Air Pollution Engineering Manual Part5 1973
Air Pollution Engineering Manual Part5 1973
Air Pollution Engineering Manual Part5 1973
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
TYPES OF RESINS<br />
RESIN KETTLES<br />
A resin is defined by the American Society for<br />
Testing Materials (ASTM) as a solid or semisolid,<br />
water-insoluble, organic substance, with<br />
little or no tendency to crystallize. Resins are<br />
the basic components of plastics and are important<br />
components of surface-coating formulations.<br />
For both uses, growth in recent years has been<br />
phenomenal; more than 5, 000 companies in the<br />
United States now produce plastics.<br />
There are two types of resins--natural and synthetic.<br />
The natural resins are obtained directly<br />
from sources such as fossil remains and tree sap.<br />
These include Congo, Batu, and East India resins<br />
I from fossils; lac from insects; and damar and<br />
I rosin from tree sap.<br />
i<br />
I<br />
i<br />
Synthetic resins can be classified by physical<br />
properties as thermoplastic or thermosetting.<br />
CHAPTER 11<br />
CHEMICAL PROCESSING EQUIPMENT<br />
resisting qualities to cross-linked molecular<br />
structures.<br />
Phenolic Resins<br />
Phenolic resins can be made from almost any<br />
phenolic compound and an aldehyde. Phenol and<br />
formaldehyde are by far the most common ingredients<br />
used, but others include phenol-fukfural,<br />
resorcinol-formaldehyde, and many similar<br />
combinations. Since a large proportion of<br />
phenolic-resin production goes into the manufacture<br />
of molding materials, the most desirable<br />
process for this manufacture will be described.<br />
Phenol and formaldehyde, along - with an acid<br />
catalyst (usually sulfuric, hydrochloric, or<br />
phosphoric acid), are charged to a steamjacketed<br />
or otherwise indirectly heated resin<br />
kettle that is provided with a reflux condenser<br />
and is capable of being operated under vacuum.<br />
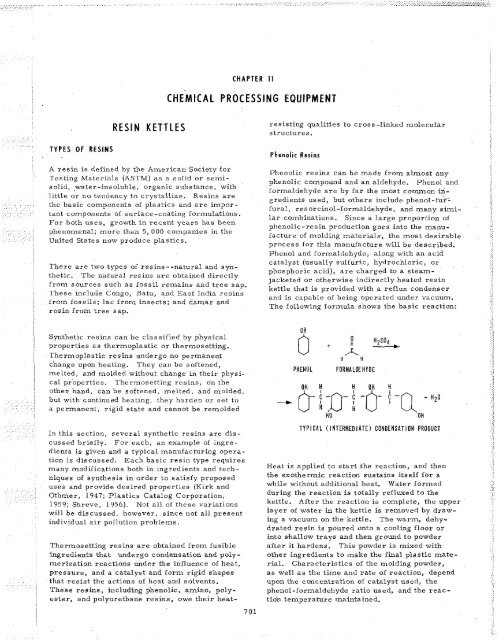
The following formula shows the basic reaction:<br />
j<br />
1<br />
1<br />
I,<br />
. . , . .<br />
... ,<br />
-<br />
Thermoplastic resins undergo no permanent<br />
change upon heating. They can be softened,<br />
melted, and molded without change in their physi<br />
cal properties. Thermosetting resins, on the<br />
other hand, can he softened, melted, and molded,<br />
but with continued heating, they harden or set to<br />
a permanent, rigid state and cannot be remolded<br />
PHENOL FORMALDEHYDE<br />
0-<br />
",, t, ,, ",, C,<br />
un n n un n<br />
--c i -n- ! -0- i -Q + H ~ O<br />
HO OH<br />
In this section, several synthetic resins are dis-<br />
cussed briefly. For each, an example of ingre-<br />
dients is given and a typical manufacturing opera-<br />
tion is discussed. Each basic resin type requires<br />
many modifications both in ingredients and tech-<br />
niques of synthesis in order to satisfy proposed<br />
uses and provide desired properties (Kirk and<br />
Othmer, 1947; Plastics Catalog Corporation,<br />
1959; Shreve, 1956). Not all of these variations<br />
will be discussed, however, since not all present<br />
individual air pollution problems.<br />
Thermosetting resins are obtained from fusible<br />
ingredients that undergo condensation and poly-<br />
merization reactions under the influence of heat,<br />
pressure, and a catalyst and form rigid shapes<br />
that resist the actions of heat and solvents.<br />
These resins, including phenolic, amino, poly-<br />
ester, and polyurethane resins, owe their heat-<br />
TYPICAL ( INTERMEDIATE) CONDENSATION PRODUCT<br />
Heat is applied to start the reaction, and then<br />
the exothermic reaction sustains itself for a<br />
while without additional heat. Water formed<br />
during the reaction is totally refluxed to the<br />
kettle. After the reaction is complete, the upper<br />
layer of water in the kettle is removed by draw-<br />
ing a vacuum on the kettle. The warm, dehy-<br />
drated resin is poured onto a cooling floor or<br />
into shallow trays and then ground to powder<br />
after it hardens. This powder is mixed with<br />
other ingredients to make the final plastic mate-<br />
rial. Characteristics of the molding powder,<br />
as well as the time and rate of reaction, depend<br />
upon the concentration of catalyst used, the<br />
phenol-formaldehyde ratio used, and the reac-<br />
tidn temperature maintained.
702 CHEMICAL PROCESSING EQUIPMENT<br />
Amino Resins<br />
The most important amino resins are the urea-<br />
formaldehyde and melamine-formaldehyde resins.<br />
The urea-Cormaldehyde reaction is simple: 1<br />
mole of urea is mixed with 2 moles of formalde-<br />
hyde as 38 percent solution. The mixture is<br />
kept alkaline with ammonia pH 7.6 to 8. The<br />
reaction is carried out at 77°F for 2 days at<br />
atmospheric pressure without any reflux.<br />
The - melamine resins are made in much the same<br />
manner except that the reactants must be heated<br />
to about 176°F initially, in order to dissolve the<br />
melamine. The solution is then cooled to 77°F<br />
for 2 days to complete the reaction.<br />
The equipment needed for the synthesis of the<br />
amino resins consists of kettles for the conden-<br />
sation reaction (usually nickel or nickel-clad<br />
steel), evaporators for concentrating the resin,<br />
and some type of dryer.<br />
The amino resins are used as molding compounds,<br />
adhesives, and protective coatings, and ior treat-<br />
ing textiles and paper.<br />
Polyester ond Alkyd Resins<br />
There is much confusion concerning the mean-<br />
ing of the two terms polyester and alkyd. Ap-<br />
parently, by chemical definition, the product<br />
obtained by the condensation reaction between<br />
a polyhydric alcohol and a polybasic acid, whether<br />
or not it is modified by other materials, is prop-<br />
erly called a polyester. All polyesters can then<br />
be divided into three basic classes: Unsaturated<br />
polyesters, saturated polyesters, and alkyds.<br />
Unsaturated polyesters are iormed when<br />
either of the reactants (alcohol and acid)<br />
contains, or both contain, a double-bonded<br />
pair of carbon atoms. The materials usu-<br />
ally used are glycols of ethylene, propylene,<br />
and butylene and unsaturated dibasic acids<br />
such as maleic anhydride and furnaric acid.<br />
A typical reaction is as follows:<br />
MALEIC ANHYDRIDE ETHYLENE GLYCOL<br />
REPRESENTATIVE SEGMENT OF CHAIN-FORMED<br />
The resulting polyester is capable of cross-<br />
linking and is usually blended wlth a poly-<br />
merizable material such as styrene. Under<br />
heat or a peroxide catalyst, or both, this<br />
blend copolymerizes into a thermosetting<br />
resin. It has recently found extensive use<br />
in the reinforced-plastics field where it is<br />
laminated with fibrous glass. It is also<br />
molded into many forms for a variety of uses.<br />
2. Saturated polyesters are made from saturated<br />
acids and alcohols, as indicated by the follow-<br />
ing reaction:<br />
H 1 ,L - - - - - - H H<br />
H - O C - 0 - C - I ' O - I !<br />
H H<br />
TEREPHTHALIC ACID ETHYLENE GLYCOL<br />
$__-__--i + H I - 0 - C - C - O - H - 4<br />
POLYESTER (REPERTING UNIT)<br />
The polyesters formed are long-chain, saturated<br />
materials not capable of cross-linking.<br />
Several of these are used as plasticizers. A<br />
special type made from ethylene glycol and<br />
terephthalic acid has been made into fiber<br />
(Dacron) and film ( ~ylara. Still others of<br />
this type with lower molecular weights are<br />
being used with di-isocyanate8 to form polyurethane<br />
resins.<br />
Alkyd resins differ from other polyesters<br />
as a result of modification by additions of<br />
fatty, monobasic acids. This is known as oil<br />
modification since the fatty acids are usu-<br />
ally in the form of naturally occurring oils<br />
such as linseed, tung, soya, cottonseed, and,<br />
at times, fish oil. The alkyds, thinned with,<br />
organic solvents, are used predominantly in<br />
the protective coating industry in varnishes,<br />
paints, and enamels.<br />
The most widely used base ingredients are<br />
phthalic anhydride and glycerol. Smaller<br />
quantities of other acids such as maleic,<br />
fumaric, and others and alcohols such as<br />
pentaerythritol, sorbitol, mannitol, ethylene<br />
glycol, and others are used. These are re-<br />
acted with the oils already mentioned to<br />
form the resin.<br />
The oils, as they exist naturally, are pre-<br />
dominantly in the form of triglycerides and<br />
do not react with the polybasic acid. They<br />
are changed to the reactive monoglyceride<br />
by reaction with a portion of the glycerol or<br />
other alcohol to be used. Heat and a cata-<br />
lyst are needed to promote this reaction,
which is known as alcoholysis. The resin<br />
is then formed by reacting this monoglyceride<br />
with the acid by agitation and sparging with<br />
inert gas until the condensation reaction prod-<br />
uct has reached the proper viscosity. The<br />
reaction takes place in an enclosed resin ket-<br />
tle equipped with a condenser and usually a<br />
scrubber, at temperatures slightly below<br />
500°F. The alcoholysis can be accomplished<br />
first and then the acid and more alcohol can<br />
be added to the kettle, or all the ingredients<br />
can be added simultaneously.<br />
An example of an alcoholysis reaction followed<br />
by reaction of the monoglyceride formed with<br />
phthalic anhydride is shown in the following:<br />
-<br />
C3 H5 (CI7 H33 COO)3 + C3 Hg (OH13<br />
Resin Kettles 703<br />
The flexible foams have found wide use in auto-<br />
mobile and furniture upholstery and in many<br />
other specialty items.<br />
By varying the ingre ents and adding other blowing<br />
agents such as Fre % ? 11, rigid foams with<br />
fine, close-cell structure can be formed. These<br />
can be formed in place by spraying techniques<br />
and are used extensively as insulating materials.<br />
Thermoplastic Resins<br />
GLYCEROL TRIESTER OF OLElC ACID GLYCERIN Polyvinyl Resins<br />
As already stated, thermoplastic resins are<br />
capable oi being reworked alter they have been<br />
formed into rigid shapes. The subdivisions in<br />
this group that are discussed here are the vinyls,<br />
styrenes, and the coal tar and petroleum base<br />
resins.<br />
H<br />
The polyvinyl resins are those having a vinyl<br />
HF - OH A !<br />
(CH=CH2) group. The most important of these<br />
-HC - 0 - C - C17 H33 +<br />
I are made from the uolvmerization oi vinvl ace-<br />
HC - OH<br />
H d<br />
MONOGLYCERIDE OF OLElC ACID PHTHALIC ANHYDRIDE<br />
H H H 9<br />
.... 0 . C. C C. 0 . C R<br />
Polyurethane<br />
H A H<br />
I<br />
CI7 H33 - C = 0<br />
REPEATING UNIT FOR OIL-MOOIFIEO ALKYO<br />
A ,<br />
tate and vinyl chloride. Other associated resins<br />
are also discussed briefly.<br />
Vinyl acetate monomer is a clear liquid made<br />
from the reaction between acetylene and acetic<br />
acid. The monomer can be polymerized in bulk,<br />
in solution, or in beads or emulsion. In the bulk<br />
reaction, only small batches can be safely bandled<br />
because oi the almost explosive violence of<br />
the reaction once it has been catalyzed by a small<br />
amount of peroxide. Probably the most common<br />
method of preparation is in solution. In this<br />
process, a mixture of 60 volumes vinyl acetate<br />
and 40 volumes benzene is fed to a jacketed,<br />
stirred resin kettle equipped with a reflux condenser.<br />
A small amount oi peroxide catalyst<br />
. .<br />
The manufacture of the finished polyurethane<br />
. .<br />
. ,<br />
.<br />
...<br />
.<br />
resin differs from the others described in that<br />
no heated reaction in a kettle is involved. One<br />
of the reactants. however. ~-. is -~ a saturated ~.. mnlu- r --,<br />
ester resin. as alreadv mentioned. . or. , more - -<br />
recently, a polvether - . resin. To iorm a flexible<br />
foam product, the resin, typically a polyether<br />
such as polyoxypropylenetriol, is reacted with<br />
tolylene diisocyanate and water in an approximate<br />
100: 42: 3 ratio by weight, along with small quantities<br />
of an emulsifying agent, a polymerization<br />
is added and the mixture is heated until gentle<br />
refluxing is obtained. Aiter about 3 hours, approximately<br />
70 percent is polymerized, and the<br />
run is transferred to another kettle where the<br />
solvent and unreacted monomer are removed by<br />
steam distillation. The wet polymer is then<br />
dried. Polyvinyl acetate is used extensively in<br />
water-based paints, and lor adhesives, textile<br />
finishes, and production of polyvinyl butyral.<br />
catalyst, and a silicone lubricant. The ingredients<br />
are metered to a mixing head that deposits<br />
the mixture onto a moving conveyor. The resin<br />
and tolylene diisocyanate (TDI) polymerize and<br />
cross-link to form the urethane resin. The TDI<br />
also reacts with the water, yielding urea and<br />
carbon dioxide. The evolved gas forms a loamlike<br />
structure. The product forms as a continuous<br />
loaf. After room temperature curing lor<br />
about a day, the loaf can be cut into desired<br />
sizes and shapes, depending upon required use.<br />
Vinyl chloride monomer under normal conditions<br />
is a gas that boils at -14°C. It is usually stored<br />
and reacted as a liquid under pressure. It is<br />
made by the catalytic combination of acetylene<br />
and hydrogen chloride gas or by the chlorination<br />
of ethylene followed by the catalytic removal of hydrogen<br />
chloride. It is polymerized in a jacketed,<br />
stirred autoclave. Since the reaction is highly exothermic<br />
and can result in local overheating and poor<br />
quality, it is usually carried out as a water emulsion<br />
to facilitate more precise control. To ensure
704 CHEMICAL PROCESSING EQUIPMENT<br />
quality and a properly controlled reaction, several with ethylene in presence of a Fridel-Crafts cataadditives<br />
are used. These include an emulsifying lyst such as aluminum chloride. During storage<br />
agent such as soap, a protective colloid such as glue, or shipment the styrene must contain a polymerizaa<br />
pH control such as acetic acid or other moderate- tion inhibitor such as hydroquinone and must be<br />
ly weak acid (2.5 is common), oxidation and re- kept under a protective atmosphere of nitrogen<br />
duction agents such as ammonium persulfate and or natural gas.<br />
sodium hisulfite, respectively, to control the oxidation-reduction<br />
atmosphere, a catalyst or initiator<br />
like henzoyl peroxide, and a chain length-con-<br />
Styrene can he polymerized in bulk, emulsion,<br />
or suspension using techniques similar to<br />
i<br />
1<br />
trolling agent such as carbon tetrachloride. The<br />
reaction is carried out in a completely enclosed<br />
vessel with the pressure controlled to maintain<br />
the unreacted vinyl chloride in the liquid state.<br />
As the reaction progresses, a suspension of latex<br />
or polymer is formed. This raw latex is removed<br />
from the kettle, and the unreacted monomer is<br />
removed by evaporation and recovered by compression<br />
and condensation.<br />
A modification oi the emulsion reaction is known<br />
as suspension polymerization. In this process,<br />
droplets of monomer are kept dispersed by rapid<br />
agitation in a water solution of sodium sulfate or<br />
in a colloidal suspension such as gelatin in water.<br />
During the the of monomer are<br />
converted to heads of oolvrner that are easily rethose<br />
mreviouslv described. The reaction is - ~<br />
exothermic and has a runawav tendencv unless I<br />
the temperature is carefully controlled. Oxygen<br />
must he excluded from the reaction since it causes 1<br />
a yellowing of the product and affects the rate of<br />
nnlvmPrira+ion. --, ---.. .-.--.<br />
i<br />
!<br />
Polystyrene is used in tremendous quantities for<br />
many .. purposes. . Because of its ease of handline, 4 u~ ,,<br />
dimensional stability, and unlimited color possi- 1<br />
bilities, it is used widely for toys, novelties, i<br />
toilet articles. houseware marts. radio and tele- ~ ~ !<br />
vision ~arts, wall tile. and other oroducts. Dis- I<br />
advantages include limited heat resistance, brit- .!<br />
tleness, and vulnerability to attack by organic<br />
solvents such as kerosine and carbon tetrachloride, i<br />
3<br />
covered and cleaned. This process is more<br />
troublesome and exacting than the emulsion reac- Petroleum and Coal Tar Resins<br />
tion hut eliminates the contaminating effects of<br />
the emulsifying agent and other additives.<br />
Petroleum and coal tar resins are the least expensive<br />
of the synthetic resins. They are made<br />
!<br />
from-the polymerization of unsaturated hydrocarbons<br />
found in crude distillate from coal tar in coke ovens ;<br />
or from cracking of petroleum. The exact chemical;<br />
nature of these hydrocarbons has not been determined,<br />
hut the unsaturates of coal tar origin are<br />
i<br />
known to be primarily cyclic while petroleum deriva<br />
tives are hoth straight- and close-chain types.<br />
Other vinyl-type resins are polyvinylidene chloride<br />
(saran@, polytetrafluoroethylene (fluoroethene),<br />
polyvinyl alcohol, polyvinyl hutyral, and others.<br />
The first two of these are made hy controlled poly-<br />
merization of the monomers in a manner similar to<br />
that previously described {or polyvinyl chloride.<br />
Polyvinyl alcohol has no existing monomer and<br />
is prepared from polyvinyl acetate by hydrolysis.<br />
Polyvinyl alcohol is unique among resins in that<br />
it is completely soluble in hoth hot and cold water.<br />
Polyvinyl butyral is made by the condensation<br />
reaction of butyraldehyde and polyvinyl alcohol.<br />
All have specific properties that make them super-<br />
ior for certain applications.<br />
Polystyrene<br />
Polystyrene, discovered in 1831, is one of the<br />
oldest resins known. Because of its transparent,<br />
glasslike properties, its practical application<br />
was recognized even then. Two major obstacles<br />
prevented its commercial development--prepara-<br />
tion of styrene monomer itself, and some means<br />
of preventing premature polymerization. These<br />
obstacles were not overcome until nearly 100<br />
years later.<br />
Styrene is a colorless liquid that boils at 145°C.<br />
It is prepared commercially from ethylbenzene,<br />
which; ,in turn, is made by reaction of benzene<br />
Most typical of the coal tar resins are those<br />
called Coumarone-Indene resin because these<br />
two compounds constitute a large portion of the<br />
distillate used for the reaction. The polymeriza-<br />
tion is initiated by a catalyst (usually sulfuric<br />
acid). After the reaction has proceeded as far as<br />
is desired, the unreacted monomer is removed<br />
by distillation. By controlling time, temperature,<br />
and proportions, many modifications of color and<br />
physical characteristics can be produced. The<br />
petroleum base distillate is polymerized in the<br />
same manner, yielding resins of slightly lower<br />
specific gravity than that of the coal tar resins.<br />
These resins are used in coating adhesives, in<br />
oleoresinous varnishes, and in floor coverings<br />
(the so-called asphalt tile).<br />
RESIN-MANUFACTURING EQUIPMENT<br />
Most resins are polymerized or otherwise reacted 1<br />
in a stainless steel, jacketed, indirectly heated ;<br />
vessel, which is completely enclosed, equipped j
with a stirring mcchanism, and generally contains<br />
an integral relluu condenser (Figure 549). Since<br />
most of the reactions prcviously iiescrihcd are<br />
exothermic, cooling coils are usually required.<br />
Some resins, such as thc phenolics, require<br />
that the kettle he under vacuum during part of<br />
the cyclc. This can be suppliecl either by a vac-'<br />
uum pump 01. by a steam or water jet ejector.<br />
Moreover, for somu reactions, that oi polyvinyl<br />
chlorirle [or example, the vessel must be capable<br />
of being operatcd uildcr pressure. This is nec-<br />
essary to kecp the norrnally gaseous monomer in<br />
a liquid state. The size of resin-processing ket-<br />
tles varies from a few hundred to several thou-<br />
sand gallons' capacity.<br />
Because oi Lhr rnany types of raw materials,<br />
ranging from gases to solids, storagc facilities<br />
vary accordingly--ethylene, a gas, is handled<br />
as such; vinyl chloride, a gas at standard condi-<br />
tions, is liquefieci easily under pressure. It is<br />
stored, therefore, as a liquid in a pressurized<br />
vessel. Most of the other liquid monomers do<br />
Res~n Kettles 705<br />
not present any particular storage problems.<br />
Some, such as styrene, must he stored under an<br />
inert atmosphere to prevent premature poly-<br />
merization. Some of the more volatile mate-<br />
rials are stored in cooled tanks to prevent ex-<br />
cessive vapor loss. Some of the materials have<br />
strong odors, and care must be taken to prevent<br />
emission of odors to the atmosphere. Solids,<br />
such as phthalic anhydride, are usually packaged<br />
and stored in bags or fiber drums.<br />
Treatment of the resin after polymerization varies<br />
with the proposed use. Resins for moldings are<br />
dried and crushed or ground into molding powder.<br />
Resins, such as the alkyd resins, to be used for<br />
protective coatings are normally transferred to<br />
an agitated thinning tank, as shown in Figure 550,<br />
where they are thinned with some type of solvent<br />
and then stored in large steel tanks equipped<br />
with water-cooled condensers to prevent loss of<br />
solvent to the atmosphere (Figure 551). Still<br />
other resins are stored in latex form as they<br />
come from the kettle.<br />
THE AIR POLLUTION PROBLEM<br />
The major sources of possible air contamination<br />
in resin manufacturing are the emissions of raw<br />
materials or monomer to the atmosphere, ernis-<br />
sions of solvent or other volatile liquids during<br />
the reaction, emissions of sublimed solids such<br />
as phthalic anhydride in alkyd production, emis-<br />
sions oi solvents during thinning of some resins,<br />
and emissions of solvents during storage and<br />
handling of thinned resins. Table 190 lists the<br />
most probable types and sources of air contami-<br />
nants from various resin-manufacturing opera- ,.<br />
tions.<br />
In the formulation of polyurethane foam, a slight<br />
excess of tolylene diisocyanate is usually added.<br />
Some of this is vaporized and emitted along<br />
with carbon dioxide during the reaction. The<br />
TDI fumes are extremely irritating to the eyes<br />
and respiratory system and are a source oi local<br />
air pollution. Since the vapor pressure of TDI<br />
is small, the fumes are minute in quantity and,<br />
if exhausted from the immediate work area and<br />
discharged to the outside atmosphere, are soon<br />
diluted to a nondetectible concentration. No<br />
specific controls have been needed to prevent<br />
emission of TDI fumes to the atmosphere.<br />
The finished solid resin represents a very small<br />
problem--chiefly some dust from crushing and<br />
grinding operations for molding powders. Generally<br />
the material is pne~rmatically conveyed<br />
from the grinder or pulverizer through a cyclone<br />
separator to a storage hopper. The fines escap-<br />
. .<br />
Figure 549. Typical resin-manufacturing unit<br />
showing process kettle and l iquid feed tanks<br />
ing the cyclone outlet are collected by a baghousetype<br />
dust collector. The collector should be de-<br />
\<br />
1<br />
(Si lmar Chemical Company, Hawthorne, Cal if.). signed for a filter velocity of about 4 fpm or less. I<br />
. ,
706 CHEMICAL PROCESSING EOUIPMENT<br />
Figure 550. Resin-thinning tanks with water-cooled condensers (Allied Chemical Carp., Plastics Div.<br />
Lynnwood. Calif.).<br />
Most of the contaminants are readily condensable.<br />
In addition to these, however, small quantities<br />
of noncondensable, odorous gases similar to those<br />
irom varnish cooking may be emitted. These are<br />
morc prevalent in the manufacture of oil-modi-<br />
fied alkyds where a drying oil such as tung, lin-<br />
seed, or soya is reacted with glycerin and phtha-<br />
lic anhydride. When a drying oil is heated,<br />
acrolein and other odorous materials are emitted<br />
at temperatures exceeding about 350°F (see<br />
Iurther discussion under Varnish Cookers). The<br />
intensity of these emissions is directly propor-<br />
tional to maximum reaction temperatures. Thus,<br />
the intensity of noncondensable gases from resin<br />
formulation should he considerably less than<br />
that of gases from varnish cooking since the re-<br />
action temperature is approximately 100°F lower.<br />
AIR POLLUTION CONTROL EQUIPMEN1<br />
Figure 551. Resin storage tanks with condensers<br />
Control of monomer and volatile solvent emis-<br />
CAI I ied chemical c ~ plastics ~ ~ 0ivision, , ~ L ~ ~ ~ .<br />
wood, Calif.),<br />
sions during storage before the reaction and of
Resin Kettles 707<br />
Table 190. PRINCIPAL AIR CONTAMINANTS AND SOURCES OF EMISSION FROM<br />
RESIN-MANUFACTURING OPERATIONS<br />
Resin<br />
I<br />
<strong>Air</strong> contaminant<br />
I<br />
Possible sources<br />
of emission<br />
Phenol~c Aldehyde odor Storage, leaks, condenser outlet,<br />
vacuum pump discharge<br />
Amino / Aldehyde odor I Storage, leaks<br />
Polyester and alkyds<br />
Polyvinyl acetate<br />
Oil-cooking odors<br />
Phthalic anhydride fumes<br />
Solvent<br />
Vinyl acetate odor<br />
Solvent<br />
Polyvinyl chloride Vinyl chloride odor 1<br />
Polystyrene<br />
Styrene odor<br />
Petroleum and coal<br />
tar resins<br />
Polyurethane resins<br />
Monomer odors<br />
Tolylene diisocyanate<br />
solvent emissions during thinning and storage<br />
after the polymerization of the resin is relatively<br />
simple. It involves care in maintaining gastight<br />
containers for gases or liquefied gases stored<br />
