T5 SOLUTIONS (Waitz) a) Draw a thermodynamic cycle on p-v and ...
T5 SOLUTIONS (Waitz) a) Draw a thermodynamic cycle on p-v and ...
T5 SOLUTIONS (Waitz) a) Draw a thermodynamic cycle on p-v and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<str<strong>on</strong>g>T5</str<strong>on</strong>g> <str<strong>on</strong>g>SOLUTIONS</str<strong>on</strong>g> (<str<strong>on</strong>g>Waitz</str<strong>on</strong>g>)<br />
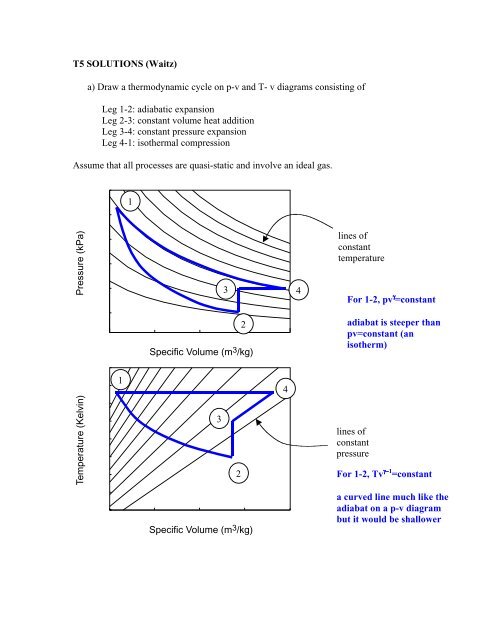
a) <str<strong>on</strong>g>Draw</str<strong>on</strong>g> a <str<strong>on</strong>g>thermodynamic</str<strong>on</strong>g> <str<strong>on</strong>g>cycle</str<strong>on</strong>g> <strong>on</strong> p-v <strong>and</strong> T- v diagrams c<strong>on</strong>sisting of<br />
Leg 1-2: adiabatic expansi<strong>on</strong><br />
Leg 2-3: c<strong>on</strong>stant volume heat additi<strong>on</strong><br />
Leg 3-4: c<strong>on</strong>stant pressure expansi<strong>on</strong><br />
Leg 4-1: isothermal compressi<strong>on</strong><br />
Assume that all processes are quasi-static <strong>and</strong> involve an ideal gas.<br />
Pressure (kPa)<br />
Temperature (Kelvin)<br />
300<br />
250<br />
200<br />
150<br />
100<br />
50<br />
0.5 0.75 1.0 1.25 1.5<br />
Specific Volume (m3/kg) 2<br />
600<br />
550<br />
500<br />
450<br />
400<br />
350<br />
300<br />
250<br />
1<br />
1<br />
3 4<br />
200<br />
0.5 0.75 1.0 1.25 1.5<br />
Specific Volume (m3 /kg)<br />
3<br />
2<br />
4<br />
lines of<br />
c<strong>on</strong>stant<br />
temperature<br />
For 1-2, pv γ =c<strong>on</strong>stant<br />
adiabat is steeper than<br />
pv=c<strong>on</strong>stant (an<br />
isotherm)<br />
lines of<br />
c<strong>on</strong>stant<br />
pressure<br />
For 1-2, Tv γγ−1 =c<strong>on</strong>stant<br />
a curved line much like the<br />
adiabat <strong>on</strong> a p-v diagram<br />
but it would be shallower
) For each leg determine if the heat <strong>and</strong> work transfers are (+), (-), or zero.<br />
Leg 1-2<br />
Leg 2-3<br />
Leg 3-4<br />
Leg 4-1<br />
Q ( +, -, or zero) W (+, -, or zero)<br />
c) Is the net work for this <str<strong>on</strong>g>cycle</str<strong>on</strong>g> positive or negative?<br />
0<br />
+<br />
+<br />
-<br />
The net work for this <str<strong>on</strong>g>cycle</str<strong>on</strong>g> is negative The area under the expansi<strong>on</strong> process is less<br />
than the area under the compressi<strong>on</strong> processes.<br />
d) What comm<strong>on</strong> purpose might you use a <str<strong>on</strong>g>cycle</str<strong>on</strong>g> like this for <strong>and</strong> why?<br />
This <str<strong>on</strong>g>cycle</str<strong>on</strong>g> could serve as a cooler or refrigerator. Overall it takes in energy in the<br />
form of heat from cold temperatures <strong>and</strong> expels energy in the form of heat from<br />
high temperatures. The net work for the <str<strong>on</strong>g>cycle</str<strong>on</strong>g> as a whole is negative, meaning that<br />
energy is put into the system to enable these transfers of heat to take place.<br />
+<br />
0<br />
+<br />
-