Practice Guidelines in Oncology - Gastric Cancer
Practice Guidelines in Oncology - Gastric Cancer
Practice Guidelines in Oncology - Gastric Cancer
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
®<br />
<strong>Practice</strong> <strong>Guidel<strong>in</strong>es</strong><br />
NCCN <strong>in</strong> <strong>Oncology</strong> – v.1.2007<br />
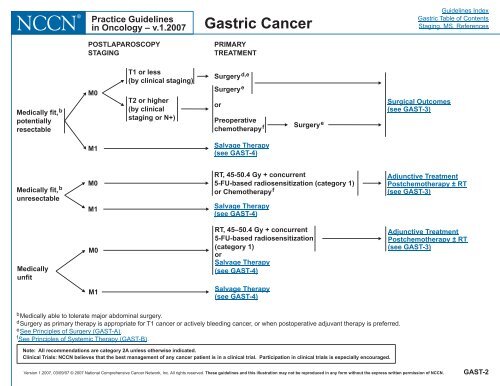
Medically fit, b<br />
potentially<br />
resectable<br />
Medically fit, b<br />
unresectable<br />
Medically<br />
unfit<br />
b<br />
d<br />
POSTLAPAROSCOPY<br />
STAGING<br />
M0<br />
M1<br />
M0<br />
M1<br />
M0<br />
M1<br />
T1 or less<br />
(by cl<strong>in</strong>ical stag<strong>in</strong>g)<br />
T2 or higher<br />
(by cl<strong>in</strong>ical<br />
stag<strong>in</strong>g or N+)<br />
<strong>Gastric</strong> <strong>Cancer</strong><br />
PRIMARY<br />
TREATMENT<br />
Surgery d,e<br />
Surgery<br />
RT, 45-50.4 Gy + concurrent<br />
5-FU-based radiosensitization (category 1)<br />
or Chemotherapy f<br />
Salvage Therapy<br />
(see GAST-4)<br />
RT, 45–50.4 Gy + concurrent<br />
5-FU-based radiosensitization<br />
(category 1)<br />
or<br />
Salvage Therapy<br />
(see GAST-4)<br />
Salvage Therapy<br />
(see GAST-4)<br />
Medically able to tolerate major abdom<strong>in</strong>al surgery.<br />
Surgery as primary therapy is appropriate for T1 cancer or actively bleed<strong>in</strong>g cancer, or when postoperative adjuvant therapy is preferred.<br />
eSee<br />
Pr<strong>in</strong>ciples of Surgery (GAST-A) .<br />
fSee<br />
Pr<strong>in</strong>ciples of Systemic Therapy (GAST-B) .<br />
Note: All recommendations are category 2A unless otherwise <strong>in</strong>dicated.<br />
Cl<strong>in</strong>ical Trials: NCCN believes that the best management of any cancer patient is <strong>in</strong> a cl<strong>in</strong>ical trial. Participation <strong>in</strong> cl<strong>in</strong>ical trials is especially encouraged.<br />
or<br />
Version 1.2007, 03/09/07 © 2007 National Comprehensive <strong>Cancer</strong> Network, Inc. All rights reserved. These guidel<strong>in</strong>es and this illustration may not be reproduced <strong>in</strong> any form without the express written permission of NCCN.<br />
e<br />
Preoperative<br />
chemotherapyf<br />
Salvage Therapy<br />
(see GAST-4)<br />
Surgery e<br />
<strong>Guidel<strong>in</strong>es</strong> Index<br />
<strong>Gastric</strong> Table of Contents<br />
Stag<strong>in</strong>g, MS, References<br />
Surgical Outcomes<br />
(see GAST-3)<br />
Adjunctive Treatment<br />
Postchemotherapy ± RT<br />
(see GAST-3)<br />
Adjunctive Treatment<br />
Postchemotherapy ± RT<br />
(see GAST-3)<br />
GAST-2