under pressure, and condensers or cooling coils<br />
on other vessels handling liquids that might vapor-<br />
ize. Since most resins are thinned at elevated<br />
temperatures near the boiling point of the thinner,<br />
resin-thinning tanks, especially, require ade-<br />
quate condensers. Aside from the necessity for<br />
control of air pollution, these steps are needed<br />
to prevent the loss of valuable products.<br />
Heated tanks used for storage of liquid phthalic<br />
and maleic anhydrides should be equipped with<br />
condensation devices to prevent losses of sub-<br />
limed material. An excellent device is a water-<br />
jacketed, vertical condenser with provisions for<br />
admitting steam to the jacket and provisions for<br />
a pressure relief valve at the condenser outlet<br />
set at perhaps 4 ounces' pressure. During stor-<br />
age the tank is kept under a slight pressure of<br />
about 2 ounces, an inert gas making the tank<br />
completely closed. During filling, the displaced<br />
gas, with any sublimed phthalic anhydride, is forced<br />
through thc cooled condenser where the phthalic is<br />
deposited on the condenser walls. After filling is<br />
completed, the condensed phthalic is remelted by<br />
passing steam through the condenser jacket.<br />
Addition of solids such as phthalic anhydride to<br />
other ingredients that are above the sublimation<br />
temperature of the phthalic anhydride causes<br />
Uncontrolled resin kettle discharge<br />
Kettle or condenser discharge<br />
Storage, condenser outlet during<br />
reaction, condenser outlet during<br />
steam distillation to recover sol-<br />
vent and unreacted monomer<br />
Leaks in pressurized system<br />
Leaks in storage and reaction<br />
equipment<br />
Leaks in storage and reaction<br />
equipment<br />
Emission from finished foam result-<br />
ing from excess TDI in formulation<br />
temporary emissions that violate most air pollution<br />
standards regarding opacity of smoke or<br />
fumes. These emissions subside somewhat as<br />
soon as the solid is completely dissolved but remain<br />
in evidence at a reduced opacity until the<br />
reaction has been completed. The emissions<br />
can he controlled fairly easily with simple scrubbing<br />
devices. Various types of scrubbers can<br />
be used. A common system that has been proved<br />
effective consists of a settling chamber, commonly<br />
called a resin slop tank, followed by an<br />
exhaust stack equipped with water sprays. The<br />
spray system should provide for at Least 2 gallons<br />
per 1, 000 scf at a velocity of 5 ips. The settling<br />
chamber can consist of an enclosed vessel partially<br />
filled with water capable of being circulated<br />
with gas connections from the reaction vessel and<br />
to the exhaust stack. Some solids and water of<br />
reaction are collected in the settling tank, the<br />
remainder being knocked down by the water sprays<br />
in the stack. Another example is shown in Figure<br />
552. Here the vapors from a polyester resin<br />
process kettle are first passed through a spray<br />
chamber-type precleaner followed by a venturi<br />
scrubber. This system effectively reduces visible<br />
emissions. Scrubber water may be recirculated<br />
or used on a once-through basis, depending<br />
~ rimaril~ upon the available waste-water disposal<br />
system. The scrubber water can be odorous<br />
and should be discharged to a sanitary sewer.<br />
Many resin polymerization reactions, for example,<br />
polyvinyl acetate by the solution method, require
708 CHEMICAL PROCES SING EQUIPMENT<br />
Figure 552. Venturi scrubber venting resin-man-<br />
ufacturinn equipment (Silmar Chemical Corporation,<br />
Hawthorne; Calif. ).<br />
refluxing of ingredients during the reaction. Thus,<br />
all reactors ior this or other reactions involving<br />
the vaporization of portions of the reactor con-<br />
tents must be equipped with suitable reflux- or<br />
horizontal-type condensers or a combination oi<br />
both. The only problems involved here are prop-<br />
er sizing of the condensers and maintaining the<br />
cooling medium at the temperature necessary to<br />
effect complete condensation.<br />
When noncondensable, odor-bearing gases are<br />
emitted during the reaction, especially with alkyd<br />
resin production as already mentioned, and these<br />
gases are in sufficient concentration to create<br />
a public nuisance, more extensive air pollution<br />
control equipment is necessary. Such equipment<br />
is discussed thoroughly under other sections con-<br />
cerning odors (Varnish Cookers and Reduction of<br />
Inedible Animal Matter) and includes equipment<br />
for absorption and chemical oxidation, adsorption,<br />
and combustion, both catalytic and direct-ilame<br />
type.<br />
INTRODUCTION<br />
VARNISH COOKERS<br />
Varnish cooking processes discussed in this sec-<br />
tion include both the heated processes used to<br />
modify natural or synthetic oils or resins which<br />
will be the film-forming vehicles in inks or<br />
coatings (i.e., varnish, paint, enamel, lacquer)<br />
and those processes completely synthesizing<br />
iilm-forming vehicles. The effect of the various<br />
processes is to shorten the drying time and im-<br />
prove the qualities of chemicals which would,<br />
without modification, dry and form a film. Some<br />
varnish cooking processes will involve the manu-<br />
facture of a resin simultaneously with the rnodi-<br />
iication of the drying oils and in the same equip-<br />
ment.<br />
Varnish cooking, until the 19301s, involved only<br />
two basic processes: heat processing of natural<br />
oils to purify them or improve their drying time<br />
ior use in coatings and manufacture of oleoresin-<br />
nus varnish by heat processing the natural oils<br />
with natural resins. Since that time synthetic<br />
resins and synthetic film-forming compounds<br />
have greatly expanded the number of heating<br />
processes employed. Many new coatings do not<br />
use the film-forming products oi varnish cooklng.<br />
As a result, the products of varnish cooking con-<br />
stitute a much lower percentage of all coating<br />
materials than they did a decade ago. Neverthe-<br />
less, varnish cooking products still are exten-<br />
sively produced for use in the manufacture of<br />
surface coatings.<br />
DEFINITIONS - PRODUCTS AND PROCESSES<br />
Table 191 lists the various oils that are processed<br />
in varnish cooking equipment and the resins, both<br />
natural and synthetic, most frequently used in the<br />
manufacture of the iilm-forming materials. Var-<br />
nish cooking operations are varied, All opera-<br />
tions of this type, however, involve the applica-<br />
tion oi heat to these materials and their resultant<br />
polymerization, depolyrnerization, melting,<br />
esterification, isomerization, etc. Some of the<br />
most common processes are defined below.<br />
Boiled Oil. Linseed oil, soybean oil or other<br />
natural oils heated with small percentages oi<br />
oxides, acetates, or other salts of lead, manga-<br />
nese, or cobalt are known as boiled oils. During<br />
the process, the oil thickens, its density in-<br />
creases, and its color darkens, principally due<br />
to polymerization hut also to some oxidation.<br />
When catalysts, certain metal oxides, activated<br />
nickel, or sulfur dioxide are added, isomeriza-<br />
tion, or conjugation, occurs. This process is<br />
periormed to accelerate the normal drying time<br />
oi the oil. While oil so treated is known as
Table 191. VARNISH COOKING INGREDIENTS<br />
(Shreve, 1967)<br />
Oils<br />
Linseed oil<br />
Tung oil<br />
Dehydrated castor oil<br />
Castor oil<br />
Fish oils<br />
Tall oils<br />
Soya oil<br />
Cottonseed oil<br />
Coconut oil<br />
Natural resins<br />
Shellac, insect secretion<br />
Rosin<br />
East India<br />
Manila<br />
Kauri, old fossil resin<br />
Copal, fossil resin<br />
Dammar, recent fossil resin<br />
Synthetic resins<br />
Phenol-aldehvde (oil-soluble)<br />
Alkyd resins<br />
Mannitol esters<br />
Pentaerythritol esters and interesters<br />
Limed rosin<br />
Ester gum<br />
Cumarone-indene<br />
Melamine and urea-formaldehvde<br />
Chlorinated rubber and diphenyl<br />
Acrylates<br />
Vinyl resins<br />
Silicones<br />
Depolymerized copals<br />
Epoxies<br />
Polyurethanes<br />
boiled oil, the oil actually is heated only to tem-<br />
peratures of 360' to 580DF, which are below the<br />
boiling point.<br />
Heat-Bodied Oil. Linseed and other natural oils,<br />
including nut, vegetable, and marine oils, heated<br />
to temperatures from 485" to 620°F are known as<br />
heat-bodied oils. The viscosity of the oil, or its<br />
"body," is increased; the amount of increase de-<br />
pends upon the kind of oil and the time and tem-<br />
perature levels to which the oils are heated.<br />
Bodying is done principally for oils to be used in<br />
enamels and printing inks and results mainly from<br />
polymerization, although some oxidation also<br />
takes place.<br />
Blown Oil. Linseed oil and other natural oils may<br />
also be bodied by bubbling air through them and,<br />
in this case, are known as blown oils. The reac-<br />
tion is mainly oxidation; however, some polymeri-<br />
zation of the oxidized molecules also occurs.<br />
During the blowing process, the oil is heated to<br />
temperatures of 212 " to 390 "F.<br />
Varnish Cookers 709<br />
Esterification. The reaction of an organic acid<br />
(or acid anhydride) with an alcohol is known as<br />
esterification. In varnish cooking, copolymer<br />
oils, such as soyhean oil-maleic anhydride, are<br />
esterified with glycerol or other polyhydric alco-<br />
hols, or the vegetable oil may first be alcoholized<br />
and then treated with the anhydride. These opera-<br />
tions produce good drying oils from less desirable<br />
ones by increasing the double bond structures of<br />
the molecules. Polymeric esters, good synlhetic<br />
film-forming materials, are prepared by csteri-<br />
lication of glycerol, fatty acids, and phrhalic<br />
anhydride. These processes are performed in<br />
closed vessels at moderate temperatures.<br />
Spirit Varnish. Most spirit varnishes do not<br />
involve varnish cooking, but are mostly solutions<br />
of resins and volatile solvents mixed at room<br />
temperature. However, one spirit varnish, gloss<br />
oil, does involve varnish cooking. Resins are<br />
cooked at moderate to high temperatures with<br />
slaked lime, and the hot calcium resinate product<br />
is thinned with petroleum spirits.<br />
Oleoresinous Varnish. These varnishes are manu<br />
factured hy heating various combinations of natural<br />
oils with various svnthetic or natural resins<br />
to hlgh - temperatures, 520 " to 650 OF. The final<br />
product is thinned with solvent, and drying agents<br />
are added after thinning.<br />
Oil Breaking. Linseed oil, or other natural oils,<br />
contain some unsaponifiable matter, usually 1 to<br />
L percent. Those oils to be used in coatings<br />
without any other treatment will he cleared of<br />
most of this unsaponifiable material by the "break-<br />
ing" process. If the oils were allowed to age,<br />
this material would eventually separate and, along<br />
with other foreign matter, would settle as foots.<br />
Heating the oil to about 450 "F accelerates this<br />
separation and the oil is said to "break."<br />
Gum Running. Some natural resins, such as<br />
kauri gum, are insoluble in oil. In order to use<br />
them, they first are heated to temperatures of<br />
570 " to 700 "F. Then they are mixed with heated<br />
oils to produce the desired varnish product. This<br />
high temperature process, called gum running,<br />
serves to depolymerize the resin.<br />
MAJOR TYPES OF VARNISH COOKING EQUIPMENT<br />
Varnish cooking processes are conducted in two<br />
types of vessels--the open-topped portable kettle<br />
and the newer, totally enclosed, stationary kettle.<br />
The open kettles are cylindrical vessels with<br />
dished or flat bottoms. They usually are trans-<br />
ported on a three- or four-wheel truck, and are<br />
heated over an open flame. This type of kettle<br />
usually varies in capacity from 185 to 375 gallons
710 CHEMICAL PROCESSING EQUIPMENT<br />
and is made of steel, copper, monel, aluminum,<br />
nickel, or stainless steel. Under most operating<br />
conditions, the kettle is charged in a loading<br />
room and then moved to the fire pit. It is heated<br />
over the fire pit and then, when the reaction is<br />
complete, transferred to another location for<br />
cooling. When the contents have cooled to the<br />
proper temperature, the kettle may be transferred<br />
to a third location for the addition of thinners<br />
and dryers or for transfer of its contents to<br />
a thinning tank. In the past, it was common to<br />
manually agitate the contents during cooking.<br />
Materials in open kettles now are seldom agitated<br />
manually. Agitation is provided by air-driven or<br />
electrically driven mixers and by sparging the<br />
contents with an inert gas, such as carbon dioxide<br />
or nitrogen. Figure 553 shows a kettle of this<br />
type. The open kettle still is employed extensively<br />
in paint manufacturing establishments.<br />
The enclosed stationary kettles are indirectly<br />
heated or cooled by jacketing or by coils. The<br />
kettles are vertical cylinders with dished tops<br />
and dished bottoms. They are constructed of an<br />
appropriate grade of stainless steel to resist cor-<br />
rosion. Some kettles are glass lined. An electri-<br />
cally driven agitator is mounted on the kettle.<br />
Batch weighing or metering equipment, pumps,<br />
and piping for the charging of liquid raw materi-<br />
als are installed with the kettle. The kettles are<br />
equipped with sealable openings through which<br />
solid or liquid materials can be charged manually.<br />
Inert gas is sparged into the bottom so as lo per-<br />
meate the entire contents of the kettle. For some<br />
types of production, condensers for reflux or<br />
vapor recovery are installed on the kettle.<br />
Figure 553. Uncontrolled open kettle far varnish<br />
cooking.<br />
Thinning for viscosity adjustment, or the addi-<br />
tion of dryers or unreacted monomers, usually<br />
is not done in the reaction kettle. Instead, the<br />
contents are pumped to other vessels designed<br />
for these purposes. These second vessels, or<br />
thinning vessels, also are closed and equipped<br />
with agitators. Jackets or coils for indirect<br />
heating or cooling, and nozzles for sparging<br />
with inert gas are installed. The thinning ves-<br />
sels are equipped with water-cooled vent con-<br />
densers mounted so that condensed solvent will<br />
drain back into the vessel. The closed station-<br />
ary kettles are almost exclusively found at chemi-<br />
cal companies engaged in manufacturing a wide<br />
variety of paint bases.<br />
THE AIR POLLUTION PROBLEM<br />
Varnish cooking processes in both open and closed<br />
kettles are carried out at temperatures ranging<br />
from 200 " to 650 "F or higher. At times, rapid<br />
cooling of the cook is necessary to control the<br />
reaction or to control the composition of a parti-<br />
cular product. At approximately 350 "F, almost<br />
all of the solid or liquid materials begin to voli-<br />
tilize and emit vapors or gases from the vessel.<br />
As long as the ingredients are held at or above<br />
this temperature, the emissions continue. Some<br />
varnish cooking operations require 3 to 10 hours<br />
or longer. The quantity, composition, and rate<br />
of emissions depend upon ingredients in the cook,<br />
maximum temperature levels, method of intro-<br />
ducing additives, degree of stirring, cooking time,<br />
and extent of air or inert gas blowing (Stenburg,<br />
1958). Total emissions for oleoresinous varnish<br />
cooking can be as high as 6 to 12 percent by<br />
weight of the materials in the kettle, and those<br />
from oil cooking and blowing, 4 to 6 percent by<br />
weight.<br />
Cooker emissions vary in composition, depending<br />
upon the ingredients in the cook. Mattiello (1943)<br />
states that compounds emitted from cooking of<br />
oleoresinous varnish include water vapor, fatty<br />
acids, glycerine, acrolein, phenols, aldehydes,<br />
ketones, terpene oils, terpenes, and carbon diox-<br />
ide. Heat-bodying of oils causes the emission of<br />
these same compounds less the phenols, terpene<br />
oils, and terpenes. Gum running yields water<br />
vapor, fatty acids, terpenes, terpene oils, and<br />
tar. Besides the air contaminants listed by Mat-<br />
tiello, some highly offensive sulfur compounds<br />
such as hydrogen sulfide, allylsulfide, butyl mer-<br />
captan, and thiophene are formed when tall oil is<br />
esterified with glycerine and ~entaerythritol.<br />
These compounds are emitted as a result of small<br />
amounts of sulfur in the tall oil. Attempts to<br />
alleviate this problem involve further refining of<br />
the tall oil to remove as much sulfur as possible.
Of all the compounds emitted, acrolein is the one<br />
most generally associated with oil cooking because<br />
of its pungent, disagreeable odor and irritating<br />
characteristics. Some of the more odorous com-<br />
pounds have very low odor thresholds; acrolein,<br />
for example, has a threshold at 1.8 ppm and some<br />
of the sulfur compounds have a threshold at about<br />
0.001 ppm.<br />
A good portion of the emissions from these opera-<br />
tions are in the form of noncondensibles, insoluble<br />
gases or vapors, or condensible vapors which<br />
tend to form submicron size droplets. Emissions<br />
in the form of particulate matter have been found,<br />
upon examination under the microscope, to be in<br />
a size range between 2 and 20 microns in diame-<br />
ter. The median size of several samples varied<br />
from 8 to 10 microns.<br />
An important source of emissions is the thinning<br />
of varnish with solvent. In most of the newer<br />
stationary enclosed kettle installations, the<br />
cooked varnish is pumped from the reaction kettIe<br />
to a thinning tank that is equipped with an integral-<br />
ly mounted condenser. In the older portable open-<br />
kettle operations, however, the thinning operation<br />
is carried out near the boiling point of the solvent,<br />
and emissions of vapor can be considerable. Con-<br />
sequently, the thinning tank can be hooded and the<br />
vapors can be ducted to the same control system<br />
that removes the fumes from the cooker. While<br />
emission of solvents from the thinning tanks con-<br />
stitutes a greater potential for formation of photo-<br />
chemical smog than emissions from the varnish<br />
cooker, emissions from varnish cookers cause<br />
greater local nuisance problems because of nox-<br />
ious odors. Both operations result in the emis-<br />
sion of visible dense white plumes.<br />
HOODING AND VENTILATION REQUIREMENTS<br />
For air pollution control to succeed, all fumes<br />
emitted from the kettles and thinning vessels<br />
must be captured and conveyed to a control device<br />
under all operating conditions without hindering<br />
producti~n.<br />
Stationary closed kettle emission volumes are<br />
entirely dependent upon the type of operation per-<br />
formed, whether the kettle is sealed shut, oper-<br />
ated under partial vacuum, or merely vented with-<br />
out sealing so that air may be drawn into it and<br />
over its contents. <strong>Air</strong> volumes necessary to ex-<br />
haust open portable kettles are considerably<br />
reduced with properly designed close-fitting hoods.<br />
Sufficient air must continuously be swept through<br />
the open kettles so as to prevent the build up of<br />
explosive concentrations of the volatiles above<br />
the surface of the liquids within the kettle and in<br />
the exhaust ductwork.<br />
Figure 554 shows two open kettles in place over<br />
Varnish Cookers 711<br />
two open furnaces. The kettles are equipped with<br />
well designed removable hoods. The hoods are<br />
provided with openings for the manual addition of<br />
materials, for thermometers, and for agitators.<br />
The openings have hinged covers so that they can<br />
be kept closed except when being used.<br />
Indraft velocities of at least 100 to 150 fpm should<br />
be provided through the face of all the openings<br />
when the largest covered area is open to prevent<br />
the escape of contaminants from the kettle during<br />
charging or observation. Good hood design pro-<br />
vides the necessary indraft velocities when gas<br />
volumes of 100 to 300 cfm are exhausted from the<br />
kettle. Because the volatiles coming from a kettle<br />
condense on the hood, the hoods for open kettles<br />
should be provided with an outer trough to collect<br />
the condensed liquids whlch will appear on the<br />
hood surfaces. This trough should have provi-<br />
sions for drainage, usually to a small container.<br />
In the past, exhaust ductwork was sized for velo-<br />
cities of 1500 to 2000 fpm. Currently, ductwork<br />
frequently is designed for higher velocities, usual-<br />
ly 3000 to 3500 fpm. Higher velocities help reduce<br />
the rate of deposition of cond'ensed vapors on the<br />
duct walls. An exhaust gas blower in the exhaust<br />
system is necessary when venting open kettles.<br />
A blower also is required for closed kettles oper-<br />
ated at atmospheric conditions rather than under<br />
pressure or vacuum. When the air pollution con-<br />
trol system includes an afterburner, reliance upon<br />
the natural draft generated by its stack can be<br />
hazardous. Positive ventilation should be pro-<br />
vided to keep the vapor composition above the<br />
kettle below explosive limits and to maintain sd-<br />
ficient velocity in the exhaust system and at the<br />
entrance to the afterburner to prevent flashback.<br />
When the exhaust system includes an afterburner,<br />
the best placement of the exhaust blower is down-<br />
stream from the afterburner. If it is installed in<br />
front of the afterburner, serious maintenance and<br />
operating problems occur because deposits quickly<br />
build on the fan blades, resulting in unbalanced<br />
operation. With the blower on the discharge side<br />
of the afterburner, blower blades remain clean<br />
and in balance. Exhaust gases are cooled by dilu-<br />
tion with ambient air before entering the blower<br />
to a temperature of about 500 'F. Blowers of<br />
standard construction are available for operation<br />
at this temperature.<br />
The exhaust ductwork is subject to severe corro-<br />
sion and to heavy fouling. Use of appropriate<br />
corrosion-resistant material such as stainless<br />
steel will overcome most corrosion problems.<br />
Fouling problems require that the ductwork con-<br />
tain a number of sealed clean-out openings for<br />
access to the interior and that a regular cleaning<br />
schedule be maintained.
712 CHEMICAL PROCESSING EQUIPMENT<br />
Figure 554. Open varnish-cooking kettles with exhaust hoods (Standard Brand Paints<br />
Company, Torrance, Calif.).<br />
Fouling has been reduced greatly or completely<br />
eliminated in some recent installations where the<br />
air pollution control device is an afterburner. In<br />
these installations, concentric type ductwork,<br />
with the outer duct insulated, has been used. A<br />
portion of the hot exhaust gas from the afterburn-<br />
er, after dilution to temperatures in the range of<br />
500" to 600 "F, is blown into the annular space<br />
between the inner and outer duct. The hot gas in<br />
the annular space heats the internal duct carry-<br />
ing the contaminated effluent from the kettles.<br />
The heated gases pass countercurrent to flow in<br />
the interior duct. The temperature of the wall of<br />
the duct carrying the contaminated effluent is<br />
raised, and condensation on the ductwork is<br />
limited. Condensation usually is restricted to<br />
low-viscosity liquids which may be carried along<br />
in the exhaust gas stream. The concentric duct-<br />
work is usually used only for the main section.<br />
Where dibasic acids, especially phthalic anhy-<br />
dride, are employed in the cooking operation,<br />
the concentric ductwork is extended to the ex-<br />
haust port on each kettle. Figure 555 illustrates<br />
one such exhaust system. Experience for 3 years<br />
with this system has demonstrated that cleaning<br />
of the duct has not been necessary. However,<br />
some provision for draining fluids from the duct<br />
should be included. Ln the installation illustrated,<br />
the downcomer duct to the afterburner is equipped<br />
with a well for fluid accumulation. The well has<br />
a removable cover, and it is cleaned after each<br />
week of operation.<br />
The cost of installing the concentric ductwork and<br />
its insulation adds approximately 150 percent to<br />
the cost of single ductwork. However, the reduc-<br />
tion of maintenance, the reduction of fire hazards,<br />
and the elimination of deposits inside the duct<br />
make the additional capital expenditure worthwhile.<br />
AIR POLLUTION CONTROL EQUIPMEN1<br />
All operations in which varnish or paint base is<br />
cooked or in which drying oils are bodied or other-<br />
wise prepared by the application of heat should be<br />
vented to air pollution control devices. From 1<br />
to 5 percent or more of the total material charged<br />
to a kettle for these processes otherwise is emit-<br />
ted to the atmosphere during operation. The<br />
material emitted includes the odorous irritating<br />
compounds previously mentioned. In addition to<br />
odors, the emissions contain considerable parti-<br />
culate matter and frequently form a highly visible<br />
plume. The control devices applicable to varnish<br />
cooking are the same as those used for controlling<br />
other sources of organic vapors, particulates,<br />
odors, and visible emissions, with some modifi-<br />
cations to meet those situations unique to the var-<br />
nish cooking operations.
