochemical<br />
and biological<br />
characterization







![Roy Forster [Kompatibilitätsmodus]](https://img.yumpu.com/7737040/1/190x135/roy-forster-kompatibilitatsmodus.jpg?quality=85)



