Practice Guidelines in Oncology - Gastric Cancer
Practice Guidelines in Oncology - Gastric Cancer
Practice Guidelines in Oncology - Gastric Cancer
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
®<br />
<strong>Practice</strong> <strong>Guidel<strong>in</strong>es</strong><br />
NCCN <strong>in</strong> <strong>Oncology</strong> – v.1.2007<br />
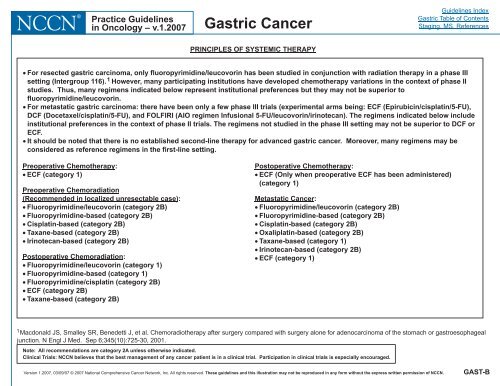
Preoperative Chemotherapy:<br />
� ECF (category 1)<br />
Preoperative Chemoradiation<br />
(Recommended <strong>in</strong> localized unresectable case) :<br />
� Fluoropyrimid<strong>in</strong>e/leucovor<strong>in</strong> (category 2B)<br />
� Fluoropyrimid<strong>in</strong>e-based (category 2B)<br />
� Cisplat<strong>in</strong>-based (category 2B)<br />
� Taxane-based (category 2B)<br />
� Ir<strong>in</strong>otecan-based (category 2B)<br />
Postoperative Chemoradiation:<br />
� Fluoropyrimid<strong>in</strong>e/leucovor<strong>in</strong> (category 1)<br />
� Fluoropyrimid<strong>in</strong>e-based (category 1)<br />
� Fluoropyrimid<strong>in</strong>e/cisplat<strong>in</strong> (category 2B)<br />
� ECF (category 2B)<br />
� Taxane-based (category 2B)<br />
<strong>Gastric</strong> <strong>Cancer</strong><br />
PRINCIPLES OF SYSTEMIC THERAPY<br />
Postoperative Chemotherapy:<br />
� ECF (Only when preoperative ECF has been adm<strong>in</strong>istered)<br />
(category 1)<br />
Metastatic <strong>Cancer</strong>:<br />
� Fluoropyrimid<strong>in</strong>e/leucovor<strong>in</strong> (category 2B)<br />
� Fluoropyrimid<strong>in</strong>e-based (category 2B)<br />
� Cisplat<strong>in</strong>-based (category 2B)<br />
� Oxaliplat<strong>in</strong>-based (category 2B)<br />
� Taxane-based (category 1)<br />
� Ir<strong>in</strong>otecan-based (category 2B)<br />
� ECF (category 1)<br />
Version 1.2007, 03/09/07 © 2007 National Comprehensive <strong>Cancer</strong> Network, Inc. All rights reserved. These guidel<strong>in</strong>es and this illustration may not be reproduced <strong>in</strong> any form without the express written permission of NCCN.<br />
<strong>Guidel<strong>in</strong>es</strong> Index<br />
<strong>Gastric</strong> Table of Contents<br />
Stag<strong>in</strong>g, MS, References<br />
� For resected gastric carc<strong>in</strong>oma, only f luoropyrimid<strong>in</strong>e/leucovor<strong>in</strong><br />
has been studied <strong>in</strong> conjunction with radiation therapy <strong>in</strong> a phase III<br />
sett<strong>in</strong>g (Intergroup 116). 1 However, many participat<strong>in</strong>g <strong>in</strong>stitutions have developed chemotherapy variations <strong>in</strong> the context of phase II<br />
studies. Thus, many regimens <strong>in</strong>dicated below represent <strong>in</strong>stitutional preferences but they may not be superior to<br />
f luoropyrimid<strong>in</strong>e/leucovor<strong>in</strong>.<br />
� For metastatic gastric carc<strong>in</strong>oma: there have been only a few phase III trials (experimental arms be<strong>in</strong>g: ECF (Epirubic<strong>in</strong>/cisplat<strong>in</strong>/5-FU),<br />
DCF (Docetaxel/cisplat<strong>in</strong>/5-FU), and FOLFIRI (AIO regimen Infusional 5-FU/leucovor<strong>in</strong>/ir<strong>in</strong>otecan). The regimens <strong>in</strong>dicated below <strong>in</strong>clude<br />
<strong>in</strong>stitutional preferences <strong>in</strong> the context of phase II trials. The regimens not studied <strong>in</strong> the phase III sett<strong>in</strong>g may not be superior to DCF or<br />
ECF.<br />
� It should be noted that there is no established second-l<strong>in</strong>e therapy for advanced gastric cancer. Moreover, many regimens may be<br />
considered as reference regimens <strong>in</strong> the first-l<strong>in</strong>e sett<strong>in</strong>g.<br />
1Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarc<strong>in</strong>oma of the stomach or gastroesophageal<br />
junction. N Engl J Med. Sep 6;345(10):725-30, 2001.<br />
Note: All recommendations are category 2A unless otherwise <strong>in</strong>dicated.<br />
Cl<strong>in</strong>ical Trials: NCCN believes that the best management of any cancer patient is <strong>in</strong> a cl<strong>in</strong>ical trial. Participation <strong>in</strong> cl<strong>in</strong>ical trials is especially encouraged.<br />
GAST-B