CONTAMINATED<br />
EXHAUST DUCT<br />
EXHAU TO AFTERBURNER<br />
BLOWE<br />
Varnish Cookers 713<br />
EXHAUST DUCT<br />
Figure 555. Varnish-cooking control system using heated concentric ductwork and an afterburner (Old Quaker<br />
Paint Co., Torrance. California).<br />
PHERE
714 CHEMICAL PROCESSING EQUIPMENT<br />
Scrubbers<br />
Scrubbers, in the past, were the only devices<br />
installed to control emissions from varnish cook-<br />
ing. The inherent hazard of fire or explosion pre-<br />
vented serious consideration of afterburners. As<br />
the control of air pollution became more critical,<br />
direct-fired afterburners were chosen to comply<br />
with the more stringent regulations. For some<br />
processes, however, scrubbers still are adequate<br />
for controlling emissions of condensibles and are<br />
less costly to install and operate than afterburners.<br />
Sampling results (test 20, Table 192) for a spray<br />
scrubber venting a closed vessel producing alkyd<br />
modified varnish, illustrated in Figure 556, indi-<br />
cated that emissions from certain processes can<br />
be controlled sufficiently to meet stringent ernis-<br />
sion limitations.<br />
Many existing scrubbers have been left in place<br />
when afterburners were installed to serve as con-<br />
densers and to provide flashback protection before<br />
the contaminated emissions enter the afterburner.<br />
The various types of scrubbers which have been<br />
used include simple spray towers, sieve plate<br />
towers, chambers or columns with series of baf-<br />
fles and water curtains, agitated tanks, and water<br />
venturi jets. Scrubbers employing spray nozzles<br />
have suffered from a major disadvantage. The<br />
excessive maintenance required to keep the noz-<br />
zles free from clogging or to replace the nozzles<br />
because of erosion by recirculated scrubbing<br />
Table 192. SUMMARY OF RESULTS OF STACK DISCHARGE TESTS, VARNISH COOKING<br />
CONTROLLED BY AFTERBURNERSANDSCRUBBERS<br />
Itsm<br />
TOW proceaa time, hr<br />
Rots.. time of teat.<br />
hr<br />
Temperature of<br />
material. OF<br />
Teat No. 3<br />
Test No. 5<br />
1. Alkyd resin u=rnish Alkyd reeh vnrniah Heat body-<br />
2. Oleorealn varniah Two kettles ing oil<br />
Two - closed<br />
GO2 L~IOB.<br />
stage of procesa Heating to<br />
maldmum temperawre<br />
ExLaust volume. scfm<br />
Temperature of<br />
exhaust. OF<br />
Particulate loading. a<br />
grlecf<br />
Rate of particuloles. iblhr<br />
Organic acids, grlsci<br />
Aldehydes-ketone., p p<br />
Hydrocarbona, ppm<br />
Ait pollution control<br />
equipment<br />
Afterburner temperature,<br />
OF<br />
Total. 950<br />
Glycerine - fatty acid8<br />
Pentaerythrifol<br />
Phthalic anhydride<br />
Closed, gas fired<br />
N2 atmoa.<br />
106 147<br />
5.20<br />
0.174<br />
67<br />
I<br />
103<br />
3 spray towers<br />
vertical afterburner<br />
1200 to I240<br />
I<br />
Linseed<br />
oil<br />
ope.<br />
I<br />
Test No. 16<br />
Phenolic rcein varnish<br />
Linseed oil<br />
Phenolic resin<br />
Two. open<br />
rf<br />
Final f cook, f cooling<br />
i L 7<br />
Heating to mardmum<br />
temperature and<br />
cooling<br />
Sinale sorav in duct I Horizontal afterburner<br />
Teat No. 20<br />
Alkyd resin varninh<br />
Soy. chinasmod oils<br />
Glycerine<br />
Phthalic anhydride<br />
Closed<br />
N2 Ltmo.. .<br />
light vacvvm<br />
Major<br />
phthalic<br />
add.<br />
2bOf<br />
90<br />
I spray<br />
in stack<br />
Minor<br />
phthalic<br />
add.<br />
Organic acids, grlscf 1 0.066 1 None / . 1 . 1 0.15 / 0.082<br />
Efrlclcnolb<br />
*art>~"lates, %<br />
Chgmlc sclds. %<br />
Aldehydes-kctone., %<br />
Hydrocarbon#, %<br />
94<br />
48<br />
73<br />
99t<br />
.See Rule 2. in appendix.<br />
b<br />
&si. - reduction of total weight.<br />
'4ccur.q ~tmited to 10 ppm.<br />
d<br />
Wution air included.<br />
'hrulySi. of combustible gam. by CCLR method. Total ppm.<br />
'with ono md tao .toam let8 in stack.<br />
97.3<br />
99.9+<br />
73<br />
85<br />
93<br />
94<br />
90d<br />
170
U<br />
GAS BURNER<br />
STEAM<br />
-<br />
F~gure 556. D~agram of a spray scrubber venting oleoresln<br />
rnanufacturlng equ~pment. Afterburners<br />
fluids has resulted in every scrubber of this type<br />
being taken out of service. Packed scrubbers<br />
have not been used in these operations because<br />
the condensed fumes rapidly plug the packing.<br />
1<br />
I<br />
Varnish Cookers 715<br />
WATER<br />
SEWER<br />
The scrubbers employed in these systems usually<br />
are designed with the flow of the contaminated gas<br />
stream countercurrent to that of the scrubbing<br />
medium. Generally, water is the scrubbing<br />
medium, and is not recirculated. Attempts have<br />
been made to use acids, bases, various oils, and<br />
solvents as scrubbing materials, but these scrub-<br />
bing solutions have not resulted in an increase in<br />
efficiency that would warrant their higher costs.<br />
Wetting agents have been added to scrubbing water,<br />
and in some special circumstances have resulted<br />
in increased collection efficiency.<br />
Condensers<br />
WATER<br />
Condensers have been used both to conserve sol-<br />
vent and to control visible emissions during the<br />
thinning operation. Varnish is thinned by the slow<br />
addition of the hot varnish to cold solvent or vice<br />
versa. Maximum solvent vaporization occurs<br />
during the initial contact between the solvent and<br />
hot varnish. Condensers must be designed to<br />
control the higher rate of emissions during the<br />
initial contact. When thinning vessels are vented<br />
directly to the atmosphere, loss of solvent, in the<br />
form of a dense white plume, is considerable. A<br />
condenser, usually a water-cooled shell-and-tube<br />
type, is mounted on the top of the thinning vessel<br />
vertically or at an angle so that the condensed sol-<br />
vent can drain back into the vessel.<br />
At one installation, a water -cooled shell-and-tube<br />
condenser having 52 square feet of transfer area<br />
and using copper tubes was mounted on a 350-gal-<br />
Ion hatch thinning tank. The condenser prevented<br />
visible emissions when 125 gallons of solvent was<br />
added to 195 gallons of varnish at 340 'F. The<br />
initial solvent flow rate was 7$ gallons per minute<br />
for 3f minutes: and the final flow rate was 12 gal-<br />
Ions per minute over an additional 14-minute<br />
period.<br />
Gas flow velocity from the condenser should be<br />
kept below 1000 fpm to minimize entrainment of<br />
condensate droplets. An entrainment separator<br />
should be installed on the discharge side of the<br />
condenser. The separator retains liquid particulates<br />
released during the initial high-volume surge<br />
of solvent vapors, which occurs when cold solvent<br />
and hot varnish first contact each other. Details<br />
on designing vapor condensers are given - in Chaater<br />
5 of this manual.<br />
Incineration of the effluent from varnish cooking<br />
processes has proved to be the most effective con-<br />
trol method and one relatively free of major main-<br />
tenance problems. Incineration has been effective<br />
for both open portable kettles and closed station-<br />
ary kettles. A direct-fired afterburner designed<br />
with appropriate parameters can reduce inlet odor<br />
concentration by 99 percent or more, and can<br />
oxidize 90 percent or more of the carbon in con-<br />
taminated effluent to carbon dioxide. Figures 555<br />
and 557 show two types of afterburners used to<br />
vent open kettle installations. Table 192 summar-<br />
izes results of stack tests conducted on direct-<br />
fired afterburners.<br />
In Los Angeles County, afterburners controlling<br />
emissions from varnish cookers have been pre-<br />
dominantly of the direct-fired type. Catalytic<br />
afterburners, however, are known to have been<br />
installed to control emissions from varnish cook-<br />
ers in other areas of the United States. Although<br />
a few catalytic units have been installed in the past<br />
in Los Angeles County, they have since been re-<br />
placed with direct-fired afterburners.<br />
One of the two most important design requirements<br />
for direct-fired afterburners is the capacity to<br />
operate at outlet temperatures of 1200" to 1400 "F<br />
under all conditions. When afterburner tempera-<br />
tures fall below 1200 "F. odor reduction is inade-<br />
quate and combustion efficiency for particulate<br />
matter and organic gases and vapors falls below<br />
90 percent an a carbon basis. The second impor-<br />
tant design requirement is the provision for direct<br />
flame contact with all vapors and gases emitted<br />
from the varnish cookers. Tests of afterburners,<br />
where direct flame contact of all the emissions<br />
did not occur, even with outlet temperatures of<br />
1400 'F and retention times greater than 0.3<br />
second, have revealed that combustion efficiencies<br />
for carbon have fallen well below 90 percent.
716 CHEMICAL PROCESSING EQUIPMENT<br />
Figure 557. Direct-fired vertical afterburner (Great<br />
Western Paint Co., a division of Western Wood<br />
Preserving Co., Los Angeles, Calif.).<br />
A serious concern in venting varnish cookers and<br />
similar process equipment to an afterburner is<br />
the danger of flashback and fire. Where scrubbing<br />
equipment is used in the exhaust system prior to<br />
entry to the afterburner, this danger is usually<br />
overcome. Newer installations of afterburners<br />
on this type of equipment have not used a scrubber<br />
upstream from the afterburner. A short section<br />
of ductwork just prior to the entrance of the after-<br />
burner is designed so that the gases and vapors<br />
passing into this section are at a velocity consi-<br />
derably greater than the ilame propagation rate<br />
of the effluent gases in the reverse direction.<br />
Generally, flame propagation rates are 18 to 20<br />
fps, but the ductwork is designed for gas veloci-<br />
ties of about 50 fps.<br />
If there are considerable deposits inside the duct<br />
at the entrance to the afterburner, these deposits<br />
will start to burn after a lengthy afterburner oper-<br />
ation. Fire control dampers, when employed in<br />
the exhaust system ductwork, require constant<br />
maintenance and inspection if they are to be effec-<br />
tive. Several fires and explosions have been<br />
traced to failure of, or slow operation of, these<br />
devices.<br />
Heat-recovery equipment should be seriously con-<br />
sidered when an afterburner is used to control<br />
emissions. Heat exchangers can be installed so<br />
that the hot exhaust gases from the afterburner<br />
are cooled to below 600 "F by transferring waste<br />
heat to either Dowtherm, water (steam), or other<br />
heat-transfer media. The recovered heat then<br />
can be used in jacketed or coil-equipped kettles<br />
or in other plant process equipment. At a recent<br />
installation, a waste heat boiler utilizing the heat<br />
of the afterburner exhaust reduced fuel costs by<br />
supplying process steam in the plant. The payout<br />
time for the waste heat boiler is expected to be<br />
2 to 4 years.<br />
SULFURIC ACID MANUFACTURING<br />
Sulfuric acid is used as a basic raw material<br />
in an extremely wide range of industrial pro-<br />
cesses and manufacturing operations. Because<br />
of its widespread usage, sulfuric acid plants are<br />
scattered throughout the nation near every indus-<br />
trial complex.<br />
Basically, the production of sulfuric acid involves<br />
the generation of sulfur dioxide (SO2), its oxidation<br />
to sulfur trioxide (SO3), and the hydration of<br />
the SO3 to form sulfuric acid. The two main processes<br />
are the chamber process and the contact<br />
process. The chamber process uses the reduction<br />
of nitrogen dioxide to nitric oxide as the oxidizing<br />
mechanism to convert the SO2 to SO3 The<br />
contact process, using a catalyst to oxidize the<br />
SO2 to SO is the more modern and the more<br />
3 '<br />
commonly encountered. For these reasons further<br />
discussion will be restricted to the contact<br />
process of sulfuric acid manufacture.<br />
CONTACT PROCESS<br />
A flow diagram of a "Type S" (sulfur-burning,<br />
hot-gas purification type) contact sulfuric acid<br />
plant is shown in Figure 558. Combustion air is<br />
drawn through a silencer, or a filter when the air<br />
is dust laden, by either a single-stage centrif-<br />
ugal blower or a positive-pressure-type blower.<br />
Since the blower is located on the upstream side,<br />
the entire plant is under a slight pressure, vary-<br />
ing from 1. 5 to 3. 0 psig. The combustion air<br />
is passed through a drying tower before it enters<br />
the sulfur burner. In the drying tower, moisture<br />
is removed from the air by countercurrent scrub-<br />
bing with 98 to 99 percent sulfuric acid at tem-<br />
peratures from 90" to 120°F. The drying tower<br />
has a topside internal-spray eliminator located<br />
just below the air outlet to minimize acid mist<br />
carryover to the sulfur burner.
WEAK hClO<br />
DRIlNG<br />
lDWER<br />
FILTERED AIR<br />
Molten sulfur is pumped to the burner where it<br />
is burned with the dried combustion air to form<br />
SO2. Normally a gas containing approximately<br />
9 percent SO2 is produced in a Type S plant. The<br />
combustion gases together with excess air leave<br />
the burner at about 1,600°F and are cooled to<br />
apprdximately 800°F in a water tube-type waste-<br />
heat boiler. The combustion gases then pass<br />
through a hot-gas filter into the first stage or<br />
"pass" of the catalytic converter at between<br />
750" and 800°F to begin the oxidation of the SO2<br />
to SO3. If the molten sulfur feed has been fil-<br />
tered at the start of the process, the hot-gas<br />
filter may be omitted. Because the conversion<br />
reaction is exothermic, the gas mixture from.<br />
the first stage of the converter is cooled in a<br />
smaller waste-heat boiler. Gas cooling after<br />
the second and third converter stages is achieved<br />
by steam superheaters. Gas leaving the fourth<br />
stage of the converter is partially cooled to ap-<br />
proximately 450 "F in an economizer. Further<br />
cooling takes place in the gas duct before the<br />
gas enters the absorber. The extent of cooling<br />
required depends largely upon whether or not<br />
oleum is to be produced. The total equivalent<br />
conversion from SO2 to SO3 in the four con-<br />
version stages is about 98 percent. Table 193<br />
shows typical temperatures and conversions<br />
at each stage of the four-stage converter. These<br />
figures vary somewhat with variations in gas<br />
composition, operating ra:e, and catalyst con-<br />
dition.<br />
The cooled SO3 combustion gas mixture enters<br />
the lower section of the absorbing tower, which<br />
is similar to the drying tower. The SO3 is ab-<br />
sorbed in a circulating stream of 99 percent<br />
sulfuric acid. The nonabsorbed tail gases pass<br />
overhead through mist removal equipment to<br />
the exit gas stack (Duecker and West, 1959).<br />
Sulfuric Acid Manufacturing 717<br />
MOLTEN<br />
SULFUR Ya"W>>w#:2:>>2.:<br />
A contact process plant intended mainly for use<br />
with various concentrations of hydrogen sulfide<br />
(H2S) as a feed material is known as a wet-gas<br />
Figure 558. ~ i o w diagram of a typical " Type S"<br />
sulfur-burning contact sulfuric acid pl.anf.<br />
~~~ ..........<br />
~~ ....<br />
WEAK IMPURE AClO<br />
Table 193. TEMPERATURES AND CONVERSIONS<br />
IN EACH STAGE OF A FOUR-STAGE CONVERTER<br />
FOR A "TYPE S" SULFUR-BURNING CONTACT<br />
SULFURIC ACLD PLANT<br />
Location of gas<br />
enter in^ 1st pass<br />
.<br />
1<br />
410 1 770<br />
Leaving 1st pass 601.8 1,115<br />
74.0<br />
Entering 2d pass<br />
Leaving 2d pass<br />
Entering 3d pass<br />
Leaving 3d pass<br />
Entering 4th pass<br />
Leaving 4th pass<br />
Total rise<br />
Temperatures,<br />
"C I "F<br />
t<br />
Equivalent<br />
conversion, 70<br />
plant, as shown in Figure 559. The wet-gas<br />
plant's combustion furnace is also used for burn-<br />
ing sulfur or dissociating spent sulfuric acid. A<br />
common procedure for wet-gas plants located near<br />
petroleum refineries is to burn simultaneously<br />
H2S, molten sulfur, and spent sulfuric acid from<br />
the alkylation processes at the refineries. In<br />
some instances a plant of this type may produce<br />
sulfuric acid by using only H2S or spent acid.<br />
In a wet-gas plant, the H2S gas, saturated with<br />
water vapor, is charged to the combustion fur-<br />
nace along with atmospheric air. The SO2<br />
formed, together with the other combustion<br />
products, is then cooled and treated for mist<br />
removal. Gas may be cooled by a waste-heat<br />
boiler or by a quench tower followed by Karhate<br />
and updraft coolers. Mist formed is removed
718 CHEMICAL PROCESSING EQUIPMENT<br />
HYOROGEN SULFIDE<br />
HEAT EXCHANGERS<br />
STAGE<br />
CATALYTIC ABSORBER<br />
...............<br />
...............<br />
...........<br />
%AX ACID STRONG AClO<br />
CDMBUSTION<br />
CHAMBER<br />
820<br />
WASTE<br />
WEAK IMPURE<br />
C<br />
NEAT HUMIOIFYING MIST DRYING ACIO TO STORAGE<br />
BOILER TOWER PRECI PITAIOR TOWER<br />
Figure 559. Flow diagram of a contact-type wet-gas sulfuric acid plant.<br />
by an electrical precipitator. Moisture is re- Table 194. SULFUR TRIOXIDE AND SULFUR<br />
moved from the SO2 and airstream with con- DIOXIDE EMISSIONS FROM TWO ABSORBERS IN<br />
centrated sulfuric acid in a drying tower. A CONTACT SULFURIC ACID PLANTS<br />
centrifugal blower takes suction on the drying<br />
tower and discharges the dried SO2 and air to<br />
the converters. The balance of the wet-gas pro-<br />
cess is essentially the same as that of the pre-<br />
viously discussed sulfur-burning process.<br />
THE AIR POLLUTION PROBLEM<br />
The only significant source of air contaminant<br />
discharge from a contact sulfuric acid plant<br />
is the tail gas discharge from the SO3 absorber.<br />
While these tail gases consist primarily of in-<br />
nocuous nitrogen, oxygen, and some carbon di-<br />
oxide, they also contain small concentrations<br />
of SO2 and smaller amounts of SO3 and sulfuric<br />
acid mist. Table 194 shows the SO2 and SO3<br />
discharged from two wet-gas sulfuric plant ab-<br />
sorbers.<br />
A well-designed contact process sulfuric acid<br />
plant operates at 90 to 95 percent conversion of<br />
the sulfur feed into product sulfuric acid. Thus<br />
a 250-ton-per-day plant can discharge 1.25 to 2.5<br />
tons of SO2 and SO3 per day. When present in<br />
sufficient concentration, SO2 is irritating to<br />
throat and nasal passages and injurious to vege-<br />
Gas flow rate,<br />
scfm<br />
Sulfur trioxide,<br />
grlscf<br />
70 by vol as SO2<br />
lblhr<br />
Sulfur dioxide,<br />
grlscf<br />
70 by vol<br />
Ib/hr<br />
Outlet of<br />
absorber No. 1<br />
9,600<br />
0.033<br />
0.002<br />
2.73<br />
2.63<br />
0.22<br />
216<br />
STACK<br />
Outlet of<br />
absorber No. 2<br />
7,200<br />
0.39<br />
2.4<br />
2.45<br />
151.2<br />
tation. SO2 concentrations greater than 0.25 ppm<br />
cause injury to plants on long exposure. The<br />
permissible limit for humans for prolonged ex-<br />
posure is 10 ppm.<br />
Tail gases that contain SO3, owing to incomplete<br />
absorption in the absorber stack, hydrate and<br />
form a finely divided mist upon contact with at-<br />
mospheric moisture. According to Fairlie<br />
(1936) the process temperature of gas going to<br />
the absorber should be on the lower side of a<br />
temperature range between 150" and 230°C.
The optimum acid concentration in the absorb-<br />
ing tower is 98.5 percent. This concentration<br />
has the lowest SO3 vapor pressure. The partial<br />
pressure of SO3 increases if the absorbing acid<br />
is too strong, and SO3 passes out with the tail<br />
gases. If a concentration of absorbing acid<br />
less than 98.5 percent is used, the beta phase<br />
of SO3, which is less easlly absorbed, is pro-<br />
duced. A mist may also form when the pro-<br />
cess gases are cooled before final absorption,<br />
as in the manufacture of oleum.<br />
Water-based mists can form as a result of the<br />
presence of water vapor in the process gases fed<br />
to the converter. This condition is often caused<br />
by poor performance of the drying tower. Effi-<br />
cient performance should result in a moisture<br />
loading of 5 milligrams or less per cubic foot.<br />
In sulfur-burning plants, mists may be formed<br />
from water resulting from the combustion of<br />
hydrocarbon impurities in the sul
720 CHEMICAL PROCESSING EQUIPMENT<br />
SO2 concentrations resulting from acid plant<br />
startups and upsets could be handled adequately<br />
by a system such as this.<br />
Table 195. SULFUR TRIOXIDE AND SULFUR<br />
DIOXIDE EMISSIONS FROM A TWO-STAGE<br />
ELECTRICAL PRECIPITATOR SERVLNG<br />
Acid Mist Removal<br />
A CONTACT SULFURIC ACID PLANT<br />
Electrical precipitators<br />
Inlet of<br />
precipitator<br />
Outlet of<br />
prec~pitator<br />
Electrical precipitators are widely used for re- Gas flow rate, scfm 13,400 13,100<br />
moval of sulfuric acid mist from the cold SO2<br />
Gas temperature, "F<br />
160 80<br />
gas stream of wet-purification systems. The<br />
wet-lead-tube type is used extensively in this<br />
service.<br />
Average gas velocity, fps<br />
Collection efficiency, a 70<br />
Moisture in gas, %<br />
GOZ, 70 (stack conditions)<br />
36. 5<br />
0.8<br />
5.9<br />
20. 6<br />
93<br />
4.1<br />
6<br />
Tube-type precipitators have also been used for<br />
treating tail gases from SO3 absorber towers.<br />
More recently, however, two-stage, plate-type<br />
02, %(stack conditions)<br />
CO, 70 (stack conditions)<br />
N2. % (stack conditions)<br />
Sulfur trioxide,<br />
9.6<br />
0<br />
83.4<br />
8.4<br />
0<br />
81.2<br />
precipitators have been used successfully. One grlscf<br />
0.062 0.0048<br />
such unit, lead lined throughout to prevent corro- lhlhr<br />
7. 1 0.54<br />
sion, is designed to handle approximately 20, 000 %by volume<br />
0.0042 0.00032<br />
cfm tail gas from a 300-ton-per-day contact<br />
sulfuric acid plant. This wet-gas plant processes<br />
H7S, - sulfur, and spent alkylation acid. Dry<br />
gas containing SO2, carbon dioxide, oxygen,<br />
nitrogen, and 5 to 10 milligrams of acid mist<br />
per cubic foot enters two inlet ducts to the precipitator.<br />
The gas flows upward through distribution<br />
tiles to the humidifying section. This<br />
section contains 5 feet of 3-inch single-spiral<br />
Sulfur dioxide,<br />
grlscf<br />
4. 1 4.1<br />
lblhr<br />
470 460<br />
70 by volume<br />
0.345 0.345"<br />
aA mechanical rectifier was supplying only 36, 000<br />
volts to the precipitator. During normal operation,<br />
silicon rectifiers suIjply 75,000 volts to the electrode<br />
wires. This should increase the acid mist collection<br />
efficiencv appreciablv.<br />
. --<br />
h~ule 53. 1 for "scavenger plants'' is applicable to<br />
tile irrigated by 800 gpm weak sulfuric acid. this plant rather than Rule 53a, which limits ernis-<br />
The conditioned gas then flows to the ionizing<br />
sions of SO2 to 0. 2 percent by volume. This plant<br />
section, which consists of about 75 grounded<br />
recovers SO2 that would otherwise be emitted to the<br />
curtain electrodes and 100 electrode wire ex-<br />
atmosphere.<br />
tensions.<br />
mechanical rectifier was, however, supplying<br />
only 36, 000 volts to the precipitator during this<br />
test. During normal operation, silicon rectifiers<br />
supply 75, 000 volts to the electrode wires.<br />
Ionized gas then flows to the precipitator section<br />
where charged acid particles migrate to the col-<br />
lector plate electrodes. There are twelve 14-<br />
by 14-foot lead plates and 375 electrode wires.<br />
The negative wire voltage is 75, 000. Acid mi-<br />
grating to the plates flows down through the pre-<br />
cipitator and is collected in the humidifying sec-<br />
tion. The gas from the precipitator section flows<br />
to a 5-foot-diameter, lead-lined stack that dis-<br />
charges to the atmosphere 150 feet above grade.<br />
The high-voltage electrode wires are suspended<br />
vertically by three sets of insulators. Horizontal<br />
motion is eliminated by four diagonally placed in-<br />
sulators, which are isolated from the gas stream<br />
by oil seals. All structural material in contact<br />
with the acid mist is lead clad. Electrical wires<br />
are stainless steel cores with lead cladding. Volt-<br />
age ia supplied from a generator with a maximum<br />
capacity of 30 kilovolt-amperes. A battery of<br />
silicon rectifiers supplies 75, 000 volts of direct<br />
current to the electrode wires.<br />
Table 195 shows the sulfur trioxide and sulfur<br />
dioxide emissions from the previously described<br />
two-stage electrical precipitator. The acid mist<br />
collection efficiency was only 93 percent. A<br />
Packed-bed separators<br />
Packed-bed separators employ sand, coke, or<br />
glass or metal fibers to intercept acid mist par-<br />
ticles. The packing also causes the particles to<br />
coalesce by reason of high turbulence in the small<br />
spaces between packing. Moderate-sized particles<br />
of mist have been effectively removed in a 12-inch-<br />
deep bed of l-inch Berl saddles with gas veloc-<br />
ities of approximately 10 fps.<br />
Glass fiber filters have not been very effective<br />
in mist removal because of a tendency on the<br />
part of the fiber to sag and mat. Nevertheless,<br />
experimental reports by Fairs (1958) on acid<br />
mist removal by silicone-treated glass wool are<br />
encouraging. A special fine-glass wool with a<br />
fiber diameter between 5 and 30 microns was<br />
used. The coarser fibers allowed adequate pene<br />
tration of the bed by the mist particles to ensure<br />
a reasonable long life and ~rovided sufficient<br />
support for the finer fibers in their trapping of<br />
the small acid mist particles.
The glass wool was treated by compressing it<br />
into a filter 2 inches thick to a density of 10<br />
pounds per cubic foot. It was then placed in<br />
a sheet metal container and heated at 500°C<br />
for 1 hour. By this treatment, the stresses<br />
in the compressed fibers were relieved, and<br />
the fiber mass could be removed from the mold<br />
without losing shape or compression. The<br />
fibers were then treated with a solution of meth-<br />
yl cblorosilane.<br />
The threshold concentration for mist visibility<br />
after scrubbing has been found experimentally<br />
by Fairs (1958) to be about 3.6 x gram<br />
SO3 per cubic foot. The discharge gases from<br />
the silicone-treated filter had an SO3 concen-<br />
tration of 1. 8 to 2. 5 x 10-4 gram per cubic<br />
foot and no appreciable acid mist plume. A<br />
faint plume became perceptible at approximate-<br />
ly weekly intervals but was eliminated by flush-<br />
ing the filter bed with water. The average tail<br />
gas-filtering rate for the treated filter was<br />
15. 6 cfm per square foot of filtering area for<br />
a pressure drop of 9-112 to 10 inches water<br />
column. According to Fairs, the effective<br />
life of the silicone fiber should be at least<br />
5,000 hours. Garnetted terylene was also<br />
used but was not as efficient as silicone-treated<br />
glass wool. It should, however, prove ade-<br />
quate for less stringent duties. Its life should<br />
be long since it does not require silicone pre-<br />
treatment. The use of untreated glass wool<br />
fiber proved unsatisfactory in reducing the<br />
opacity of the acid mist plume.<br />
Table 196 shows the SO2 and acid mist emis-<br />
sions from the outlet of a typical silicone-<br />
treated, glass fiber mist eliminator. This<br />
control unit processes absorber discharge gas<br />
from a contact sulfuric acid plant. The acid<br />
mist collection efficiency for the fiber glass<br />
mist eliminator was 98. 9 percent. A success-<br />
ful application of a mist eliminator using treat-<br />
Table 196. EMISSIONS OF SULFUR DIOXIDE<br />
AND ACID MIST FROM THE OUTLET OF<br />
A SILICONE-TREATED, GLASS FIBER MIST<br />
ELIMINATOR SERVING A CONTACT<br />
SULFURIC ACID PLANT<br />
Mist<br />
I inlet I<br />
Concentration, . - erlscf 0.035<br />
Concentration, ppm<br />
ZOO<br />
25<br />
Weight, lhlhr<br />
45<br />
0.5<br />
Collection efficiency, 70 I 98.9<br />
Gas<br />
1<br />
flow rate, scfm<br />
Avg gas velocity, fps<br />
Gas temoerature. ~F<br />
Sulfuric Acid Manufacturing 721<br />
1,300<br />
160<br />
ed fiber (Figure 560) bas been made by the<br />
Monsanto Chemical Company (Brink, 1959).<br />
exact treatment given to the fiber is not available<br />
since it is the property of the inventor, J. A.<br />
Brink, Jr.<br />
FIBER PRCKING<br />
Figure 560. Brink fiber mist eliminator (Brink,<br />
1959).<br />
Wire mesh mist eliminators<br />
Wire mesh mist eliminators are usually con-<br />
structed in two stages. The lower stage of<br />
wire mesh may have a bulk density of about<br />
14 pounds per cubic foot, while the upper stage<br />
is less dense. The two stages are separated<br />
by several feet in a vertical duct. The high-<br />
density lower stage acts as a coalescer. The<br />
reentrained coalesced particles are removed<br />
in the upper stage. Typical gas velocities for<br />
these units range from 11 to 18 fps. The kinet-<br />
ic energy of the mist particle is apparently too<br />
low to promote coalescence at velocities less<br />
than 11 fps, and reentrainment becomes a<br />
problem at velocities greater than 18 fps. The<br />
tail gas pressure drop through a wire mesh<br />
mist installation is approximately 3 inches<br />
water column.
722 CHEMICAL PROCESSING EQUIPMENT<br />
Exit sulfuric acid mist loadings of less than 5<br />
milligrams per cubic foot of gas are normally<br />
obtained from wire mesh units serving plants<br />
making 98 percent acid. No type of mechan-<br />
ical coalescer, however, has satisfactorily<br />
controlled acid mists from oleum-producing<br />
plants. Corrosion possibilities from concen-<br />
trated sulfuric acid must be considered in se-<br />
lecting wire mesh material. The initial cost of<br />
wire mesh equipment is modest. The value of<br />
recovered sulfuric acid is usually sufficient to<br />
pay the first investment in 1 or 2 years (Duecker<br />
and West, 1959).<br />
Ceramic filters<br />
Porous ceramic filter tubes have proved success-<br />
ful in removing acid mist. The filter tubes are<br />
usually several feet in length and several inches<br />
in diameter with a wall thickness of about 318 inch.<br />
The tubes are mounted in a horizontal tube sheet,<br />
with the tops open and the bottoms closed. The<br />
tail gases flow downward into the tubes and pass<br />
out through porous walls. Appreciably more fil-<br />
tering area is required for the ceramic filter<br />
than for the wire mesh type. The porous ceramic<br />
filter is composed of small particles of alumina<br />
or similar refractory material fused with a binder.<br />
The maintenance costs for ceramic tubes is con-<br />
siderably higher than those for wire mesh filters<br />
because of tube breakage. Initial installation<br />
costs are also considerably higher than those for<br />
wire mesh. A pressure drop of 8 to 10 inches<br />
water column is required to effect mist removal<br />
equivalent to that of a wire mesh filter. Thus,<br />
operating costs would also be appreciable (Duecker<br />
and West, 1959).<br />
Sonic agglomeration<br />
The principle of sonic agglomeration is also<br />
used to remove acid particles from waste-gas<br />
streams. Sound waves cause smaller particles<br />
in an aerosol to vibrate and thereby coalesce<br />
into larger particles. Conventional cyclone sep-<br />
arators can then be used for removal of these<br />
larger particles. One installation treating exit<br />
stack gases from a contact acid plant has been<br />
reported to remove 90 percent by weight of acid<br />
in the gas stream. The tail gases leaving the<br />
sonic collector contained 2 to 3 milligrams of<br />
100 percent sulfuric acid mist per cubic foot. A<br />
nuisance factor must be taken into consideration,<br />
however, since some of the sound frequencies<br />
are in the audible range (Duecker and West, 1959).<br />
5 microns in size. A considerable amount of<br />
the larger size acid mist particles may he re-<br />
moved; however, the visibility of the stack<br />
plume is not greatly affected, since the smallest<br />
particle size contributes most to visibility. Vane-<br />
type separators operate at relatively high gas<br />
velocities and thus make better use of the parti-<br />
cles' kinetic energy. They have been found to be<br />
moderately effective for contact plants having<br />
wet-purification systems in reducing stack plume<br />
opacities (Duecker and West, 1959).<br />
SULFUR SCAVENGER PLANTS<br />
INTRODUCTION<br />
A sulfur scavenger plant scavenges elemental<br />
sulfur from the waste gases and fuel gases pro-<br />
duced in an oil refinery. Elemental sulfur has a<br />
wide variety of industrial uses and the amount of<br />
sulfur produced by scavenger plants is becoming<br />
increasingly important. As the demand for sul-<br />
fur increases and as natural sulfur deposits are<br />
depleted or their recovery becomes uneconomical,<br />
the price of sulfur will rise. Accordingly, the<br />
'recovery of sulfur from refinery gases containing<br />
high concentrations of hydrogen sulfide (H2S)<br />
becomes increasingly economical. From an air<br />
pollution standpoint, the vital function of the sul-<br />
fur scavenger plant is to prevent or reduce the<br />
emission of sulfur compounds to the atmosphere.<br />
SULFUR IN CRUDE OIL<br />
A11 crude oils contain sulfur and sulfur compounds<br />
in widely varying amounts. Some crudes contain<br />
as little as 0.1 percent while others contain 5.0<br />
percent or more, with most crudes having between<br />
1 and 2 percent by weight of sulfur and sulfur com-<br />
pounds. It is beneficial to refinery operations to<br />
remove the sulfur compounds because of their<br />
deleterious effects. The major effects are illus-<br />
trated in Table 197.<br />
In a variety of crude oil refining processes, most<br />
of the sulfur and sulfur compounds in the original<br />
crude oil are converted to H2S. This H2S gas is<br />
contained in the overhead off-gas streams from<br />
these processes and, after separation of ~roduct<br />
gas fractions, remains in the final waste gases<br />
which usually are used as fuel in refinery heaters.<br />
The burning of these gases results in large quanti-<br />
ties of sulfur dioxide (SO2) being formed and emit- 1<br />
Miscellaneous devices ted from the heater stacks. To reduce emissions<br />
Simple baffles and cyclone separators are not<br />
of SO2 from this source, it is necessary to remove<br />
as much H2S as possible from these gases<br />
effective in collecting particles smaller than before they are used as heater fuel.
Sulfur Scavenger Plants 721<br />
Table 197. EFFECT OF SULFUR COMPOUNDS DURING REFINING<br />
Purpose Primary Effect of contaminant<br />
contaminants<br />
Reduce Mercaptans Offensive product odors 0. 002% max. tolerable con-<br />
odor centration<br />
Reduce<br />
corrosion<br />
Reduce oper-<br />
ational pro-<br />
blems<br />
Improve<br />
gasoline octane<br />
Avoid catalyst<br />
poisoning<br />
Elemental S Attacks iron, copper, etc., forming sulfides<br />
Hzs Attacks zinc, copper, iron<br />
Mercaptans Mildly corrosive<br />
1 Corrosion compounds<br />
I<br />
H2S<br />
Iron sulfide<br />
Sulfur compounds<br />
Improve color, Disulfides<br />
reduce gum<br />
I<br />
Thiophenol<br />
(mercaptan)<br />
Plug exchangers, collect on fractionator trays;<br />
spontaneous combustion during cleanout<br />
Highly toxic (dangerous during cleanout)<br />
Foaming in amine systems (reduced efficiency)<br />
Foaming in caustic wash systems<br />
Oxidation (in simlight) to form acids and haze in gas-<br />
oline~<br />
Catalyst in gum formation reactions<br />
Disulfides In the listed order of severity, sulfur compounds<br />
Sulfides reduce the effectiveness of TEL in raising octane<br />
Thiophene ratings<br />
Elemental S In reforming operations, sulfur compounds are con-<br />
Sulfur compounds verted to H2S which may react with catalyst to form<br />
sulfides<br />
Extend lube Sulfur in gasoline Sulfur compounds formed during combustion drastic-<br />
oil life ally reduce lube oil life<br />
REMOVAL OF H2S FROM REFINERY WASTE GASES RNHZ H2S G RNH3HS<br />
There are numerous methods by which H2S can be<br />
separated from hydrocarbon gas streams, and<br />
other methods are in the development stage. The<br />
three methods which are in common use in the<br />
petroleum industry are illustrated in Table 198.<br />
As noted in the table, the iron-sponge process is<br />
not practical for large gas streams or gas streams<br />
with a high H2S content. This process is used<br />
most commonly in desulfurizing natural gas<br />
streams, and the carbonate or amine absorption<br />
processes are used most commonly in petroleum<br />
refinery operations. Of the latter two processes,<br />
the amine is the most popular since refinery waste<br />
gases generally have H2S concentrations well<br />
suited to this process, and a greater removal effi-<br />
ciency is obtained than by the carbonate process.<br />
Both DEA (diethanolomine) and MEA (monoethano-<br />
lamine) are used, with DEA being preferred since<br />
chemical degradation and make-up rates are lower.<br />
Amine solutions will absorb both HzS and CO2<br />
according to the following reactions:<br />
Absorption of H2S occurs at 100 O F or below, and<br />
rejection of sulfide is active at 240 "F. The amine<br />
desulfurization process, therefore, involves con-<br />
tacting the sour (sulfur bearing) gas stream with<br />
a cool amine solution to absorb the H2S and then<br />
regenerating the amine and stripping the H2S from<br />
the amine solution by heating. A typical amine<br />
H2S removal system is shown in Figure 561.<br />
The treated gas leaving the amine desulfuriaation<br />
process will be used as refinery fuel or be burned<br />
in a flare. It is necessary, therefore, to ensure<br />
proper capacity and funcdoning of the amine sys-<br />
tem. The efficiency of an operating amine system<br />
can readily be determined by laboratory analysis<br />
of the sour inlet gas, treated outlet gas, and acid<br />
outlet gas streams. It may also be necessary to<br />
verify the adequacy of a proposed new amine sys-<br />
tem, and the following parameters may be used<br />
for this purpose:
724 CHEMICAL PROCESSING EQUIPMENT<br />
Progress<br />
Iron sponge<br />
Hot potassium<br />
carbonate<br />
Arnine<br />
(MEA or DEA)<br />
Table 198. REMOVAL OF H2S FROM REFINERY GAS<br />
I Gas to be<br />
treated<br />
I process<br />
stages<br />
Advantages<br />
of method<br />
1. Low gas volume<br />
2. Low H2S content<br />
1. High volume<br />
2. Very sour gas<br />
(5 to 50% H2S)<br />
1. High volume<br />
2. Low or intermediate<br />
H2S content<br />
COOL WATER<br />
I I<br />
1. Reaction to<br />
2. Revivification<br />
or<br />
3. Repacking<br />
1. Absorption<br />
2. Regeneration<br />
by decrease in<br />
pressure<br />
1. Absorption<br />
2. Regeneration<br />
by heat striping<br />
I<br />
1. Low initial cost<br />
2. High efficiency<br />
for gas streams<br />
with low H2S content<br />
1. Will reduce H2S<br />
to less than 0.1% in<br />
very sour gas streams<br />
2. Extensive heat exchange<br />
equipment not<br />
required<br />
3. Absorbent (K2CO3)<br />
does not decrade<br />
readily.<br />
1. Will reduce H2S<br />
to less than 1 grain<br />
I100 ft3 in sour gas<br />
stream<br />
2. DEA preferred over<br />
MEA due to degradation<br />
of MEA by carbonyl<br />
sulfide and carbon<br />
disulfide in gas stream<br />
1. Actual H2S/DEA ratio versus H2SIDEA<br />
equilibrium ratio<br />
2. corrosion limitation<br />
3. absorber column sizing.<br />
Figure 561. Amine desulfurization process. standard parameters.<br />
The following example illustrates the calculations<br />
used in verifying the adequacy of a proposed<br />
amine (DEA) desulfurization system:<br />
Given:<br />
Total sour gas feed, scf/day<br />
= 20x106<br />
Specific gravity of sour gas = 0.8<br />
H2S in sour gas feed, weight % = 2.5<br />
DEA solution circulation rate, gal. /min 1.160<br />
DEA concentration, % = 20<br />
Absorber temperature, O F =lo0<br />
Absorber pressure, psig =I20<br />
Absorber diameter, feet = 5.0<br />
Absorber tray spacing, feet = 2.0<br />
Problem:<br />
Verify performance of proposed system against
Sulfur Scavenger Plants 725<br />
Solution: H2S IDEA (actual) =<br />
1. Actual H2S/DEA ratio: 1907 lb Hzs<br />
2. 5% 20 x lo6 scf/day<br />
Total H2S in feed = --<br />
100 24 hrlday 2. Corrosion limitation:<br />
1<br />
x - 1870 lblhr Total H2S =<br />
1 14 f b<br />
= 0. 109<br />
17,500 lb DEA - (OK)<br />
1907 lbIhr = 56. 0 mol/hr<br />
34 lb/mol<br />
Total H2S in DEA (based on 98% regeneration)<br />
Total DEA = 17' 500 lbIhr - 166.5 mol/hr<br />
= (1870)(0. 02) = 21 1b/hr 105.1 lblmol<br />
Total H2S to absorber = 1907 lb/hr<br />
Total DEA flow (20% solution) = (160 gallmin)<br />
x (60 minlhr) = 9600 gallhr<br />
H2S/DEA ratio (maximum recommended) =<br />
mol<br />
0. 4 - mol<br />
20% DEA = 1920 gallhr<br />
H2S DEA ratio (actual) = -- 56 0.336 (OK)<br />
166.5 - --<br />
80% Hz0 = 7680 gallhr<br />
3. Absorber column sizing:<br />
Total DEA flow = (1920 gal/hr)(9.09 lblgal) +<br />
. Experience has shown that a satisfactory<br />
absorber tower cross-sectional area may be<br />
(7680 gal/hr)(8. 34 lblgal) derived from the following equation (Connors,<br />
1958):<br />
HzSIDEA ratio = - x 100 = 2. 34% (by<br />
81,500<br />
weight)<br />
Partial pressure H2S = (120 psi + 14. 7 psi*)<br />
x (760 mm Hgl14.7 psi) x (2. 34%/100) =<br />
163 mm Hg<br />
lb H2S<br />
HzSIDEA equilibrium = 0. 23 -<br />
lb DEA<br />
(from Figure 562)<br />
HzS PRESSURE, mm.Hg (GAS PHASE)<br />
Figure 562. Equilibrium plats of H2S in 20% DEA<br />
aqueous solution.<br />
*Atmospheric pressure.<br />
where<br />
A = absorber cross-sectional area, ft2<br />
B =Brown tray spacing factor<br />
(24 in. ) = 0. 82<br />
C = Barton plate correction<br />
factor (120 psig) = 1. 56<br />
D = specific gravity of sour gas = 0. 80<br />
K : specific gravity of liquid<br />
PEA) = 1. 09<br />
P = gas pressure, psia = 134.7<br />
Q = acid gas volume, lo6 scflday = 20. 0<br />
T = temperature, 'R = 560<br />
Required absorber diameter, Dr = (23.35)*<br />
-<br />
= 4. 83 ft<br />
-<br />
Actual absorber diameter, Da = 5.0 ft (OK)
726 CHEMICAL PROCESSING EQUIPMENT<br />
THE AIR POLLUTION PROBLEM<br />
The acid gas stream from typical refinery amine<br />
(or potassium carbonate) units will contain we11<br />
over 95 percent acid gas (HZS and carbon dioxide).<br />
I£ desired, there are processes for selective re-<br />
moval of the C02 from the acid gas, but this is<br />
not essential for processing the gas. The remain-<br />
der of the stream will be largely hydrocarbons,<br />
with small amounts of carried-over amine. With-<br />
out air pollution control equipment, this highly<br />
toxic and undesirable gas stream would have to be<br />
burned to convert the H2S to SO2, which is less<br />
toxic but also highly undesirable. <strong>Air</strong> pollution<br />
control equipment is required, therefore, for<br />
reduction of H2S emission to the atmosphere with-<br />
out a corresponding substitution of SO2 emission.<br />
This may be done by conversion of H2S to elemen-<br />
tal sulfur.<br />
AIR POLLUTION CONTROL EQUIPMENT<br />
There are numerous processes by which H2S<br />
can be removed from hydrocarbon gas streams<br />
by conversion to free sulfur, but these are pri-<br />
marily natural gas purification processes. The<br />
sulfur scavenger plant, however, serves solely<br />
to produce free sulfur from EZS streams which<br />
have been removed from hydrocarbon streams by<br />
other (amine, etc.) methods. The standard sulfur<br />
scavenger plant is based on the Claus process<br />
which involves oxidation of one-third of the H2S<br />
to SO2 and then catalytically reacting the remain-<br />
ing H2S with the SO2 to form water and free sul-<br />
fur:<br />
and<br />
The second (catalyzed) reaction usually is accom-<br />
plished in two stages, but more recently, three<br />
stages are being used for more complete conver-<br />
sion. Final off-gas is incinerated to prevent un-<br />
reacted HZS from being emitted into the atmos-<br />
phere. This results in some SO2 emission, al-<br />
though only a fraction of that which would have<br />
been emitted upon combustion of all the HZS.<br />
A variation of the basic Claus (split-stream) pro-<br />
cess is the partial-combustion process wherein<br />
the acid gas stream is partially oxidized by con-<br />
trolling the supply of air. The reaction gas is<br />
cooled to condense sulfur vapors and comingled<br />
with air and a slip-stream of acid gas, which are<br />
burned "in-line" so as to reheat the mixture be-<br />
fore it is passed to the first converter. This<br />
reaction gas is again cooled to condense sulfur<br />
vapors before being reheated by "in-line" com-<br />
bustion of additional slip-stream acid gas and<br />
being passed to the second catalytic converter.<br />
Again, the reaction gas is cooled for condensation<br />
of sulfur and then passed to a coalescer for<br />
removal of the remaining sulfur mist before being<br />
incinerated. All condensed sulfur drains to mol-<br />
ten sulfur storage, from which it may be pumped<br />
out and shipped in the molten state or may be<br />
solidified, by cooling in vats or blocks, for han-<br />
dling in the solid state. The basic split-stream<br />
process is illustrated in Figure 563, and the<br />
more commonly used partial-oxidation process<br />
is illustrated in Figure 564.<br />
However high the efficiency of a scavenger<br />
,$ant, the plant will be a source of SO2 air pol-<br />
lutlon slnce remaining sulfur compounds in the<br />
final vent gas will be incinerated to SO2 and then<br />
discharged through a stack to the atmosphere.<br />
Operation of the scavenger plant, however, re-<br />
duces the amount of SO2 emission in proportion<br />
to the amount of sulfur which is recovered, and<br />
this reduces overall emission of pollutants. In<br />
Los Angeles County. APCD Rule 53. 1 specifies<br />
that the scavenger plant must "substantially"<br />
reduce the amount of pollutants which would<br />
otherwise be emitted. In practice, a well-<br />
designed and properly operated scavenger plant<br />
can be expected to recover more than 90 percent<br />
of the sulfur contained in the acid gas feed. The<br />
criterion for evaluating scavenger plants has,<br />
therefore, been the achievement of a recovery<br />
efficiency of 90 percent or greater. Even at<br />
satisfactory plant efficiency, however, the SO2<br />
concentration in incinerated flue gas may exceed<br />
allowable limits and it may, therefore, be neces-<br />
sary to add dilution air to the incinerated gas<br />
(see Figures 563 and 564) before discharge to the<br />
atmosphere.<br />
The efficiency of an operating scavenger plant<br />
may be determined by methods similar to that<br />
illustrated by the following example:<br />
Given:<br />
Test period, hours<br />
- 24<br />
Total feed gas volume, scf = 600,000<br />
Feed gas composition:<br />
H2S, mol 70 - 65. 0<br />
Hydrocarbons, mol 70 - 2.0<br />
Sulfur produced, short tons = 15. 0<br />
Problem:<br />
Calculate efficiency of sulfur recovery.
ACID GAS<br />
I I<br />
Sulfur Scavenger Plants 727<br />
2/3 STREAM FLUE GAS<br />
1/3 STREAM<br />
OXIDATION<br />
AIR BLOWER<br />
STEAM<br />
OXIDATION UNIT AND<br />
WASTE HEAT BOILER<br />
NO 1<br />
F~gure 563. Sulfur scavenger plant using the spllt-stream process (Giusti, 1965).<br />
Solution: 4. reheater 2: in-line combustion (or other<br />
1. Available sulfur in feed - gas<br />
means) to reheat gases to 450 "F<br />
5. converters 1 and 2: each to have sufficient<br />
600, 000 ft3<br />
379 ft3/mol<br />
0. 65 32 lblrnol<br />
2000 lb/ton<br />
= 16.45 tons<br />
bauxite catalyst that the<br />
ratio of H7S flow (cfs) to<br />
catalyst volume (f't3) does<br />
2. Sulfur recoyery efficiency not exceed 2:l.<br />
15. 0 tons x 100 = 91. 2%<br />
16. 45 tons<br />
In addition to supplying the correct amount of<br />
oxygen (air) to permit conversion reactions to<br />
take place, optimum operating conditions also<br />
must be maintained. The waste beat boiler, cool-<br />
ers, reheaters, and converters must be sized and<br />
operated to achieve conditions approximating<br />
those shown below:<br />
1. Waste heat boiler: boiler feed water to cool<br />
reaction gases Lo 450 "F<br />
2. coolers 1, 2, and 3: each to have capacity to<br />
cool gases to 300 "F<br />
3. reheater 1: in-line combustion (or other<br />
means) to reheat gases to 475 "F<br />
The above conditions can be achieved by various<br />
combinations of vessel sizing and/or flow rates<br />
and need not be further illustrated.<br />
Incineration Requirements<br />
Even in an efficient scavenger plant, a portion of<br />
the H2S in the acid gas is unreacted and passes<br />
through the plant. The final vent gas is composed<br />
of the products of reaction (other than recovered<br />
sulfur) and the inert nitrogen contained in the air<br />
used for supply of reaction oxygen, plus the com-<br />
ponents of the feed stream which do not take part<br />
in the conversion reactions and the unreacted H2S.<br />
Because of the remaining HZS, this vent gas must<br />
be incinerated to convert the H2S to SO2 before<br />
the vent gas is discharged to the atmosphere.<br />
Although the oxidation of H2S is exothermic, it<br />
may be necessary to supply additional heat at the
728 CHEMICAL PROCESSING EQUIPMENT<br />
SLIP STREW"<br />
STEAM<br />
If If<br />
"3<br />
4<br />
LI)<br />
- m<br />
0<br />
4<br />
WASTE HEAT<br />
BOILER - AIR<br />
4<br />
AIR<br />
L<br />
I<br />
2 %<br />
C<br />
"7<br />
- Z<br />
4<br />
I<br />
OXIDATION<br />
-<br />
UNIT<br />
CONDENSER<br />
OXIDATION MOLTEN !<br />
AIR BLOWER AIR FUEL GAS SULFUR -. I<br />
DILUTION<br />
AIR<br />
STORAGE<br />
PIT<br />
F~gure 564 Sulfur scavenger plant uslng the partlal ox~dat~on process (Giustl, 1965)<br />
incinerator in order to maintain incineration tem-<br />
perature for the total vent gas stream. Determina-<br />
tion of incineration requirements is illustrated by<br />
the following example for a scavenger plant simi-<br />
lar to that shown in Figure 563 and for the same<br />
feed stream used in the preceding example. All<br />
data are for a 24-hour period.<br />
Given:<br />
Feed composition:<br />
Total feed, scf = 600,000<br />
H2S, mol %<br />
- 65<br />
CO2, mol %<br />
- 3 3<br />
CH4, mol 70<br />
- 1.5<br />
CzH6, mol %<br />
- -<br />
Sulfur recovery efficiency, 70 = 91. 2<br />
Free sulfur carry-over (0. l%), lb = 30. 0<br />
Final vent gas temperature. "F = 320<br />
Problem A:<br />
Determine quantities of unreacted products pass-<br />
ing through the unit to the incinerator.<br />
Solution:<br />
1. Weight of unreacted H2S<br />
H 2 S - 100 - 91.2 = 8.8%<br />
- 0.5 = 3.77 mol/hr<br />
-<br />
\
Sulfur Scavenger Plants 729<br />
2. Weight of unreacted GO2 6. Reaction quantities for hydrocarbon oxidation :<br />
Problem B:<br />
Using basic reaction formulas, determine quantities<br />
of reaction products from oxidation of both<br />
H2S and hydrocarbons.<br />
Solution:<br />
0.79N2<br />
N2 = 3.14 mollhr x = 11. 8 mollhr<br />
0.2102 -<br />
GO2 = l(0. 99) f 2(0. 33) = 1. 65 mol/hr<br />
HzO= 2(0. 99) t 3(0. 33) = 2.molIhr<br />
7. Total reaction products (other than sulfur):<br />
--<br />
1. Basic reaction formulas for conversion of H2S: from 3 from 6<br />
HzS<br />
3<br />
- 02 -SO2<br />
2<br />
i Hz0<br />
H20 = 60.1 t 2.97 = 63. mollhr<br />
3<br />
3HZS + - 02-<br />
2<br />
2. Weight of reacted H2S :<br />
3S t 3H20 Problem C:<br />
Determine quantity of free sulfur carry-over.<br />
Solution:<br />
1. Weight of sulfur carrv-over<br />
= 60.1 mollhr<br />
15.0 tons (0.001)~2000<br />
S2 = lblton<br />
3. Weight of H2S reaction products (other than<br />
-<br />
sulfur) :<br />
24 hr x 64 lb/mol<br />
= 0.02 mollhr<br />
Oxidation of H2S to SO2 is rapid and essentially<br />
3 mol<br />
HO-- x 60.1 mollhr = 60.1 mol/hr<br />
2 - 3 mol<br />
complete at temperatures over 1050 "F, provided<br />
there is adequate air supply and mixing. For<br />
given operating conditions, additional fuel gas and<br />
air requirements may be determined and effectiveness<br />
of incineration may be verified.<br />
4. Reaction formulas for oxidation of<br />
hydrocarbons :<br />
CH4 t 202-C02 + 2H20<br />
7<br />
I CZH6 + 02- 2C02 i 3H20<br />
I<br />
5. Weight of hydrocarbons in feed stream:<br />
i = 0. 99 mollhr<br />
i<br />
= 0. mollhr<br />
Given:<br />
Vent gas temperature, "R = 320 t 460 = 780<br />
Incineration pressure, psia = 14.7<br />
Proposed incineration temperature. "R = 1700<br />
Excess air to be supplied, % = 25<br />
Dimensions of incinerator chamber = 4 ft diam<br />
x 8 it long<br />
Heat value (refinery gas), ~tu/ft~ = 800<br />
Temperature refinery gas and air, "F = 100<br />
Problem D:<br />
Determine air required for incineration of vent<br />
gas stream.
730 CHEMICAL PROCESSING EQUIPMENT<br />
Solution: COz=21.70 + 1.65 = 23.35 mollhr<br />
Composition,<br />
mol/hr Oxidation reaction<br />
3<br />
H2S = 3.77 HZS + - 02 -SOZ + H20<br />
2<br />
GO2= 23.35 C02 C02<br />
N2 = 113. 0 (from reaction) = 113.00 mol/hr<br />
N2 = 21.45 (from<br />
incineration) = 21.45mollhr<br />
<strong>Air</strong> = 6.78 (excess, from<br />
incineration) = 6.78 mollhr<br />
H20= 63.07 H20 Hzo 2. Total heat required<br />
Hi (at initial<br />
N2 =<br />
124. 80<br />
215. Oi2 N2<br />
SO2<br />
H2(1700 'F) - temperature) = Btulmol<br />
25,350 16,200 (780 "R) 9,150<br />
1. Theoretical O2 required<br />
3<br />
02 = 2(0. 02) + $3.77) = 5.70 mollhr<br />
2. Amount N2 for required 02<br />
79%<br />
N = 5.70 x - = 21.45 mollhr<br />
2 21%<br />
3. Excess air for 25% excess<br />
<strong>Air</strong> = 0. 25<br />
Problem E:<br />
(5' 70<br />
(0.21)<br />
= 6. 78 mol/hr<br />
Determine heat evolved from incineration of vent<br />
gases.<br />
Solution: Heat<br />
value,<br />
mollhr x lb/mol x Btullb = Btulhr<br />
----<br />
S2 0.02 64 3.984 5.100<br />
HZ 3.77 2 51,593 389,000<br />
S 3.77 3 2 3,984 480,500<br />
Total evolved heat 874,600<br />
Problem F:<br />
Calculate heat required to raise other (reacted)<br />
vent gases from 320 "F to 1240 "F.<br />
Solution:<br />
1. Total vent gases, after oxidation of comhustibles<br />
(from quantities in Problems A, C, and<br />
D, and reaction equations in Problem D)<br />
SO, = Z(0.02) + lf3.77) = 3.81 mollhr<br />
H20 34,450 21,650 (780 "R) 12,800<br />
C02 14,270 3,510 (780 a) lo, 760<br />
N2 9,600 2, 850 (780 "R) 6.750<br />
N i 9,600 1, 310 (560 OR) 8,290<br />
<strong>Air</strong> 9,680 1, 310 (560 "R) 8, 370<br />
3. Total heat required for incineration<br />
mollhr x Btulmol = Btu/hr<br />
s02 3. 81 9,150 34,800<br />
Hzo 66.84 12,800 856,000<br />
C02 23. 35 10,760 255,000<br />
N2<br />
113.00 6.750 763, 000<br />
N 2 21.45 8.290 177, 800<br />
<strong>Air</strong> 6.78 8, 370 56,700<br />
Total<br />
4. Additional heat required<br />
= 2,143, 300<br />
H = Heat required to raise products to 1240<br />
"F, minus incineration-evolved heat.<br />
H : 2,143, 300 - 874,600 = 1,268,700 Btulhr<br />
5. Refinery gas required for additional heat<br />
H = 800 Btu/ft3 x 379 ft3/mol = 313,000<br />
Btulmol<br />
1,268,700 Btu/hr = 4. O5<br />
Weight required =<br />
313,000 Btulmol mollhr<br />
Hz0 = (3.77) + 63. 07 = 66.84 mollhr Use 5. 0 mol/hr (for safety factor)
Sulfur Scavenger Plants 731<br />
When burning refinery gas to obtain the required<br />
additional heat necessary to achieve the proposed<br />
Total = 2, 390,600 Btulhr<br />
incineration temperature, additional air for corn- 3. Anticipated incinerator temperature<br />
bustion of this gas must be supplied. The required<br />
air can be determined by calculations similar T - T -<br />
2 1- -<br />
to those above. A final incineration heat balance<br />
M C ~<br />
can then be made, and the actual incineration<br />
temperature can be predicted. It is also neces- Where<br />
sary that the incinerator mixing velocity be such<br />
that the retention time exceeds 0. 3 second to T2 = final temperature, "F<br />
ensure adequate time for conversion of H2S to<br />
SO2. TI = initial temperature, "F<br />
Given: AQ = heat added, Btulhr<br />
Refinery gas essentially 75% CH4 and 25% C2H6. W = weight of gas, lb<br />
Excess air for combustion = 25%<br />
Problem G:<br />
Determine air requirement for fuel gas combus-<br />
tion and make final heat balance of combined vent<br />
gases, fuel gas, and air for fuel gas. Determine<br />
predicted incineration temperature and check<br />
against desired 1240 "F. Verify incinerator<br />
mixing velocity and retention time.<br />
Solution:<br />
1. <strong>Air</strong> requirement for 5. 0 mollhr fuel gas<br />
7 5<br />
02 = 2(-x 5. 0 mollhr) = 7. 5 mollhr<br />
100<br />
7 25<br />
02 = - (-x 5. 0 mollhr) = 4. 375 mol/hr<br />
2 100<br />
Required O2 = 7.5 + 4. 375 = 11. 875 mollhr<br />
Required N2 = - O' 79 x 11. 875 = 44.7 mollhr<br />
0. 21<br />
25 11. 875<br />
Excess air = - x --- mollhr = 14. 14<br />
100 0.21<br />
mollhr<br />
Additional C02 and H20 from fuel gas<br />
H20 = 2(3. 75) t 3(1. 25) = 11. 25 mol/hr<br />
C = specific heat of gas.<br />
P<br />
Assumed:<br />
(149)<br />
TI = 300 "F (vent gas at 320 "F; fuel and air<br />
at 100 O F )<br />
Cp = 0.27 (based on weighted average of C<br />
P<br />
for major vent gas components)<br />
Incinerator heat loss = 15%<br />
W, weight of gases (Problem F and 1 above)<br />
mol/hr x lblmol = lb/hr<br />
C02 = l(3. 75) t 2(1. 25) = 6. 25 mol/hr (checks with assumed temperature of 1240 "F,<br />
allowing for heat-loss);. - ,<br />
2. Total heat added<br />
! 4. Incinerator mixing velocity:<br />
I<br />
1<br />
.~.. ,<br />
Incineration heat (Problem E) = 874.600 Btulhr<br />
Volume of gases<br />
mol<br />
Btu<br />
Fuel gas heat = 5. 0 - x 379 Gl ft3 x 800 - to incinerator<br />
hr<br />
= 311.57 mollhr x 379 ft3/hr<br />
ft3
7 32 CHEMICAL PROCESSING EQUIPMENT<br />
Area of<br />
incinerator<br />
- 3. 14(4)2 = 12. 56 ft2<br />
- 4<br />
119,700 ft3/hr 1<br />
Mixing velocity = 3600 sec/hr 12.56 ft<br />
5. Retention time:<br />
= 2. 65 fps<br />
Length of incinerator = 8. 0 ft<br />
Retention time = O ft = 3 seconds (OK)<br />
2. 65 ft/sec<br />
Stack Dilution <strong>Air</strong><br />
As shown in the preceding problems, unconverted<br />
HzS and carried-over sulfur are burned in the<br />
incinerator to SO2. However, even when the<br />
scavenger plant is operating efficiently, the vol-<br />
ume concentration of SO2 in the flue gas from the<br />
incinerator may be high. To meet acceptable con-<br />
centration levels at the stack, dilution air may<br />
be added at the base of the stack. The required<br />
amount of dilution air may be determined as<br />
illustrated in the following problem.<br />
Maximum allowable SO2 = 0. 2 vol %<br />
Solution:<br />
1. Undiluted SO2 concentration<br />
3. 81<br />
volume 70 = - x 100 = 1.207%<br />
311.57<br />
2. Required dilution air<br />
Total volume required - 3. 8lmollhr x 379 ftlmol'<br />
for 0. 2% SO2<br />
60 minlhr x 0.002<br />
Total incinerator gas =<br />
= 12,040 cfm<br />
311. 57 mollhr x 379 ft3/mol = 1970 cfm<br />
60 minlhr<br />
Required air = total volume - incinerated gas<br />
: 12, 040 - 1970 = 10,070 cfm<br />
Use 11,-cfm blower<br />
In some localities, there may be no established<br />
criterion for maximum allowable SO2 concentra- ;<br />
tion in the flue gas. Nevertheless, SO2 is itself<br />
toxic and it is necessary to ensure that safe<br />
ground level concentrations are not exceeded.<br />
Additionally, some of the SO2 may be oxidized<br />
to SO3 which, in turn, could combine with atmo- I<br />
spheric moisture to form acid mist and lead to I<br />
extensive ground level damage. The additionof<br />
1<br />
dilution air, in addition to reducing SO2 concen-<br />
1<br />
tration, has the added advantage of "quenching"<br />
the incinerated gases and greatly reducing SO3<br />
formation.<br />
Incinerator Stack Height<br />
Where there is no set limit for stack SO2 concentration,<br />
an attempt may be made to keep<br />
ground level concentration to an acceptable level<br />
by providing a high stack. The required stack<br />
height may be determined by methods illustrated<br />
in the following example:<br />
i<br />
!<br />
Example<br />
Problem:<br />
Determine the SO2 concentration in the flue gas<br />
and the amount of dilution air required to lower<br />
the concentration to an acceptable level.<br />
Given:<br />
Total SO2 (from Problem G)= 3. 81 mollhr<br />
Given:<br />
Total flue gas volume, cfm<br />
SO2 concentration, vol %<br />
Allowable SO2 at 500 ft from base<br />
of stack, ppm<br />
Wind velocity, mph<br />
Solution:<br />
= 2000<br />
=0.5<br />
= 3<br />
= 10<br />
1<br />
I 1<br />
I<br />
!<br />
i<br />
!<br />
I<br />
Total gas = 311.57 mollhr The general concentration equation is<br />
I<br />
1<br />
where<br />
C = ground concentration, it3 50~/10~ ft3 air i<br />
M = SO2 emission rate, tonslday<br />
V = wind velocity, mph<br />
X = distance from stack base, ft !<br />
K = e (-20 HIX)<br />
(Values of K are plotted in Figure 565. )<br />
2000 cfm x 0.005 1440 min/day<br />
M =<br />
5. 93 ft31lb 2000 lblton<br />
= 121 tonslday<br />
1<br />
1 i<br />
I<br />
i<br />
!<br />
i
VALUE OF COEFFICIENT K<br />
stack for any stack height and wind veincity (Steinbock,<br />
1952).<br />
Stack height (from Figure 565). H = 130 ft<br />
Maximum ground level concentration will occur<br />
roughly at a distance of ten times the stack height<br />
from the stack. Since this distance derives from<br />
the stack height not yet determined, the required<br />
stack height to limit the ground concentration at<br />
any point must be determined. The height may be<br />
determined from Figure 566 by the following steps:<br />
1. Draw a vertical line at 121 tons per day until<br />
it intersects the 10-mph curve.<br />
2. From this intersection, draw a horizontal line.<br />
3. Draw a vertical line from 3 ppm mtil it inter-<br />
sects the horizontal line.<br />
RATE OF EMISSION OF POLLUTANTS, tons/day<br />
MAXIMUM SOZGROUND CONCENTRATION, ppm<br />
Figure 566. Maximum ground concentration of sulfur<br />
dioxide discharged from stacks of various heights<br />
(Steinbock. 1952).<br />
Sulfur Scavenger Plants 733<br />
4. The pocnt of intersection is the required stack<br />
height.<br />
By interpolation of height curves, H = 230 ft.<br />
Plant Operational Procedures<br />
Even in properly designed plants, operational<br />
problems which result in decreased sulfur re-<br />
covery efficiency and consequent increase in H2S<br />
vented to the incinerator are often encountered.<br />
Most of these problems stem from either varia-<br />
tion in the feed gas stream or from inadequate<br />
reaction air and/or cooling water controls.<br />
Problems in these areas, in turn, affect the cata-<br />
lyst and may drastically reduce conversion<br />
efficiency.<br />
Based upon a fixed feed stream composition, the<br />
correct amount of oxidation air can readily be<br />
controlled by flow controllers. However, feed<br />
gas composition monitoring equipment is expen-<br />
sive and difficult to maintain and is therefore<br />
not generally used. The H2S content can be<br />
readily checked, and this is generally done on a<br />
periodic basis, with air supply adjustment as<br />
necessary.<br />
A more difficult problem is an increase in the<br />
hydrocarbon content, which may not be detected<br />
until the imbalance in feed is reflected in ahnor-<br />
ma1 temperature differentials in the waste heat<br />
boiler, coolers, and/or reactors. It is necessary,<br />
therefore, that operating temperatures he con-<br />
stantly monitored to detect hydrocarbon fluctua-<br />
tions. If not, the excess hydrocarbons will dis-<br />
rupt the oxygen supply and not permit full H2S<br />
conversion to SO2 in the first-stage reaction.<br />
Secondly, if not controlled, temperatures may<br />
rise to the point of equipment damage and pos-<br />
sible breakdown. Thirdly, the excess hydrocar-<br />
bons may not be fully oxidized in the controlled<br />
air supply and, particularly when the hydrocar-<br />
bons are heavier than C3 fractions, carbon may<br />
be deposited on the catalyst and thus reduce con-<br />
version efficiency.<br />
As discussed earlier, a two-stage conversion<br />
plant should attain a sulfur recovery efficiency of<br />
at least 90 percent. Many modern plants will<br />
yield 94 to 96 percent, and the efficiencies may<br />
he raised to as high as 98 percent where a third<br />
stage is used. Although the addition of a third<br />
stage to an existing two-stage plant may not be<br />
economical solely on a sulfur recovery basis,<br />
the provision of a third stage in new plants is<br />
relatively inexpensive. In either case, this third<br />
stage is highly desirable from an air pollution<br />
standpoint since large amounts of pollutant are<br />
represented by each percentage point of recovery<br />
efficiency. Also, the third stage can act as a<br />
stand-by for the other stages during upset con-
7 34 CHEMICAL PROCE :SSING EQUIPMENT<br />
ditions. As activity of catalyst in the first stage<br />
is reduced (by surface deposits of sulfur or car-<br />
bon), abnormal quantities of HzS will pass<br />
through. However, this can then be reaCtkd in<br />
the subsequent stages and high efficiencies can<br />
still be maintained until the first stage catalyst<br />
is reactivated. With the additional flexibility of<br />
three stages, any one of the stages can be by-<br />
passed for replacement of inactive catalyst.<br />
Generally, however, catalyst is reactivated rather<br />
than replaced, and this is done during periodic<br />
plant maintenance shutdown. Sour gas feed is<br />
shut off and fuel gas is burned with theoretical<br />
air in the combustion chamber until all sour gas<br />
is purged from the reaction system, Thereafter,<br />
air supply is raised to 25 percent excess for a<br />
period and then to 50 percent excess. In doing so,<br />
the hot excess air burns surface carbon and<br />
sulfur from the reactor catalyst, and this is<br />
reflected by a temperature rise across the re-<br />
actor. When the temperature becomes equal at<br />
inlet and outlet, surface material has been fully<br />
oxidized and the catalyst is reactivated.<br />
In all areas where either oil refining or sour<br />
natural gas processing operations take place,<br />
the sulfur scavenger plant may be one of the most,<br />
if not the most, important single air pollution<br />
control method. The provision of such plants,<br />
properly designed and efficiently operated, will<br />
prevent the emission to the atmosphere of intoler-<br />
ably high amounts of material which is toxic to<br />
both man and his environment.<br />
Inasmuch as the gas treating plants, as well as<br />
the sulfur recovery facilities, are processing a<br />
highly odorous and toxic gas, it is of utmost<br />
importance that the area be designed for easy<br />
cleaning oi spills, and that an efficient plant<br />
housekeeping program be followed.<br />
Tail Gas Treofmenf<br />
As previously discussed, the main portion of the<br />
sulfur from the crude oil can be removed and recovered.<br />
Recent attention to further reduced<br />
ambient air quality standards for sulfur oxides<br />
reauires added treatment to the tail u gases from<br />
the scavenger plants. Conversion efficiencies<br />
must be raised from the 90 to 95 percent range<br />
to a level greater than 99 percent.<br />
Two approaches are available to achieve the addi-<br />
tional clean-up. One requires the tail gas efflu-<br />
ent from the sulfur plant to be totally in an oxi-<br />
dize? *tate. The gas is then conditioned and<br />
treated by chemical scrubbing. The treated gas,<br />
containing less than 100 ppm sulfur compounds,<br />
discharges to the atmosphere, the chemical solu-<br />
tion is regenerated, and the resulting sulfur<br />
oxides are recycled back to the sulfur recovery<br />
units. This process was developed by Wellman-<br />
Power Gas, Inc.. of Lakeland, Florida.<br />
The other approach to this problem is to provide<br />
the tail gas in a reduced state of H2S before chemi-<br />
cally scrubbing. It is necessary to minimize car-<br />
bon disulfide and carbonyl sulfides to prevent high<br />
degradation of the scrubbing solution. Two pro-<br />
cesses of this type are known as the Beavon Stret-<br />
ford (R. M. Parsons Go. of Los Angeles, Califor-<br />
nia) and the Cleanair (The Pritchard Companies of<br />
Kansas City, Missouri). Both processes are<br />
capable of reducing the sulfur compounds to less<br />
than 100 ppm.<br />
Various other approaches to tail gas treatment<br />
are in development stages. Many involve chemi<br />
cal scrubbing of the gaseous streams although<br />
adsorption and molecular separation processes<br />
are being evaluated.<br />
PHOSPHORIC AClD MANUFACTURING<br />
During the past 20 years, the use of phosphorus-<br />
containing chemical iertilizers, phosphoric acid,<br />
and phosphate salts and derivatives has increased<br />
greatly. In addition to their very large use in<br />
fertilizers, phosphorus derivatives are widely<br />
used in food and medicine, and for treating water,<br />
plasticizing in the plastic and lacquer industries,<br />
flameproofing cloth and paper, refining petroleum,<br />
rustprooiing metal, and lor a large number of<br />
miscellaneous purposes. Most of the phosphate<br />
salts are produced for detergents in washing<br />
compounds.<br />
With the exception of the iertilizer products,<br />
most phosphorus compounds are derived from<br />
orthophosphoric acid, produced by the oxidation<br />
of elemental phosphorus. At present, elemental<br />
phosphorus is manufactured on a large enough<br />
scale to be classed as a heavy chemical and is<br />
shipped in tank cars from the point of initial<br />
manufacture, where the raw materials are inex-<br />
pensive, to distant plants for its conversion to<br />
phosphoric acid, phosphates, and other compounds.<br />
PHOSPHORIC AClD PROCESS<br />
Generally, phosphoric acid is made by burning<br />
phosphorus to form the pentoxide and reacting<br />
the pentoxide with water to form the acid. Spe- ~<br />
cifically, liquid phosphorus (melting point 112'F) !<br />
is pumped into a refractory-lined tower where it<br />
is burned to form phosphoric oxide, P4Ol0, which<br />
is equivalent algebraicly to two molecules of the<br />
theoretical pentoxide, PZ05, and is, therefore,<br />
commonly termed phosphorus pentoxide:
An excess of air is provided to ensure complete<br />
oxidation so that no phosphorus trioxide (P203)<br />
or yellow phosphorus is coproduced. The reac-<br />
tion is exothermic, and considerable heat must<br />
be removed to reduce corrosion. Generally,<br />
water is sprayed into the hot gases to reduce<br />
their temperature before they enter the hydrating<br />
section.<br />
Additional water is sprayed countercurrently to<br />
the gas stream, hydrating the phosphorus pent-<br />
oxide to orthophosphoric acid and diluting the<br />
acid to about 75 to 85 percent:<br />
The hot phosphoric acid discharges continuously<br />
into a tank, from which it is periodically rc-<br />
moved for storage or purification. The tail gas<br />
from the hydrator is discharged to a final col-<br />
lector where most of the residual acid mist is<br />
removed before the tail gas is vented to the air.<br />
A general flow diagram for a phosphoric acid<br />
plant is shown in Figure 567.<br />
Figure 567. General flow diagram for phosphoric<br />
acid production.<br />
The raw acid contains arsenic and other heavy<br />
metals. These impurities are precipitated as<br />
sulfides. A slight excess of hydrogen sulfide,<br />
sodium hydrosulfide, or sodium sulfide is add-<br />
ed and the treated acid is filtered. The excess<br />
hydrogen sulfide is removed from the acid by<br />
air blowing.<br />
The entire process is very corrosive, and<br />
special materials oi construction are required.<br />
Stainless steel, carbon, and graphite are com-<br />
monly used for this severe service.<br />
Special facilities are required for handling<br />
elemental yellow phosphorus since it ignites<br />
Phosphoric Acid Manufacturing 735<br />
spontaneously on contact with air at atmospher-<br />
ic temperatures and is highly toxic. Phosphorus<br />
is always shipped and stored under water to pre-<br />
vent combustion. The tank car of phosphorus is<br />
heated by steam coils to melt the water-covered<br />
phosphorus. Heated water at about 135°F is<br />
then pumped into the tank car and displaces the<br />
phosphorus, which flows into a storage tank.<br />
A similar system using hot displacement water<br />
is irequently used to feed phosphorus to the<br />
burning tower.<br />
THE AIR POLLUTION PROBLEM<br />
A number oi air contaminants, such as phosphinc,<br />
phosphorus penlosiiie, hydrogen suliide, and phos-<br />
phoric acid niist, may be released by the phosphor-<br />
ic acid process.<br />
Phosphine (pH3), a very toxic gas, may be formed<br />
by the hyrlralysis of metallic phosphides that exist<br />
as impurities in the phosphorus. When the tank<br />
car is opened, the phosphine usually ignites spon-<br />
taneously hut only momentarily.<br />
Phosphorus pcntoxide (P4010), created when<br />
ptiosphorus is burned with excess air, forms<br />
an extremely dense fume. Our military forces<br />
takc advantage oi this property by using this con-<br />
pound to form srnolie screens. The fumes are<br />
submicron in size and are 100 percent opaque.<br />
Except for this military use, phosphorus pent-<br />
oxide is never released to the atmosphere unless<br />
phosphorus is accidentally spilled and exposed<br />
to air. Since handling elemental phosphorus is<br />
extremely hazardous, stringent safety precau-<br />
tions are mandatory, and phosphorus spills are<br />
very infrequent.<br />
Hydrogen sulfide (H2S) is released from the acid<br />
during treatment with NaHS to precipitate sulfides<br />
of antimony and arsenic and other heavy metals.<br />
Removal of these heavy metals is necessary for<br />
manufacture of good grade acid. H2S is highly<br />
toxic and flammable. Health authorities recom-<br />
mend a maximum allowable concentration of this<br />
gas of 20 ppm for an 8-hour exposure. The odor<br />
threshold is 0. 19 ppm (Gillespie and Johnstone,<br />
1955). In practice, however, H2S is blown from<br />
the treating tank and piped to the phosphorous-<br />
burning tower where it is burned to SO2. Source<br />
test information indicates that the concentration<br />
of SOZ in the gaseous effluent from the acid tower<br />
scrubberwillnot exceed 0.03 volume percent. Evo-<br />
lution of H2S is also minimized by restricting the<br />
amount of NaHS in excess of thatneeded to precipitate<br />
arsenic and antimony and other heavy metals.<br />
The manufacture of phosphoric acid cannot he<br />
accomplished in a practical way by burning<br />
phosphorus and bubbling the resultant products<br />
through either water or dilute phosphoric acid
736 CHEMICAL PROCESSING EQUIPMENT I<br />
(Slaik and Turk, 1953). When water vapor comes<br />
into contact with a gas stream that contains a<br />
volatile anhydride, such as phosphorus pentoxide,<br />
an acid mist consisting of liquid particles of var-<br />
ious sizes is formed almost instantly. An investi-<br />
gation (Brink, 1959) indicates that the particle<br />
size of the phosphoric acid aerosol is small,<br />
about 2 microns or less, and that it has a median<br />
diameter of 1.6 microns, with a range of 0. 4 to<br />
2.6 microns.<br />
The tail gas discharged from the phosphoric acid<br />
plant is saturated with water vapor and produces<br />
a 100 percent opaque plume. The concentration<br />
of phosphoric acid in this plume may be kept<br />
small with a well-designed plant. This loss<br />
amounts to 0.2 percent or less of the phosphorus<br />
charged to the combustion chamber as phosphorus<br />
pentoxide.<br />
HOODING AND VENTILATION REQUIREMENTS<br />
All the reactions involved take place in closed<br />
vessels. The phosphorus-burning chamber and<br />
the hydrator vessel are kept under a slight neg-<br />
ative pressure by the fan that handles the effluent<br />
gases, as shown in Figure 520. This is neces-<br />
sary to prevent loss of product as well as to pre-<br />
vent air pollution.<br />
The hydrogen sulfide generated during the acid<br />
purification treatment must be captured and collected,<br />
and sufficient ventilation must be provided<br />
to prevent an explosive concentration, for<br />
hydrogen sulfide has a lower explosive limit of<br />
4. 3 percent. The sulfiding agent must be carefully<br />
metered lnto the acld to prevent excessively<br />
rap~d evolution of hydrogen sulfide.<br />
AIR POLLUTION CONTROL EQUIPMENT<br />
The hydrogen sulfide can he removed by chem-<br />
ical absorption or by combustion. Weak solu-<br />
tions of caustic soda or soda ash sprayed<br />
countercurrently to the gas stream react with<br />
the hydrogen sulfide and neutralize it:<br />
NaZC03 t H2S - NaHC03 t NaHS<br />
The hydrogen sulfide may also he oxidized in<br />
a suitable afterburner:<br />
The phosphoric acid mist in the tail gas is<br />
commonly removed by an electrical precip-<br />
itator, a venturi scrubber, or a Brink fiber<br />
mist eliminator (Brink, 1959). All are very<br />
effective in this service.<br />
The Tennessee Valley Authority has used elec-<br />
trical precipitators for many years to reduce<br />
the emission of phosphoric acid mist (Striplin,<br />
1948). Severe corrosion has always been a<br />
problem with these precipitators. Published<br />
data (Slaik and Turk, 1953) indicate that the<br />
problem has been partially solved by reducing<br />
the tail gas temperature to 135' to 185°F.<br />
The acid discharged amounts to about 0. 15 per-<br />
cent of the phosphorus pentoxide charged to the<br />
combustion chamber as phosphorus. The rela-<br />
tively low gas temperatures and consequently<br />
infrequent failure of the wires are given as the<br />
reason for the high mist recovery from the gas<br />
Stream.<br />
The TVA replaced one of the electrical precip-<br />
itators wlth a venturi scrubber in 1954. The<br />
venturi scrubber is constructed of stainless<br />
steel and is 14 feet 6 inches high, with a 30-<br />
inch-diameter inlet and outlet and a 11-112-<br />
inch-diameter throat (Barber, 1958). The<br />
scrubber is followed by a centrifugal entrain-<br />
ment separator. Stack analyses of emissions<br />
from this production unit are summarized in<br />
Table 199.<br />
Table 199. STACK ANALYSES OF EMISSIONS<br />
FROM A PHOSPHORIC ACID PLANT<br />
WITH A VENTURI SCRUBBER<br />
Phosphorus burning rate, lhlhr 2,650<br />
Temperature, *F<br />
Vaporizer outlet 1,650<br />
Burner outlet 880<br />
Venturi scrubber outlet 195<br />
Stack gas 175<br />
Pressure drop, in. WC<br />
Across venturi scrubber 25.2<br />
Across entrainment separator 1.9<br />
Emissions as % of phosphorous burned 0.2<br />
In 1962, the TVA constructed a stainless steel<br />
phosphoric acid unit that has an adjustable ven-<br />
turi scrubber, followed by a packed scrubber,<br />
and a wire mesh mist eliminator. When the<br />
venturi scrubber is adjusted to give a pressure<br />
drop of 37 inches of water column or higher,<br />
losses of P205 from the unit amount to only<br />
about 5 pounds per hour at phosphorus-burn-<br />
ing rates up to 6,000 pounds per hour.<br />
Considerable research and development work<br />
by the TVA demonstrated that good recovery of<br />
phosphoric acid mist could be achieved by intro-<br />
ducing water vapor into the hot gases from the<br />
combustion of phosphorus, passing the mixture<br />
through a packed tower, and condensing it<br />
(Slaik and Turk, 1953).
. .<br />
Soap, Fatty Acid, and Glycerine Manufacturing Equipment 737<br />
A large-scale plant using a Raschig ring-packed The Brink (1959) [iber mist eliminator is a relatower<br />
Sollowed by three gas coolers was built. tively new type of collector that has been used<br />
1<br />
1<br />
Overall p21aspRorus pcntoxide recovery exceeded<br />
99. 9 percent, hut the process was eventually<br />
alianiloneil bccausr of the ercessivc rate of corrosuccessfully<br />
on sulfuric acid mist, oleurn, phosphoric<br />
acid, ammonium chloride fume, and various<br />
organics. Collectors of this type have been dission<br />
of the gas coolers. cussed in the preceding section of this chapter.<br />
This same process, with a second packed scrubber<br />
or glass fiber-packed iiltcr unit for acid mist re-<br />
moval replacing the gas cooler, is used by a number<br />
of phosphoric acid producers throughout the country.<br />
Thcsr plants routinely operatc with phosphorus<br />
pentoxiclr recovery efiiciencies in exccss of 99. 8<br />
perccnt. H visible pliosphoric acid plume still<br />
rzmains, though the phosphorus content has been<br />
rccluced to lcss than 0. 1 grain pcr scS. A plant<br />
such as this is in opcration in Los Angeles County<br />
and is shoxm in Figure 568. Thc plume contains<br />
a large pcrcentagc of watcr vapor and does not<br />
violatc local air pollution prohibitions. Stack<br />
analyscs ol emissions irom this plant are shown<br />
in Table 200.<br />
The packed scruhber must be tl~oroughly and uniformly<br />
wctted with either water or weak acid and<br />
must have uniform gas distribution to achieve high<br />
collection cificiency. Gooci gas distribution is also<br />
niandatory iar glass fiber filter units, and a superficial<br />
gas velocity of less than 100 fpm is recommended.<br />
At one plant owned by Monsanto Chemical Company,<br />
the stack plume was very persistent and visible.<br />
Thirty milligrams of fine sulfuric acid mists per<br />
standard cubic foot and 80 to 200 milligrams of<br />
phosphoric acid particles per standard cubic foot<br />
were emitted from the stack. To correct the<br />
situat~on, a gas absorption apparatus followed<br />
by a flber mist collector was installed. Collection<br />
efficiencies of 99 percent on particles less<br />
than 3 microns in diameter and of 100 percent<br />
on larger particles were achieved. The stack<br />
plume, which consists of 15 percent water vapor,<br />
disappears within 40 to 50 feet of the stack on<br />
dry days and within 150 feet on wet days. No<br />
maintenance problems or changes in pressure drop<br />
through the apparatus have been encountered.<br />
SOAP, FATTY ACID, AND GLYCERINE<br />
MANUFACTURING EQUIPMENT<br />
INTRODUCTION<br />
Soap for washing and emulsifying purposes bas<br />
been manufactured and used for over two thou-<br />
sand years. Traditionally, soap has been manu-<br />
factured in batches by saponifying natural oils<br />
and fats with a solution of caustic soda, salting<br />
out the soluble soap formed, and drawing off the<br />
dilute glycerol produced. Shortly before World<br />
War 11, major changes started to occur in the<br />
industry. Pretreatment of the fats and oils was<br />
introduced and changes were made in plant pro-<br />
cedures and in finishing of the soap. Since World<br />
Was 11, with the advent of synthetic detergents,<br />
soap use has declined precipitously until its pro-<br />
duction today constitutes less than 20 percent of<br />
the combined production of soaps and detergents<br />
(Silvas, 1969). Figure 569 illustrates the vast<br />
change in relative production of soap and deter-<br />
gent since 1944. The manufacture of detergents<br />
is discussed in another section of this manual.<br />
When the direct neutralization of fatty acids by<br />
soda ash and/or caustic soda was introduced as<br />
a soap-making process, fat splitting, or hydrolysis,<br />
became a basic operation of the soap industry.<br />
Prior to 1955. the soap industry generated<br />
its own supply of fatty acids for use in soap making<br />
by splitting of natural fats and oils and provided<br />
fatty acids to other chemical process industries.<br />
Since 1955, however, fattv acids have<br />
also been synthesized from petroleum products,<br />
F~gure 568. Phosphor~c acld plant wlth a Raschlg so that today fatty acids are produced synthetlcalrine-packed<br />
scrubber. ly in greater quantities than by spllttlng natural
7 38<br />
CHEMICAL PROCESSING EQUIPMENT<br />
Table 200. STACK ANALYSES OF EMISSIONS FROM A PHOSPHORIC ACID<br />
PLANT WITH TWO RASCHIG RING-PACKED SCRUBBERS<br />
Phosphorus burning rate, lh/hr<br />
Gas rate, stack outlet, scfm<br />
Gas temperature, stack outlet, "F<br />
Diameter of first packed scrubber,ft<br />
Height of first scrubber's Raschig ring packing, ft<br />
Diameter of final packed scrubber, ft<br />
Height of final scrubber's Raschig ring packing, ft<br />
Final scrubber's superficial velocity, fpm<br />
P205 emitted, gr/scf<br />
P205 emitted, lblhr<br />
Emissions as 70 of phosphorus burned<br />
RAW MATERIALS<br />
Report series No.<br />
C-167 A C-167 B<br />
The soap industry applies the term "oi1"to those<br />
natural fats which are liquid at ambient conditions,<br />
excluding hydrocarbon oils obtained from petro-<br />
leum. "Fats, " in the soap industry, refers to<br />
all natural oils and fats, liquid or solid. Soap<br />
is produced almost exclusively from these natu-<br />
ral fats and oils.<br />
Ordinary soluble soaps are classifiable in a num-<br />
ber of ways. They may be generally classed as<br />
toilet soaps, household soaps or industrial soaps.<br />
Traditionally, sodium soaps have been called hard<br />
soaps, and potassium soaps called soft soaps.<br />
Today such classification is no longer meaningful,<br />
as the hard or soft quality of soap is much more !<br />
0 drpident on the type and quality of fats and oils j<br />
1944 1948 1952 1956 1960 1364<br />
used to make the soap.<br />
YEAR i<br />
Figure 569. Production of soap and detergents in the<br />
United States, 1944 to 1968 (Chemical Week, 1969).<br />
fats and oils. The soap industry, however, still<br />
uses fatty acids produced almost exclusively by<br />
splitting natural oils and fats, and still supplies<br />
a significant amount of fatty acids to other chemi-<br />
cal process industries. The soap industry had<br />
been the principal supplier of glycerine to chemi-<br />
cal process industries. However, glycerine is<br />
now produced synthetically, and presently the<br />
soap industry supplies only about one-half of the<br />
total glycerine consumed in this country.<br />
Metallic soaps have uses entirely different from<br />
those for ordinary soaps, so that theyare not in<br />
direct competition. These soaps are alkaline<br />
earth, metal, or heavy-metal salts of fatty acids.<br />
They are made either by heating fatty acids with<br />
metallic oxides, carbonates, etc.. or by the re-<br />
action of soluble ordinary soap with solutions of<br />
heavy-metal salts. Their manufacture will not<br />
be discussed in this section.<br />
1,875<br />
12,200<br />
175<br />
8.5<br />
12<br />
2 0<br />
3<br />
47<br />
0.095<br />
9.9<br />
0.23<br />
895<br />
3,420<br />
162<br />
8. 5<br />
12<br />
2 0<br />
3<br />
13<br />
0.108<br />
3.2<br />
0. 16<br />
The properties of soaps are directly related to the !.<br />
type of fatty acids used. The most desirable fatty i.<br />
acids are lauric, myristic, paluitic, stearic, and !<br />
1 .<br />
olsic, which are acids having 12 to 18 carbon<br />
I'<br />
atoms. These acids constitute the bulk of the<br />
fatty acids found in tallow and coconut oil. As a<br />
1'<br />
result, many soaps are combinations of these two i<br />
oils, usually in ratios of 3 or 4 parts of tallow to 1<br />
1 part coconut oil. Because of its favorable acid !<br />
content, availability, and low price, tallow is the 1<br />
most predominant fat used for soap making. It<br />
constitutes 80 percent or more of the total fats<br />
used by the soap industry. Greases, the rendered j<br />
fats from hogs and other small domestic animals,<br />
are the next most often consumed fatty material<br />
(Shreve, 1967). Other natural oils, including<br />
marine oils, may also he used in the soap making<br />
processes. Many of the marine oils are used in i<br />
special applications. They represent only an !<br />
insignificant portion of the total fats used.<br />
Sodlum hydroxide is the saponifying alkali used<br />
for most soap manufacture. Potassium hydroxide
Soa~. Fattv Acid. and Glvce? .ine Manufacturing Equipment 739<br />
still is used to some degree, and because potas-<br />
sium soaps are more soluble than the sodium<br />
soaps, they are used, or blended with sodium<br />
soaps, for making liquid soap solutions. Miner-<br />
als, including soda ash, caustic potash, sodium<br />
silicate, sodium bicarbonate, and trisodium<br />
phosphate, are used extensively as builders or<br />
fillers. Used in smaller quantities, but of ex-<br />
ceeding importance as synergetic soap builders,<br />
are tetrasodium pyrophosphate and sodium tri-<br />
polyphosphate. Carboxylmethylcellulose (CMC)<br />
also is an additive for most heavy-duty soaps.<br />
Finished soaps also may contain small quantities<br />
of chemicals used as preservatives, pigments,<br />
dyes, and perfumes, as well as antioxidants or<br />
chelating compounds. Bar toilet soaps and pow-<br />
der or granular laundry soaps may be manufac-<br />
tured as a combination of synthetic detergents<br />
and the neutralized fatty acid soluble soaps.<br />
Detergents used are either anionic or nonionic,<br />
but not cationic.<br />
FATTY AClD PRODUCTION<br />
Fatty acid production from natural fats may be<br />
performed by any one of several processes. The<br />
processes all result in "splitting" or hydrolysis<br />
of the fat. This may be represented as:<br />
I<br />
H-C -OH<br />
I I<br />
-<br />
H - COOR + 3H70-3RC00 + H - C -OH<br />
I<br />
H - COOR<br />
FAT<br />
I<br />
H-C-OH<br />
FATTY GLYCEROL<br />
AClD<br />
Three current processes for splitting or hydro-<br />
lyzing fats to produce fatty acids and glycerol<br />
utilizing either batch or continuous processes<br />
are detailed and compared in Table 201. Several<br />
older process methods, such as panning and<br />
pressing procedures, fractional distillation, and<br />
solvent crystalization, no longer are used. Of<br />
the three current processes, the continuous high-<br />
pressure hydrolysis process is the one most often<br />
used by the soap industry. A flow diagram of the<br />
process is shown in Figure 570.<br />
In the continuous high-pressure hydrolysis pro-<br />
cess, fat and water, both in liquid phase, are<br />
heated in contact with each other to temperatures<br />
in excess of about 400 "F, and some of the water<br />
becomes dissolved in the fatty matter. The pro-<br />
portion of water that becomes dissolved in the<br />
fatty layer increases rapidly with rise in tempera<br />
ture, causing a reduction in the aqueous layer.<br />
At temperatures approaching 550 "F, depending<br />
upon the type of oil used, the aqueous phase<br />
merges into the fatty phase, leaving but a single<br />
liquid phase (Ittner, 1942). In practice, the<br />
equipment is operated at temperatures and pres-<br />
sures where the two components show consider-<br />
able mutual solubility but below the temperatures<br />
where only one phase exists. The glycerine<br />
formed is continuously removed in the water<br />
stream, and at the same time the product fatty<br />
acids are removed as a separate stream.<br />
The equipment used for this process is a vertical<br />
column (Figure 570). The fats, in liquid form,<br />
are first vacuum deaerated, which prevents dar-<br />
kening, and then pmnped into the bottom of the<br />
column through a sparge ring. Deaerated, de-<br />
mineralized water is pumped at high temperature<br />
and high pressure into the top of the tower. High-<br />
pressure steam, at pressures of 700 to 750 psi,<br />
is introduced into the tower either along with the<br />
oil or directly into the reaction zone at the center<br />
of the tower, or, in some cases, at both the top<br />
and bottom of the tower. Tower operating pres-<br />
sures are usually 650 to 800 psi, and temperature<br />
of the fats is usually around 485 ' to 500 "F. The<br />
oil droplets travel up the column, while the water-<br />
glycerine solution flows down the column. The<br />
fatty acids then pass overhead to a flash tank for<br />
the removal of entrained water, or they may be<br />
decanted from the water after cooling and then<br />
passed to a settling tank where further separation<br />
occurs.<br />
As shown in Figure 570, the glycerine and water<br />
solution, called sweetwater, is drawn from the<br />
bottom of the hydrolyzer tower and is passed<br />
through a series of evaporators, or to a flash<br />
tank to remove some of the water, and then to<br />
storage as crude glycerine.<br />
The fatty acids produced are characteristic of<br />
the particular type oils being processed. Distil-<br />
lation is employed frequently on- stream with con-<br />
tinuous hydrolization to further refine fatty acids.<br />
When chemical and industrial products are manu-<br />
factured from these fatty acids, fractional distil-<br />
lation is used. When soap stock is produced.<br />
simple distillation in a continnous vacuum-type<br />
still is used as shown in Figure 570.<br />
In the vacuum still, the boiling fatty acids pass<br />
overhead through a series of condensers. They<br />
are then either drawn off and pumped to storage<br />
as fatty acids or passed through a line mixer<br />
where caustic soda or soda ash is added to pro-<br />
duce the salt of the fatty acids, which is a soluble<br />
soap. The soap stock thus produced is then held<br />
in storage for use in the various soap manufac-<br />
turing and finishing operations.
740 CHEMICAL PROCESSING EQUIPMENT<br />
Table 201. COMPARISON OF THREE CURR<br />
Temperature, "F<br />
Pressure, psig<br />
Catalyst<br />
Time, hr<br />
Operation<br />
Equipment<br />
Hydrolyzed<br />
Advantages<br />
Disadvantages<br />
Twitchell<br />
212 to220<br />
0<br />
AIkyl-aryI sulfonic acids<br />
or cycloaliphatic sulfonic<br />
acids, both used with sul-<br />
furic acid, 0. 75 to 1. 25%<br />
of the charge<br />
12 to 48<br />
Batch<br />
Lead-lined, copper-lined,<br />
monel-lined, or<br />
wooden tanks<br />
85 to 98 % hydrolyzed:<br />
5 to 15 % glycerol solution<br />
obtained, depending on<br />
number of stages and<br />
type of fat<br />
Lorn temp-rature and<br />
pressure; adaptable to<br />
small scales; low first<br />
cost because of relatively<br />
simple and inexpensive<br />
equipment<br />
Catalyst handling; long<br />
reaction time; fat stocks<br />
of poor quality must<br />
often be acid-refined to<br />
avoid catalyst poison-<br />
ing; high steam consump-<br />
tion; tendency to form<br />
dark-colored acids;<br />
need for more than one<br />
stage for good yield and<br />
high glycerin concentra-<br />
tion; not adaptable to<br />
automatic control;<br />
:NT FAT-SPLITTING PROCE:<br />
Batch autoclave<br />
300 to350 (450 without cataly<br />
75 to 150 (425 to 450 without<br />
catalyst)<br />
Zinc<br />
calcium.<br />
or magne-<br />
sium<br />
oxides.<br />
1 to 2%<br />
5 to 10<br />
Batch<br />
Copper or stainless-steel<br />
autoclave<br />
85 to 98 %hydrolyzed;<br />
10 to 15 % glycerol,<br />
depending on<br />
number of stages and<br />
type of fat<br />
Adaptable to small<br />
scale; lower first cost<br />
for small scale than<br />
continuous process;<br />
faster than Twitchell<br />
High first cost; catalyst<br />
handling; longer<br />
reaction time than<br />
continuous process;<br />
not so adaptable to<br />
automatic control as<br />
continuous; high<br />
labor cost; need for<br />
more than one<br />
stage for good yield<br />
and high glycerin<br />
concentration<br />
5 (Shreve. 1967)<br />
Continuous<br />
counter current<br />
485 approx<br />
600 to700<br />
Optional<br />
2 to 3<br />
Continuous<br />
Type 316 stainless<br />
tower<br />
97 to 99 %, hydolyzid;<br />
10 to 25 % glycerol,<br />
depending on type<br />
of fat<br />
Small floor space;<br />
uniform product<br />
quality; high<br />
glycerin concen-<br />
tration; low<br />
labor cost; more<br />
accurate and<br />
automatic control;<br />
constant utility<br />
load<br />
High first cost;<br />
high temperature<br />
and pressure;<br />
greater operating<br />
skill<br />
The fatty acids, which may contain considerable soap stock. The hydrogenation operation is us=unsaturated<br />
organic acids, can be further pro- ally on-stream with the hydrolysis operation.<br />
cessed by hydrogenation. Hydrogenation, with<br />
the use of a catalvst, saturates the double bonds<br />
GLYCERINE PRODUCTION<br />
of the unsaturated fatty acids. The process helps The saponification of natural oils can be repre<br />
to eliminate objectionable odors and hardens the sented by the following reaction:
Soap, Fatty Acid, and Glycerine Manufacturing Equipment<br />
Figure 570. Flow diagram of a continuous process for hydrolysis of natural fats.<br />
H H<br />
I I<br />
H - COOCR H-C -OH<br />
I I<br />
H - COOCR + 3NaOH -+3NaCOOR + H - C - OH<br />
I I<br />
H - COOCR H-C-OH<br />
I I<br />
FAT SOAP GLYCEROL<br />
Whether soap is manufactured by the older method<br />
of saponification of natural oils illustrated by the<br />
preceding reaction or by the newer method of<br />
direct saponification of fatty acids, glycerine is<br />
always an accompanying product.<br />
Figure 571 illustrates a typical soap plant glyc<br />
erine purification operation. The crude weak<br />
glycerine solution derived from the hydrolysis<br />
process is refined to produce both commercial<br />
and pharmaceutical grades of glycerine. The<br />
processing of the glycerine obtained from the<br />
continuous hydrolysis process is a much easier<br />
operation than the processing of the spent soap<br />
741<br />
lye glycerine from the kettle or batch processes<br />
of soap making. The sweetwater drawn from<br />
the bottom of the hydrolizer column has a concentration<br />
of about 12 percent glycerol. This<br />
sweetwater usually is so hot that, upon passing<br />
through three evaporators in sequence, the glycerol<br />
concentration increases to about 75 to SO<br />
percent by weight: This crude glycerine then is<br />
held in a settling tank for at least 48 hours at<br />
elevated temperatures to reduce whatever fatty<br />
impurities are still present. It then is distilled<br />
under vacuum (60 mm Hg absolute) at temperatures<br />
of approximately 400 'F. Small amounts I<br />
of caustic are added to the still feed to saponify the !<br />
!<br />
I
742 CHEMICAL PROCESSING EQUIPMENT -<br />
v<br />
STILL FEED TANK<br />
Y<br />
STEAM<br />
CAUSTIC<br />
FOOTS<br />
HOT WATER<br />
F~gure 571. Flow diagram for glycerine manufacture from hydrolys~s sweetwaters<br />
small amounts of fatty acid impurities which are<br />
present so that they will not boil off. The overhead<br />
product glycerine from the vacuum still then is<br />
condensed in a three-stage condensing system<br />
with progressively lower temperatures at each<br />
stage. The staged condensation yields different<br />
grades of glycerine. The highest temperature<br />
first-stage condensate usually contains 99 percent<br />
glycerol. Lower quality grades are collected<br />
from the lower temperature condensers. The<br />
glycerine is purified by bleeching and filtration<br />
or ion exchange.<br />
In the making of soap by alkaline saponification<br />
of fats, glycerine always is formed and common-<br />
ly is recovered in solution in the soap lye. The<br />
spent lye removed from the saponification process<br />
averages around 4 to 5 percent glycerine when<br />
removed directly, and may exceed 10 percent<br />
when other washing processes are used. The<br />
spent lye, in addition to the glycerine, contains<br />
roughly 10 percent by weight of salt and some<br />
small amount of soaps that are still soluble in<br />
the lye.<br />
Figure 572 illustrates a typical spent soap lye<br />
processing plant. The first step is the purifica-<br />
-L<br />
TO EJECTORS<br />
tion of the lye solution removed from the saponifi-<br />
cation operation. The lye is neutralized by trrat-<br />
ing with mlneral acids to form a salt. The neu-<br />
tralized solution is heated and agitated to precip-<br />
itate any remaining soap. After filtration, the<br />
solution then is evaporated. Vacuum evaporation,<br />
either in a series of batch vessels or continuously<br />
in cone bottom vessels, causes the salt crystal-<br />
lization point to be reached.<br />
As the salt concentration increases, salt crystal-<br />
lizes out and separates. In the continuous vessel,<br />
a portion of the separated salt is intermittently<br />
removed from the bottom. In the batch separation,<br />
the salt is removed from the slurry by pumping it<br />
through filters or centrifuges. Recovered salt is<br />
reused in the soap making process. The concen-<br />
trated glycerine is boiled down to remove even<br />
more salt until a concentrated crude soap lye<br />
glycerine is obtained. At this stage the crude<br />
glycerine constitutes 80 to 82 percent by weight<br />
of the solution with approximately 2 percent by<br />
weight of nonvolatile organic matter, the remain-<br />
der being a mild salt solution in water. Further<br />
treaixnent of this crude glycerine follows the same<br />
procedures used with the crude glycerine obtained<br />
from fat splitting operations.
FATTY ACID OR<br />
HIGH F.A. STOCK<br />
BOILING TANK<br />
SOAP MANUFACTURING<br />
Soap, Fatty Acid, and Glycerine Manufacturing Equipment<br />
SPENT SOAP LYE<br />
FROM SOAP MAKING<br />
SURGE TANK<br />
Figure 572. Spent soap lye plant for recovery of crude glycerine.<br />
The soap-making processes, either those utiliz-<br />
ing the alkaline saponification of fats and oils or<br />
those employing the saponification of fatty acids.<br />
are variously batch or continuous. The kettle or<br />
full-boiled process is a batch process which fol-<br />
lows the historical and traditional soap-making<br />
methods since the beginning of the industry. This<br />
process involves several steps or operations in a<br />
single kettle or, in large operations, a series of<br />
kettles. The kettles or pans used in these pro-<br />
cesses vary considerably in size depending upon<br />
production requirements. Small operations or<br />
producers of specialty soaps may employ a kettle<br />
which will only produce a few hundred pounds of<br />
soap. Large commercial producers of soaps may<br />
use kettles which will produce 150,000 pounds of<br />
soap per batch.<br />
4<br />
><br />
Y<br />
743<br />
The steps or operations performed include sapon-<br />
ification of the fats and oils by boiling in a caustic<br />
solution using live steam, followed by "graining"<br />
or precipitating the soft curds of soap out of the<br />
aqueous lye solution by adding sodium chloride<br />
salt. The soap solution then is washed to remove<br />
glycerine and color body impurities to leave the<br />
settled or "neat" soap to form on standing. Neat<br />
soap is the almost pure soap produced in the full-<br />
boiled process and remains as the upper layer of<br />
soap from which "nigre" soap and lye solutions<br />
have settled. The steps described above in the<br />
full-boiled process, including that of the final<br />
settling, can require a period of several days.<br />
The smaller kettles using this process may re-<br />
quire up to 24 hours per batch, while the larger<br />
kettles may require up to a full week to complete<br />
a batch.
744 CHEMICAL PROCESSING EQUIPMENT<br />
Other batch processes of saponification of fats<br />
and oils, still used for small production runs of<br />
specialty soaps, include the semiboiled process,<br />
the cold process, the autoclave process, the<br />
methyl ester process and the jet saponification<br />
process.<br />
Two proprietary processes for continuous sapon-<br />
ification of natural oils are used by some soap<br />
manufacturers. These are the Sharples Process<br />
and the Mon Savon Process. Both processes,<br />
while dissimilar, eliminate the large kettles and<br />
lengthy process time required by the old tradi-<br />
tional batch operations. All processes, however,<br />
accomplish the same steps of soap manufacture.<br />
The manufacture of soap by direct saponification<br />
of fatty acids is easily accomplished in continuous<br />
processes. However, many plants employ con-<br />
ventional soap kettle processes. Batch saponifi-<br />
cation also is performed in mixing kettles, com-<br />
monly called crutchers. Fatty acids obtained by<br />
continuous hydrolysis are usually neutralized with<br />
50 percent caustic soda continuously in a high-<br />
speed mixer-neutralizer to form soap. The neat<br />
soap produced is discharged at 200°F into an<br />
agitated blending tank to even out any inequalities<br />
of neutralization. The neat soap contains approx-<br />
imately 30 percent water at this stage. This soap<br />
stock then is held at an elevated temperature for<br />
use in the various soap finishing operations.<br />
SOAP FINISHING<br />
-<br />
Soap is finished for consumer use in various<br />
forms such as liquid, powder, granule, chip,<br />
flake, or bar. Part of the finishing operation<br />
for soap is the addition of various ingredients to<br />
accomplish the purposes for which the final product<br />
is designed. Toilet bars of the purest type<br />
of soap will have the minimum of additional ingredients.<br />
Heavy-duty laundry soaps will have a<br />
with whatever markings are desired, and wrapped<br />
for shipment.<br />
Most bar soap today is manufactured by a second<br />
process, the "milling" process. Milled soaps,<br />
as they are called by the industry, usually are<br />
manufactured in one of two processes. In the<br />
older and still more commonly used process<br />
shown in Figure 573, the soap stock is batched<br />
in a mixer, called a "crutcher", with other ingredients.<br />
The batch is then flowed onto chill<br />
rolls, and then flaked off and passed through a<br />
steam-heated hot:& dryer. The flakes can be<br />
packaged as flake soap or ground and packaged as<br />
powder. When soap bars are made, the flakes<br />
from the dryer are 'Lplodded'l (mixed in a screw<br />
or sugar tubular mixer) or mixed with final ingredients<br />
such as perfume. The plodded material<br />
then is fed to a roll mill. The flaky soap produced<br />
by the roll mill then is plodded again to throughly<br />
mix ingredients and improve texture and is extruded<br />
in a continuous bar shape for cutting,<br />
stamping, and wrapping.<br />
li<br />
MIXER<br />
STEAM<br />
maximum of other ingredients added. All soap, - -<br />
after finishing, contains some water, usually between<br />
10 and 30 percent, because anhydrous soap<br />
PLODDER<br />
ROLL<br />
would be too insoluble to use easily. The finished<br />
soap product contains perfume which, while fre-<br />
t - - quently not apparent, has been mixed in with the<br />
soap to disguise somewhat unappealing odors.<br />
CUTTER<br />
MIXER<br />
MILL<br />
v -<br />
STAMPER<br />
PACKAGING<br />
FLAKE<br />
PACKAGING<br />
BAR<br />
PACKAGING<br />
Bar soap is produced in three general processes.<br />
The oldest process, the framing -. process, seldom<br />
is used today except for some special types of<br />
soap. In this process, liquid soap, after mixing<br />
or crutching with other necessary ingredients, is<br />
Figure 573. Flow diagram of milling soap finishing<br />
n. - r- n- - - r . ~ ~ ~<br />
poured as a semiliquid paste into large vertical In the second and more recent milled soap promolds.<br />
The soap hardens upon cooling in these cess, the basic blended soap stock is pumped<br />
molds. The sides of the molds or frames are through atomizing nozzles against the inside wall<br />
removed and the soap is cut by mechanical saw- of a vacuum chamber and dropped from the chaming<br />
processes into rough shapes and sizes of the ber into a plodder. Figure 574 is a diagram of<br />
hars. They are then stamped into the final shape, this process. The plodded soap is immediately
t<br />
LIQUID MIXER<br />
SOAP<br />
STOCK<br />
I<br />
-<br />
PLODDER CUTTER<br />
Soap, Fatty Acid, and Glycerine Manufacturing Equipment 745<br />
MINOR INGREDIENTS TO VACUUM<br />
- VACUUM<br />
n FLASH<br />
CHAMBER<br />
t<br />
STEAM<br />
MIXER<br />
PERFUME, ETC.<br />
Figure 574. Flow diagram of vacuum flash drying<br />
process for bar soap production.<br />
mixed with the necessary additional ingredients<br />
and then passed through a series of roll mills<br />
and plodders until it is extruded in a continuous<br />
bar for cutting, stamping, and wrapping.<br />
A third process, illustrated in Figure 575, pro-<br />
duces aerated soap bars. Neat soap is heated<br />
under pressure and then water is flashed off.<br />
<strong>Air</strong> is mixed with the soap, perfume is added, and<br />
the paste chilled and then extruded continuously.<br />
After cutting to rough shape, the bars are "aged"<br />
or cooled, and then stamped and wrapped.<br />
Soap also is finished for marketing in flake or<br />
chip form. In manufacturing this type of product,<br />
the same procedures are followed as were de-<br />
scribed for the framing process. The only ex-<br />
ception is that after hot air-drying, the soap is<br />
not milled or plodded.<br />
Soap powder formerly was produced by grinding<br />
the chips coming from the hot air-dryer discussed<br />
above and shown in Figure 573. This method of<br />
soap powder manufacture has been highly unsat-<br />
isfactory since it produced a product containing<br />
excessive fines. However, this process is still<br />
used occasionaly in some soap plants. Soap<br />
powders now are manufactured almost exclusive-<br />
ly by first crutching the soap stock with the fillers<br />
and other additives to produce the final composi-<br />
tion and then spray drying the slurry mix. The<br />
spray drying of soap and the spray drying of syn-<br />
L<br />
TI<br />
LIQUID SOAP (<br />
I STOCK I ( I<br />
A<br />
1-i VAPOR<br />
FLASH TANK 1<br />
"<br />
I<br />
I I<br />
CONTIYUOUS<br />
I CRUTCHER -<br />
FREEZER-<br />
I I<br />
CUTTER<br />
04<br />
AGING ROOM<br />
H-H~I<br />
CUTTER STAMPER PACKAGING<br />
Figure 575. Flow diagram of aerated soap har<br />
production.<br />
thetic detergent compounds are very similar pro-<br />
cesses. Spray drying is discussed in much great-<br />
er detail in the section of this manual dealing<br />
with detergents.<br />
Liquid soaps very rarely are manufactured today<br />
except for some very specialized products such<br />
as "pure soap hair shampoo. " Top quality liquid<br />
soap is blended in tanks with the other ingredients<br />
desired and then packaged in standard bottle<br />
filling equipment.<br />
THE AIR POLLUTION PROBLEM<br />
All chemical processes and some of the other<br />
operations involved in the making of soap, pro-<br />
duction of fatty acids, and the purification of<br />
glycerine have odors as a common air pollution<br />
problem. Blending, mixing, drying, packaging,<br />
and other physical operations are subject to the<br />
air pollution problems of dust emissions.<br />
Odors may be emitted from equipment used for<br />
the following operations:
746 CHEMICAL PROCESSING EQUIPMENT<br />
1. Receiving and storage of animal and vege-<br />
table oils<br />
2. saponification of fats and oils or of fatty<br />
acids<br />
3. hydrolieation of natural fats and oils to<br />
produce fatty acids<br />
4. distillation of fatty acids<br />
5. hydrogenation of fatty acids<br />
6. concentration and distillation of glycerine.<br />
During their receiving and storage, natural fats<br />
and oils are heated to temperatures not over 150°~<br />
to reduce viscosity for pumping. The fats and oils<br />
used by most soap manufacturers are of high qual-<br />
ity, and odors usually do not cause a local nuisance<br />
unless the equipment is located adjacent to homes<br />
or businesses.<br />
Fats and oils are heated during the direct saponi-<br />
fication, resulting in the emission of odors.<br />
These odors may cause a local nuisance, depend-<br />
ing upon the location of the equipment in the com-<br />
munity, and may require control equipment.<br />
Odors also are emitted during the deaeration and<br />
hydrolization of fats and oils to produce fatty acids.<br />
Distillation and hydrogenation of the fatty acids<br />
emit odors. Fortunately, the flash deaeration of<br />
fats and oils is performed under a vacuum, pro-<br />
duced usually by water or steam jets. Steam jets<br />
are followed by contact barometric condensers<br />
which in effect serve as scrubbers. This arrange-<br />
ment of equipment provides satisfactory odor con-<br />
lrol so that the installation of additional control<br />
equipment usually is not necessary. All stages of<br />
these operations are vented similarly. Figure 576<br />
illustrates several vacuum ejector and condenser<br />
systems.<br />
The flash dehydration of glycerine has been found<br />
to emit only mild odors. Equipment for glycerine<br />
distillation under vacuum is vented through steam<br />
jets and barometric condensers. Emissions again<br />
are very light and odors do not cause any nuisance<br />
problems.<br />
In soap-finishing operations, dust can be emitted<br />
from equipment performing the following opera-<br />
tions: Addition of powdered and fine crystalline<br />
materials to crutchers, mechanical sawing and<br />
cntting of cold frame soap, milling and plodding<br />
soap, air drying of soap in steam-heated dryers,<br />
milling, forming, and packaging. Although dust<br />
emissions from these equipment sources rarely<br />
violate existing air pollution regulations, the dust<br />
emissions cause an internal plant hygiene problem.<br />
Various pieces of process equipment must there-<br />
fore be vented to control equipment for worker<br />
comfort and safety.<br />
There are, however, other equipment sources of<br />
dust emissions which usually exceed air pollution<br />
regulations. The sources are: Grinding of soap<br />
chips, pneumatic conveying of powders, and spray<br />
drying of soap. Installation of control equipment<br />
is necessary for compliance with air pollution<br />
regulations as well as worker comfort and safety.<br />
The production of soap powder by spray drying<br />
creates the largest single source of dust in the<br />
manufacture of soap. Spray drying is designed to<br />
produce relatively coarse granules followed by<br />
highly efficient separation of soap granules from<br />
the drying air before the air is vented to the at-<br />
mosphere. Most towers for the spray drying of<br />
soap are the concurrent type where both the heat-<br />
ed air and soap slurry spray are introduced at<br />
the top of the tower. Heated air and soap granu-<br />
les are separated at the bottom of the tower in a<br />
baffled area which causes the granule-laden air to<br />
make sharp 180" turns. Most of the soap is de-<br />
posited in the baffled section and drops into the<br />
cone bottom of the spray dryer. However, a few<br />
soap particles still remain in the heated air after<br />
passage through the baffled area. The particles<br />
range in size from 2 to 200 microns, with a medi-<br />
ian particle size of over 20 microns. <strong>Air</strong> pollution<br />
control equipment is required before venting the<br />
contaminated air to the atmosphere.<br />
The hot soap granules removed from the bottom<br />
of the spray dryer must be cooled to prevent cak-<br />
ing and then screened and stored or sent to pack-<br />
aging equipment. The most common way to cool<br />
soap granules is by pneumatic conveying of the<br />
soap granules to elevated locations for gravity<br />
flow through screens into storage or packaging<br />
equipment. Cyclone separators or gravity settling<br />
chambers are used to remove the soap granules<br />
from the conveying air. Soap particles venting<br />
- -<br />
from the separator or settling chamber are finer<br />
in size than the soap particles in the exhaust air<br />
from the spray drying tower and are in such concentrations<br />
that they must be collected by control<br />
equipment in order to comply with air pollution<br />
regulations.<br />
AIR POLLUTION CONTROL EQUIPMENT<br />
The elimination of odors from the manufacture of<br />
raw soap, fatty acids, and glycerine can be accom-<br />
plished by scrubbers such as water ejectors or<br />
barometric condensers. Figure 576a shows a<br />
contact type scrubber which successfully vents<br />
odorous emissions from a vessel used for dehy-<br />
drating blends of tallow and foots oils by heating<br />
them above 200'F. This water jet contact scrubber
-<br />
60°F WATER<br />
8 PSlG<br />
6-in. dla. DUCT<br />
50 - 100 ft.<br />
1%-tn. INLET<br />
I I TO HOTWELL<br />
50,000 to 100,000 odor units per 113<br />
Soap, Fatty Acid, and Glycerine Manufacturing Equipment<br />
?-in OUTLET<br />
a. Barometric condenser and fan venting odorous oil b. Vacuum still which also provides odor reduction.<br />
blending operations.<br />
C. Flash tank which provides both jet exhaust<br />
and odor reduction.<br />
DRUM DRIER<br />
MAKEUP WATER PUMP<br />
d. Drum dryer which provides both vapor removal<br />
and odor reduction.<br />
Figure 576. Steam and water ejector and barometric condenser combinations which also effect odor reduction<br />
(condenser) reduces odor levels by over 90 per- Dust emissions from equipment used in the soap-<br />
cent. Tbe odor-containing gases vented from this finishing operations other than spray drying can<br />
scrubber are in very low volumes. The residual be controlled by dry filters and baghouses. Mois-<br />
odors are diluted in the atmosphere well below ture content of the dust-laden air is well below<br />
their threshold levels in traveling through the saturation and close to ambient so that condensa-<br />
atmosphere for only a short distance from the tion in the baghouse is not a problem. Dust col-<br />
scrubber exhaust. lected in filters or baghouses can be recycled<br />
747
748 CHEMICAL PROCESSING EQUIPMENT<br />
to the process. Methods for hooding and ventila- equipment of higher collection efficiency than the<br />
tion of equipment emitting dust and the design of cyclones must be used in place of the cyclones or<br />
baghouses or filters are discussed in Chapter 4 be installed in series on the exhaust from the last<br />
of this manual. cyclone when this occurs. A baghouse presents<br />
problems because the exhaust air is usually saturated<br />
at tempzratures of 100" to 150 "F and, at<br />
<strong>Air</strong> pollution control equipment for soap spray this saturated condition, caking and blinding of the<br />
drying towers is designed specifically for the op- bag fabric can occur unless a special heated bagerating<br />
parameters of the particular tower. These house design is employed. Figure 577 is a flow<br />
parameters include: Materials sprayed, tower diagram illustrating the control of a soap spray<br />
operating temperature, tower dimensions, gas drying operation. Multistage centrifugal scrubbers<br />
velocities, and others. Because of the relatively or venhri scrubbers have proven to be satisfac~<br />
large size of the particulates in soap drying, high- tory when additional control is required. Recirefficiency<br />
cyclones installed in series may be sat- culation of scrubbing liquid has not been employed<br />
isfactory in controlling emissions. Cyclones per- because soap slurry can cause severe foaming<br />
mit the recovery of materials for reuse in the<br />
'<br />
problems. Details on the design of scrubbers are<br />
process. However, small particulates may escape given in Chapter 4 of this manual. Further discollection<br />
by the second cyclone and may be in cussion which is applicable to spray drying towers<br />
such concentrations and quantities as to cause for soap also can be found in the following section<br />
emissions which violate regulations. Control on synthetic detergents.<br />
F~gure 577. Flow d~agram of soap spray drylng process wlth cyclones and baghouse for air pollution control.
Synthetic Detergent Surfactant Manufacturing Equipment<br />
SYNTHETIC DETERGENT SURFACTANT<br />
MANUFACTURING EQUIPMENT<br />
INTRODUCTION<br />
Surfactants are organic compounds that encom-<br />
pass in the same molecule two dissimilar struc-<br />
tural groups, e. g. , a water-soluble (hydrophilic)<br />
and a water-insoluble (hydrophobic) group. The<br />
composition, solubility properties, location, and<br />
relative sizes of these dissimilar groups in rela-<br />
tion to the overall molecular configuration deter-<br />
mine the surface activity of the compound (Kirk<br />
and Othmer, 1969). Every surfactant possesses<br />
detergent, dispersing, emulsifying, foaming,<br />
solubilizing, and wetting properties in varying<br />
degrees. The predominant property of any parti-<br />
cular surfactant dictates its use. Those surfact-<br />
ants with strong detergent properties when in<br />
aqueous solutions are used as the active agents<br />
in formulating various synthetic detergent pro-<br />
ducts. Detergent surfactant production for use<br />
in comp2unding of laundry and other cleaning com-<br />
pounds represents over 80 percent of all synthetic<br />
surfactant production. The remaining synthetic<br />
surfactants produced, of all types, are used in<br />
various industrial and chemical processes.<br />
Soap, as discussed earlier in this chapter, is a<br />
detergent type surfactant derived from saponification<br />
of natural oils and fats. It is generally<br />
not included in the present meaning of the term<br />
"detergent. I' Detergent is now almost exclusive<br />
'ly considered to apply to the synthetic organic<br />
surfactants. Synthetic detergents were first<br />
commerciallv emoloved in the textile industrv<br />
A ,<br />
during the 1930's. Just prior to World War 11,<br />
some commercial production of laundry products<br />
containing detergents was started. Since the<br />
close of World War 11, products incorporating<br />
detergents greatly increased in use with a concomitant<br />
decline in the use of soap. By 1968,<br />
synthetic detergent products accounted for 83<br />
percent of the total combined production of soap<br />
and detergent cleaning compounds in the United<br />
States (Silvas, 1969). Figure 569 graphically<br />
illustrates this change in relationship.<br />
Surfactants are classified into four categories<br />
on the basis of their hydrophilic grouping: (1)<br />
Anionic surfactants have hydrophilic groups that<br />
are carboxylates, sulfonates, sulfates, or phos-<br />
phates, e. g., -OSO3- or S03-; (2) cationic sur-<br />
factants have hydrophilic groups that are primary,<br />
secondary, and tertiary amines and quaternary<br />
ammonium groups, e. g., -N(CH3)3f or C5~5~f-;<br />
(3) nonionic surfactants have hydrophilic groups<br />
that are hydroxyl groups and polyoxyethylene<br />
chains, e. g.. -(OCHZCHZ), OH; (4) amphoteric<br />
or zwitterionic surfactants include more than<br />
one solubiliaing group of differing types.<br />
749<br />
Table 202 shows the total 1966 U. S. production of<br />
surfactants divided among several major catego-<br />
ries. All soaps, both those for cleaning and<br />
washing products and those for metallic soaps<br />
for industrial and chemical processes, are in-<br />
cluded under carboxylates in the table. The over-<br />
whelming bulk of surfactant is produced by the i<br />
soap and detergent manufacturing companies, and<br />
consists almost exclusively of the anionic types.<br />
Limited amounts of anionic surfactants used in<br />
detergent cleaning and washing compounds are<br />
produced by petrochemical or chemical manufac-<br />
turing companies. The other two major catego-<br />
ries of synthetic surfactants, cationic and non-<br />
ionic, are almost entirely produced by chemical<br />
manufacturing companies. Their total produc-<br />
tion is relatively minor compared to anionic pro-<br />
duction. Therefore, cationic and nonionic sur-<br />
factants will not be discussed in this section.<br />
Row Materials<br />
The hydrophobic portion of most anionic surfac-<br />
tants is a hydrocarbon containing 8 to 18 carbon<br />
atoms in a straight or slightly branched chain.<br />
In certain cases, a benzene ring may replace<br />
some of the carbon atoms in the chain, e.g.,<br />
C12H25-, C9H19. C6H4-. The hulk of anionic<br />
surfactants are made with dodecylbenzene, or<br />
commonly termed "detergent alkylate, 'I as the<br />
hydrophobic group. Prior to 1965, the alkylates<br />
were produced by reacting benzene or its homo-<br />
logs with branched-chain olefins such as propy-<br />
lene trimer or tetramer. Since 1965, the alky-<br />
lates used for detergents are "soft", or biode-<br />
gradable, and are made from long straight-chain<br />
normal paraffins, which are combined with ben-<br />
zene by the FriedelLCrafts reaction. Most deter-<br />
gent alkylates are produced at petroleum refiner-<br />
ies or petrochemical plants. Their production<br />
will not he discussed in this section. Some<br />
anionic detergents are made with normal fatty<br />
alcohols. Most of these alcohols are produced<br />
by large soap and detergent plants by hydro-<br />
genation of the fatty acxds obtained by the hydro-<br />
lization of natural oils and fats. Synthetic alco-<br />
hols are also used. The production of natural<br />
or synthetic alcohol will not be discussed in this<br />
section.<br />
The hydrophilic grouping in the anionic synthetic<br />
detergent surfactants is either a sulfonate or a<br />
sulfate. Sulfur trioxide or one of its hydrates,<br />
sulfuric acid or oleum, is reacted with alkylate<br />
or fatty alcohols to organic acids which are later<br />
neutralized to form salts. Neutralization is ac-<br />
complished hy empLoying sodium hydroxide, so-<br />
dium bicarbonate, or other sodium bases to form<br />
the sodium salts of the sulfonate or sulfate. Other<br />
neutralization procedures employ ammonia, po-<br />
tassium, diethanolamine, or triethanolamine to<br />
form their respective salts.
750 CHEMICAL PROCESSING EQUIPMEI'JT<br />
Processes<br />
Table 202. TOTAL U. S. PRODUCT-ION OF SURFACTANTS, DIVIDED<br />
I.V'IO hlA.IOK CAILtiOlllES. ALL FIGUIlL'S ARE FOR 100 I'LKCES I'<br />
SURI'ACL-I\C I'IVL' 11A ILKIALS<br />
(Kirk-Othmer, 1969)<br />
Nonionic-total all types<br />
Cationic-total all types<br />
Type<br />
Anionic synthetics<br />
Sulfonic acids and salts<br />
Sulfuric acids esters and salts (sulfates)<br />
Others<br />
Total anionic synthetic<br />
Amphoteric-total all types<br />
Carboxylic acids and salts-soaps and others<br />
Total-all surfactants<br />
There are several separate and distinctive pro-<br />
cesses for sulfonation or sulfation of various or-<br />
ganic bases to produce the detergents most com-<br />
monly manufactured. These processes variously<br />
employ oleum, sulfur trioxide in liquid or in va-<br />
port phase, sulfuric acid in high concentration,<br />
or chlorosulfuric acid.<br />
The attachment of the sulfonic acid group, 502<br />
OH, to a carbon atom of a hydrocarbon group<br />
(RH) is termed sulfonation. As<br />
with sulfur trioxide RH + S03-RSO2OH<br />
with sulfuric acid RHt H2SOqzRS020H<br />
with oleum t Hz0<br />
with chlorosulfuric RH t ClSO3H-RSOzOH<br />
acid (sulfone) t H CI~<br />
Sulfation in detergent manufacturing denotes the<br />
attachment of an SO2OH group to an oxygen atom<br />
of an alcohol group. As<br />
with sulfur trioxide ROH t SO3-ROS020H<br />
with sulfuric acid<br />
with oleum ROH + HSOtROS020H<br />
with chlorosulfuric ROHt C1S03H-ROSOzOH<br />
acid (sulfone) 1 t HC11<br />
Production,<br />
pounds<br />
963,812,000<br />
111,925,000<br />
12,956,000<br />
1P88,693,000<br />
2,903,503,000<br />
Oleum is the most frequently used reactant for<br />
sulfonation. Oleum of 20 or 25 percent strength<br />
(20 or 25 parts by weight of sulfur trioxide liquid<br />
dissolved in 75 or 80 parts by weight or concen-<br />
trated sulfuric acid) is most frequently employed.<br />
During the early period of commercial production<br />
of synthetic detergents, all sulfonation processes<br />
employed 20 percent oleum in batch operations.<br />
Today almost all sulfonation of alkylate with oleum<br />
is performed in continuous operations. There are<br />
two typical processes: (1) Oleum sulfonation and<br />
(2) oleum sulfonation and sulfation.<br />
OLEUM SULFONATION I<br />
~<br />
The most frequently encountered oleum sulfonation<br />
is the patented packaged plant illustrated in<br />
Figure 578. Although the feed equipment and discharge<br />
equipment may differ, the essential suli<br />
I<br />
i<br />
fonation and neutralization processes are the same<br />
for all of these plants. Oleum and alkylate are<br />
precisely metered and introduced to a centrifugal<br />
!<br />
mixing pump. The reacting mixture is then cooled<br />
in heat exchangers. Some reacting product !<br />
recirculates to the mixing pump. The discharged<br />
I<br />
reacting product enters a digester tube section to<br />
allow the reaction to go to completion. The pro-<br />
duct is then diluted with water which hydrolizes<br />
sulfuric acid anhyrides and makes possible sub-<br />
sequent layer separation of spent sulfuric acid<br />
from the sulfonic acid. The layer separation<br />
step, which would normally require several hours,<br />
is effected in only a few minutes by adding sul-<br />
fonic acid product to the large mass of recycling<br />
spent acid. A centrifugal mixing pump is used
Synthetic Detergent Surfactant Manufacturing Equipment<br />
WATER ALKALI 1<br />
SULFONIC<br />
SULFONATION DILUTION CONCENTRATION NEUTRALIZATION<br />
Figure 578. Sulfonation of detergent alkylate with oleum in package plant with vacuum deodorizing<br />
(Chemithon Corp., Seattle, Wash.).<br />
to add dilution water, and the pump recirculates<br />
part of the dilute sulfonic acid along with part of<br />
the spent acid from the separation vessel. The<br />
spent acld is pumped from the bottom of the<br />
separation vessel, and sulfonic acid is pumped<br />
from the top. At this point in the process, differ-<br />
ences from one installation to another are found.<br />
Various arrangements of equipment provide for:<br />
Neutralization of the sulfonic acid followed by<br />
storage: deodorization by vacuum treating follow-<br />
ed either by storage or neutralization and storage;<br />
storage of the sulfonic acid without any further<br />
treating.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
Emissions of highly visible white opacity occur<br />
when oleum, formerly termed fuming sulfuric<br />
acid, is charged to a vessel and the displaced<br />
vapors are allowed to escape to the atmosphere.<br />
The visible emissions result from the reaction of<br />
sulfur trioxide vapor with water vapor in the air<br />
to form sulfuric acid particles in mist form. The<br />
particle size of this mist is directly comparable to<br />
the sulfuric acid mist in tail gases from sulfuric<br />
acid manufacturing. The particles of this mist<br />
are all less than 2 microns and 10 percent by<br />
weight are less than 1 micron. The threshold of<br />
visibility for this mist has been found experimen-<br />
tally to be of the order of 3.6 x grains/ft3<br />
(0.0203 mg/ft3), the precise value depending upon<br />
the temperature and humidity of the atmosphere<br />
(Fairs, 1958). Dense white emissions up to 100<br />
percent opacity occur from vents of storage tanks<br />
and process vessels during filling operations<br />
with oleum.<br />
Continuous sulfonation occurs in this equipment<br />
in a closed system without venting (except for<br />
751<br />
emergency relief). The sulfonic acid separation<br />
vessel is not vented, and the discharge of sul-<br />
fonic acid product and the waste sulfuric acid,<br />
after separation, to storage tanks does not cause<br />
emissions of more than trace opacities from vents<br />
of these tanks. When the sulfonic acid is neutra-<br />
lized, no visible emissions occur from the pro-<br />
cess equipment or from the final storage vessel.<br />
Depending upon the reagents used, the presence<br />
of long alkyl chains in the alkylate can lead to<br />
some dealkylation. Dealkylation results in the<br />
formation of small amounts of long-chain olefins<br />
which can cause product odor problems. To over-<br />
come odors, some plants deodorize the sulfonic<br />
acid product in a vacuum tank. The thresholds<br />
of the odorous materials removed depend upon<br />
the organic base feed stock. The ejectors and<br />
barometric condensers used for producing the<br />
vacuum scrub out odorous compounds from the<br />
gaseous stream. The odorous materials are<br />
skimmed from the water in the hot well. After<br />
skimming, the water may safely be cooled with-<br />
out creating odor emissions from the cooling<br />
tower.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
The transfer of liquid sulfur trioxide into vessels<br />
creates the same dense visible emissions as de-<br />
scribed for the transfer of oleum. When oleum<br />
and liquid SO3 were first introduced in industrial<br />
operations, control of emissions was attempted<br />
with simple water or caustic solution traps or<br />
scrubbers, but without success. Scrubbers and<br />
packed towers employing concentrated sulfuric<br />
acid as the scrubbing or absorbing medium were<br />
next employed. Many of these control installa-<br />
tions succeeded in reducing the opacity of the<br />
visible mist emissions, but did not reduce them
~.<br />
. .. .<br />
752 CHEMICAL PROCESSING EQUIPMENT<br />
to a point that their corrosive effects on adjoin-<br />
ing equipment were eliminated.<br />
tured by Monsanto Company. Efficient operation<br />
of the filter is dependent upon adequately contact-<br />
ing the off-gases with water before entering the<br />
filter to convert all sulfur trioxide vapor to sul-<br />
furic acid mist for filtration. This is accomplish-<br />
ed by water sprayed concurrent with the gas flow.<br />
A 20 percent oleum was charged to the vented<br />
vessel at a rate of approximately 1000 pounds per<br />
minute, and the filter reduced the dense white<br />
emissions to a barely visible plume.<br />
Sulfur trioxide liquid is usually stored, shipped,<br />
and handled at a temperature between 90' and<br />
100 "F. Commercial stabilized SO3 has a melting<br />
point of about 62.2"F and a boiling point of<br />
about 112 "F. It is best handled and stored at<br />
92.2"F. Pure SO3 has a vapor pressure of 1 atmosphere<br />
at its boiling point, but the pressure<br />
will vary, at some lower value, from one supplier<br />
to another depending on the stabilizers employed.<br />
The vapor pressure of SO3 over 20 percent<br />
oleum, however, is only 0.8 mm Hg at<br />
OLEUM SULFONATION AND SULFATION<br />
35 "C (0.24 ounces~in.~ at 95°F). The vapor<br />
readily reacts either with water or water vapor<br />
to form sulfuric acid. Because it is a vapor<br />
phase reaction, and the sulfuric acid formed<br />
immediatley condenses to a liquid, the acid droplets<br />
are very small. Effective collection of these<br />
mists from tanks and vessels receiving SO3 or<br />
oleum will require exceedingly high-energy<br />
scrubbers or very high-efficiency filters. An<br />
Another continuous sulfonation process empLoys<br />
both sulfonation of an alkylate and sulfation of an<br />
alcohol in a two-stage series reaction with oleum<br />
in both stages. Figure 580 is a schematic flow<br />
diagram of this process. This process is proprietary<br />
and is operated by The Procter and Gamble<br />
Manufacturing Company.<br />
effective high-efficiency filter was constructed<br />
by Fairs (1958). The filter was made by com-<br />
Process<br />
pressing silicone treated ultrafine glass fibers<br />
to a bulk density of 10 pounds per cubic foot. Provision<br />
was made to throughly humidify the acid<br />
bearing exhaust gases before passing them to this<br />
filter.<br />
In this process, 20 percent oleum is continuously<br />
introduced to a mixing pump along with alkylate.<br />
The process proceeds according to what is termed<br />
the "dominant bath" principle to control the heat<br />
of sulfonation conversion and maintain the temp-<br />
A high-efficiency filter installation is illustrated<br />
in Figure 579. This oleum tank vent control consists<br />
of a Brink Mist Eliminator filter, manufacerature<br />
at a steady 130'F. A high recirculation<br />
ratio (volume of recirculating material divided<br />
by the volume of throughput) of at least 20: 1 is<br />
maintained ~~ to ~rodnce a favorable system. The<br />
discharged sulfonic mixture is passed through<br />
TO ATMOSPHERE coils to provide sufficient time for sulfonation to<br />
reach the desired high conversion. In the second<br />
stage of the process, fatty tallow alcohol, more<br />
oleum, and the first-stage sulfonic product are<br />
mixed in a centrifugal pump. This stage also<br />
OLEUM TANK<br />
HIGH-EFFICIENCY<br />
employs the dominant bath principle with a high<br />
recirculation ratio. The discharged sulfonicsulphate<br />
product then passes to a continuous line<br />
mixer where a sodium hydroxide solution is introduced<br />
for neutralization. Again, the dominant<br />
bath principle with a high recirculation ratio is<br />
used in the neutralization step. The surfactant<br />
slurry then is pumped to storage. This process<br />
makes no attempt to separate and remove any<br />
unreacted sulfuric acid as waste acid. All residual<br />
sulfuric acid is neutralized to sodium sulfate.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
SEWER<br />
The Procter and Gamble process requires an entirelv<br />
closed system. Venting - to the atmosphere<br />
Figure 579. High-efficiency mineral fiber filter installed<br />
ta vent acid mists from oleum storage tank;<br />
back flow not shown (Brink Filter. Monsanto Co.. St.<br />
iou!~. MO.).<br />
occurs only when the surfactant slurry is discharged<br />
into a storage vessel. Gaseous emissions<br />
from the storage vessel are minimal, and no air<br />
pollution control equipment is required.
CAUSTIC<br />
Synthetic Detergent Surfactant Manufacturing Equipment 753<br />
Figure 580. Continuous series sulfonation-sulfation with oleum, ending with neutralization, employing circulating<br />
heat-exchanging dominant bath to control heat (The Procter and Gamble Manufacturing Co., Long Beach, Calif.).<br />
SULFUR TRlOXlDE VAPOR SULFONATION<br />
When liquid detergent compounds were introduced<br />
as household products, it became necessary to<br />
reduce the sodium sulfate content of the surfac-<br />
tant. Sulfonation with oleum resulted in excess-<br />
ively high sodium sulfate content when the sur-<br />
factant was neutralized and used in liquid deter-<br />
gent products. The high sulfate content (11 to 12<br />
percent) caused cloudiness in the liquid deter-<br />
gent products and the sulfate settled out in the<br />
bottom of the containers. Sulfonation with pure<br />
sulfur trioxide vapor provided neutralized sur-<br />
factants with much lower sodium sulfate content<br />
(1 to 3 percent) and thus became an important<br />
process. The first ma'or installations of SO3<br />
vapor sulfonation equipLent included sulfur burn-<br />
ers for production of SO3. However, with the<br />
commercial development and availability of<br />
stabilized SO3 at low costs, many installations<br />
eliminated the SO3 production equipment (SO3<br />
production is discussed earlier in this chapter).<br />
Earliest processes for SO3 vapor sulfonation were<br />
batch operations. Several proprietary continuous<br />
operations are now used for this process. The<br />
continuous processes employ several types of<br />
reactors. One reactor is a mechanically agitated<br />
unit, and two others are film type reactors. The<br />
processes are similar and cause similar air<br />
pollution problems. SO3 is vaporized and diluted<br />
in a dry air stream (dew point 40 'F) to 29 to 8<br />
percent by volume before entering the reactor.<br />
Either alkylates or alcohols may be used in the<br />
processes. Figure 581 is a schematic flow dia-<br />
gram of one installation utilizing a film reactor.<br />
In this process, air is compressed, cooled, and<br />
dehydrated, then accurately metered to the reac-<br />
tion column. Simultaneously, stabilized liquid<br />
SO3 is weighed, then metered to a steam heat<br />
exchanger to be vaporized. Metered air is mix-<br />
ed with the SO3 vapor just before entering the<br />
reaction column, Al+late, alcohol, or other<br />
organic materials are metered and pumped to the<br />
reaction column. The reactor is a vertical cclumn<br />
designed for concurrent flow from top to bottom of<br />
the dilute SO3 vapor in air and the organic liquid.<br />
Cooling water flows on the outside of the reactor<br />
from the bottom to top in an opposite direction to<br />
the descending reactants. The sulfonic acid dis-<br />
charges from the bottom of the column to a cen-<br />
trifugal separator where liquid sulfonic acid is<br />
drawn from the bottom, and gas is vented from<br />
the top of the separator to the atmosphere (other<br />
installations may recycle the discharged gas and<br />
only vent some bleed-off). The liquid sulfonic<br />
acid then passes through a degassing column,<br />
maintained under vacuum, to remove unreacted<br />
SO3 vapors in the liquid phase. Depending upon<br />
the product manufactured, the product is pumped<br />
either to storage or neutralization tanks or to an<br />
"ageing tank" maintained under slight vacuum<br />
where it is agitated and cooled by water coils to<br />
ensure completion of the sulfonation reaction.<br />
It is then pumped either to storage or to neutra-<br />
lization equipment. Trace amounts of sulfuric
754 CHEMICAL PROCESSING EQUIPMENT<br />
I<br />
COMPRESSED AIR AIR AIR TO<br />
DRYER<br />
t<br />
POLLUTION CONTROL<br />
COOL WATER<br />
.-... - VAPORIZER I I I I<br />
LlUUlU<br />
SULFUR TRlOXlDE I I I<br />
STEAM 'V<br />
- -<br />
ALKYLATEORALCOHOL I I l l<br />
V<br />
INTERNAL SEPARATOR<br />
- I<br />
SULFONIC ACID<br />
VACUUM LINE PRODUCT<br />
COOL WATER<br />
- --. I&<br />
VACUUM<br />
OR<br />
DEGASSER<br />
NEUTRALIZER<br />
r TO STORAGE<br />
OR PROCESS<br />
NEUTRALIZER WATER<br />
~<br />
Figure 581. Sulfonation with sulfur trioxide vapor using a f i lrn reactor (Texti lana Corp., Hawthorne, Cal if.).<br />
acid anhydrides may be present in the sulfonic<br />
product, and form sodium sulfate when neutra-<br />
lized. Fatty alcohols must be neutralized immed-<br />
iately after the reaction with SO3 because sulfuric<br />
acid esters hydrolize rapidly. Some sulfonic<br />
acid is diluted with water to eliminate anhydrides<br />
before being stored. Neutralization of the sulfonic<br />
acid or sulfate is accomplished batchwise in an<br />
agitated water-cooled tank or in a dominant bath<br />
continuous neutralizer. The sulfonic acid or sul-<br />
fate products may be neutralized by sodium or<br />
potassium alkalies or by ammonia.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
COOLING TANK<br />
(AGING)<br />
In the SO3 vapor sulfonation process described,<br />
there are three point sources from which air<br />
contaminants can be emitted. The principal<br />
source of emission is the venting of gas separated<br />
from the sulfonic product upon discharge from the<br />
reactor. When the film reactor equipment was<br />
first installed, the emissions were believed to be<br />
_<br />
:<br />
.<br />
.,<br />
'=<br />
SURGE<br />
COOL<br />
WATER<br />
I<br />
STEAM<br />
TO HOTWELL<br />
__,.<br />
r<br />
QD I TO STORAGE<br />
mostly air with a small amount of SO3 vapor as<br />
acid mist. Control of this emission was attempt- i.<br />
ed by employing a mist eliminator followed by a<br />
packed scrubber using 99 percent sulfuric acid<br />
as the scrubbing medium. This method of control<br />
did not eliminate the visible emission ranging<br />
from 50 to 100 percent opacity. Upon investiga-<br />
I<br />
i<br />
tion, the emission was found to be almost com-<br />
pletely water soluble and to cause suds in water.<br />
The sulfuric acid in the scrubber did not in-<br />
crease in strength as would be the case if SO3<br />
were absorbed in the scrubber. Infrared spectro-<br />
meter analysis of the gaseous emissions indicated :<br />
the principle constituent of the visible emission to ~<br />
be sulfonic acid. Fine mists are produced in the i<br />
reactor and may be formed in several ways. Some 1<br />
alkylate may vaporize in excess of normal equili-<br />
brium conditions due to localized hot zones in the<br />
reactor to later condense in the gas phase with<br />
the SO3 gas to produce sulfonic acid. Since the<br />
sulfonic acid is produced under conditions below<br />
its dewpoint, it immediately condenses from the<br />
!.<br />
1<br />
, ,
Synthetic Detergent Surfactant Manufacturing Equipment 755<br />
gas phase as a very fine mist. Some water is<br />
produced by side reactions. The water vaporizes<br />
and reacts with the SO3 gas in the vapor phase<br />
to form sulfuric acid below its dewpoint result-<br />
ing in a fine mist (Brink, et al. 1966).<br />
The other two paints of emissions have not been<br />
found to cause emissions of excessive opacities.<br />
One of these points is the exhaust from the single-<br />
stage steam ejector employed to provide vacuum.<br />
The ejector exhaust was found to contain little if<br />
any air contaminants and is not controlled. The<br />
third point of emission is the by-pass discharge<br />
from the reactor. The by-pass discharges liquids<br />
from the reactor during start-up procedures to a<br />
sump lor disposal to the sewer system. When<br />
carefully operated to avoid wasting materials or<br />
product, the start-up by-pass discharge is used<br />
for only a very short period, usually less than<br />
1 minute. Visible air contaminants from this<br />
source have been observed only during these<br />
short periods.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
The visible mist emitted from the vent of the re-<br />
actor is not readily controlled by scrubbing. In-<br />
cineration of the off-gasses eliminates visible<br />
emissions but results in emission of sulfur oxides.<br />
The high-efficiency filter discussed previously for<br />
SO3 acid mist control may be expected to control<br />
emissions from the reactor. The volume of ex-<br />
haust gas is determined by the operating para-<br />
meters of the sulfonation equipment. Pressure<br />
drop through the high-efficiency filter arranged<br />
for horizontal gas flow with velocities of 15 to 30<br />
fpm ranges between 5 and 8 inches of water col-<br />
umn. Provisions should he made for back-wash-<br />
ing of the filter elements.<br />
SULFUR TRlOXlDE LIQUID SULFONATION<br />
Since liquid sulfur trioxide with even a trace of<br />
moistur 2 present forms solid polymers at room<br />
temperatures, it was not a commerically feasible<br />
reactant until it was stabilized by addition of small<br />
quantities of suitable compounds. First processes<br />
employing liquid SO3 resulted in surfactants of<br />
poor color or with unpleasant odors. Liquid SO3<br />
added to undiluted alkylate causes heavy dealkyla-<br />
tion with the formation of odorous long-chain ole-<br />
fins. Pilot Chemical Company developed a com-<br />
mercial process employing the liquid, shown<br />
schematically in Figure 582. The process is op-<br />
erated in batches and employs liquid sulfur diox-<br />
ide as a diluent and as a refrigerant to maintain<br />
low batch temperatures (30°F) during the highly<br />
exothermic sulfonation reaction.<br />
Alkylate to be sulfonated is metered into the re-<br />
actor, and then the liquid SO2 and SO3 are meter-<br />
ed to the reactor. The heat generated by the sul-<br />
fonation reaction causes evaporation of the SOz,<br />
which holds the reaction mass at low temperature.<br />
The reactor is maintained under negative condition<br />
by pumping the SO2 vapor from it. The reaction<br />
is completed after several hours, and the sulfonic<br />
product is then heated to 85°F to drive off reemin-<br />
ing SO2 liquid as a vapor. The SOZ vapor removed<br />
from the reactor is compressed, scrubbed with<br />
concentrated sulfuric acid to remove any SO3,<br />
condensed, and returned to storage. The sulfonic<br />
product discharged from the reactor passes to a<br />
vacuum stripper to remove traces of SO2 and then<br />
to either storage or a neutralization tank. The<br />
vacuum pump for the vacuum stripper discharges<br />
to the compressors and returns any removed SO2<br />
to storage.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
The Pilot Chemical process emp?oys an entirely<br />
closed system u?til the sulfonic product is dis-<br />
charged to storage. The product in storage does<br />
not cause any air contaminant emissions other<br />
than very dilute and mild odors. The only air<br />
pollution problem found from the operation is<br />
from the necessary purge venting of the liquid<br />
SO2 storage tank. The tank must be continuously<br />
purged whenever the compressor is discharging<br />
SO2 back to it from the sulfonation reaction. This<br />
prevents any build-up of air which enters the<br />
system by leakage into the compressor and other<br />
equipment. SO2 vapors are present in the gas<br />
flow from the purge vent line.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
SO2 vapor emitted from the storage tank receiv-<br />
ing recovered SO2 from this process is readily<br />
controlled by absorption in a caustic solution.<br />
Moderate energy scrubbers such as packed<br />
columns and spargers using 10 percent sodium<br />
hydroxide solution as the scrubbing medium are<br />
effective. An air pollution control scrubber is<br />
schematically shown in Figure 583. At this<br />
installation, gas flow rate from the tank is limi-<br />
ted to approximately 1 to 2 cfm by a needle valve<br />
for the purge operation. SO2 vapor is sparged<br />
beneath the caustic solution contained in two<br />
scrubber vessels connected in series. The caus-<br />
tic solution is sampled regularly and maintained<br />
above 5 percent concentration. The same system<br />
can serve to vent the storage tank when it is fill-<br />
ed with liquid SO2 from a tank truck. The SO2<br />
vapor displaced during filling is returned to the<br />
truck tank so that the purge line vents only a low<br />
gas flow to the scrubber. The knock-out pot in<br />
the purge line between the tank and the scrubber<br />
principally serves to prevent back-flow of caustic<br />
solution to the SO2 storage tank.
756 CHEMICAL PROCESSING EQUIPMENT<br />
SULFONIC AClD<br />
TO<br />
NEUTRALIZATION<br />
OR<br />
STORAGE<br />
Figure 582. Sulfonation with liquid sulfur trioxide diluted with liquid sulfur dioxide (Pilot<br />
Chemical Co., Santa Fe Springs, Calif.).<br />
CHLOROSULFURIC AClD SULFATION<br />
Chlorosulfuric acid as an acid reactant requires<br />
considerably different process equipment than<br />
any of the other processes discussed previously.<br />
The reaction is carried out with fatty alcohols.<br />
It is employed to produce surfactants used in for-<br />
mulating detergent products of high quality. Fig-<br />
ure 584 is a schematic flow diagram illustrating<br />
this process. Fatty alcohols are transferred<br />
from storage by a vacuum applied to the reactor.<br />
In the reactor, the alcohols are first cooled to the<br />
point of solidification by circulation of refrigera-<br />
ted water in the reactor jacket. The reactor is<br />
purged with inert gas (nitrogen) and chlorosulfuric<br />
acid is then slowly metered into the reactor. This<br />
reaction is characterized by the highly unbalanced<br />
pattern of heat and gas evolution. When the acid<br />
is first added to the alcohol, the exothermic for-<br />
mation of slkoxonium chloride occurs, and the<br />
hydrogen chloride generated by the sulfonation re-<br />
action is retained in the reactant mass. As acid<br />
addition continues, the HC1 is endothermically<br />
evolved as a gas and released from the reacting<br />
mass with considerable foaming. The heat evo-<br />
lution is highest at the start of the reaction, with<br />
approximately 60 percent of the total heat<br />
generated when only 20 percent of the total acid<br />
reagent has been added. Inert gas must be con-<br />
tinuously bled from the reactor along with the<br />
generated HC1. Off-gases containing HC1 pass to<br />
a falling film reactor for recovery of the HCI by<br />
absorption in water to form hydrochloric acid as<br />
a valuable byproduct. When the reaction has<br />
reached completion, the sulfated alcohol is trans-<br />
ferred to another vessel for neutralization. So-<br />
dium hydroxide, triethanolamine, or ammonia is<br />
used for neutralization. The sulfated alcohol must<br />
be neutralized immediately after completion of<br />
the reaction to avoid hydrolization.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
The receiving, storage, and handling of chloro-<br />
sulfuric acid results in more severe air pollution<br />
problems than those encountered with liquid So3<br />
and oleum. Chlorosulfuric acid is a fuming liquid<br />
which is extremely corrosive and reacts violently<br />
with water to produce hydrochloric and sulfuric<br />
acid. It is not normally exposed to air, and inert
Synthetic Detergent Surfactant Manufacturing Equipment 757<br />
NEEDLEVALVE<br />
SULFUR DIOXIDE RETURN SULFUR<br />
F-A AIR AND SULFUR<br />
( LIQUID SULFUR DIOXIDE<br />
TANK<br />
CAUSTIC<br />
OVER<br />
:&--- -x--<br />
pH GUAGE . .<br />
TO SEWER<br />
LIQUID<br />
Figure 583. <strong>Air</strong> pollution control system venting<br />
liquid sulfur dioxide tank receiving recycled sulfur<br />
dioxide from sulfonation process (Pi lot Chemical Co.,<br />
Sante Fe Springs, Calif.).<br />
gas under positive pressure is used in place of<br />
atmosphere when charging or discharging any<br />
containers or vessels. Venting of the displaced<br />
gas from vessels when filling with chlorosulfuric<br />
acid produces visible emissions of the acid mist.<br />
The chlorosulfation process evolves HC1 gas which<br />
is vented along with inert gas to a falling film ab-<br />
sorber. Most of the HC1 is absorbed in water,<br />
but a small amount of unabsorbed HCl is carried<br />
out with the inert gas to the atmosphere, causing<br />
visible emissions of acid mist. Hydrochloric<br />
acid produced by absorption of the HCl in water<br />
(usually 25 to 28 percent solution) is transferred<br />
to storage. Displaced vapors vented during fill-<br />
ing of the storage tank also consist of visible<br />
acid mist. The balance between the reactor op-<br />
eration and the absorber operation is critical if<br />
absorption of the HCl gas is to be reasonably com-<br />
plete. If absorption is inefficient, larger quanti-<br />
ties of acid mists are emitted from the storage<br />
tank vent. If inert gas flow volume to the reac-<br />
tor is excessively high, the absorption efficiency<br />
for HCl decreases. The flow of HCl from the re-<br />
actor is not constant. Therefore the feed water<br />
rate and other absorption variables must be con-<br />
trolled to keep the byproduct acid concentration<br />
from the absorber constant in spite of varying<br />
HCl gas loads.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
HCI acid mist displaced during filling of the stor-<br />
age tank is readily controlled with a caustic solu-<br />
tion scrubber or packed column. Two simple<br />
submersion type scrubbers illustrated in Figure<br />
585 serve to control emissions with high efficiency.<br />
The volume of HCl solution discharged from the<br />
chlorosulfation unit absorber is quite low, and<br />
this scrubber contains an excess of caustic for<br />
neutralizing all the HC1 vented during the batch<br />
operation. The caustic solution is tested after<br />
each batch operation to maintain the pH of the<br />
solution at 8 or greater.<br />
Sulfoalkylation is a condensation-type reaction<br />
producing a more complex sulfonate from a sim-<br />
ple one. One sulfoalkylation process, the manu-<br />
facture of N-acyl-N-alkyltaurate, is performed<br />
in the same equipment schematically illustrated<br />
in Figure 584 for a chlorosulfation process.<br />
Fatty acids are moved to the reactor, and phos-<br />
phorous trichloride is added as a reactant to pro-<br />
duce a fatty acyl chloride. Inert gas sparging is<br />
used, and hydrogen chloride is released by the<br />
reaction to be vented from the reactor with the<br />
inert gas. In this reaction, phosphoric acid is<br />
also formed in the reactor and settles out at its<br />
bottom. When the reaction is complete, remain-<br />
ing HCl is purged with the inert gas, and the<br />
phosphoric acid is drawn off. The fatty acyl<br />
chloride product of the first reaction is then re-<br />
acted with N-methyltaurine (taurine is 2-amino-<br />
ethane sulfonic acid) to form the final detergent<br />
surfactant (one of the "Igepon TI1 series of Gen-<br />
eral Aniline and Film Corporation). The HCl and<br />
inert gas vented from the reactor during the first<br />
reaction flows to the falling film absorber for<br />
HCl recovery. The surfactant is pumped from<br />
the reactor to a neutralization tank for final<br />
treatment with a caustic solution.<br />
Another sulfoalkylation process is a reaction be-<br />
tween fatty acids and isethionate, the sodium<br />
salt of isethionic acid (Z-hydroxyethane sulfonic<br />
acid), as the sulfating agent, and fatty acids in the<br />
presence of a catalyst to produce 8-sulfoesters.<br />
These detergents are sensitive to hydrolysis and<br />
are used only in specialty products. Hydrolysis<br />
does not deter their use in personal products.<br />
The sodium salt of 2-sulfoethyl ester of lauric<br />
acid or of coconut acid is used in manufacturing<br />
synthetic detergent bars.<br />
In this process, isethionic acid and coconut fatty<br />
acid are metered to a closed reactor, and a catalyst<br />
in ~owder form is added. The mix is agitated<br />
and circulated through a heat exchanger to<br />
add heat to the reactant mass. Inert gas is sparged<br />
into the reactor to prevent discoloration of pro-
758 CHEMICAL PROCESSING EQUIPMENT<br />
Flgure 584. Chlorosulfonatian af fatty alcohols and recovery af hydrogen chloride (Textilana Carp.,<br />
Hawthorne, Calif.).<br />
a. Low-volume hydrogen chlorlde ac~d mlst<br />
vented from chlorosulfonat~on process.<br />
VACUUM RELIEF VALVE<br />
b. Low-volume hydrogen chloride ac~d mlst vented<br />
from hydrogen chlorlde storage tank.<br />
Flgure 585. Submersion type scrubbers uslng caustlc solution (Textllana Corp., Hawthorne, Cal~f.).
Synthetic Detergent Product Manufacturing Equipment 759<br />
duct. Fatty acid vapor, water vapor, and inert<br />
SYNTHETIC DETERGENT PRODUCT<br />
gas are vented from the reactor through a condenser<br />
to a seaarator tank. Fattv acids seoara- MANUFACTURING EQUIPMENT<br />
ted are recycled to the reactor, the water is sent<br />
to the sewer, and the inert gas . is returned to a<br />
gas holder. The reaction is completed after several<br />
hours, and the product is then pumped to a<br />
jacketed vacuum stripper tank. The temperature<br />
is maintained, tallow fatty acid and more catalyst<br />
are added, and inert gas is sparged into the tank.<br />
The vessel is held under slight vacuum for a short<br />
period while the contents are agitated. The vacuum<br />
is then increased to strip any remaining unreacted<br />
fatty acid. Water vapor, inert gas, and<br />
fatty acid vapors are vented through a condenser.<br />
The condensate from the condenser consists primarily<br />
of coconut fatty acid and is returned to<br />
storage. The fresh surfactant is discharged<br />
through a line mixer where a small amount of water<br />
is added for cooling purposes. It is then transferred<br />
to a holding vessel for use in formulation<br />
of toilet bars. The water content flashes off in<br />
the holding vessel.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
The first of the two reactions involved in the production<br />
of alkyltaurates, the fatty acyl chloride<br />
reaction, also creates HCI acid mist emissions<br />
similar to those from the chlorosulfonation reaction.<br />
The second reaction, with the N-methyltaurine.<br />
is accomplished in the vessel without<br />
venting until the reaction is complete.<br />
The vacuum system employed in the second process<br />
to oroduce the sulfoester consists of compound<br />
steam ejectors with barometric condensers.<br />
The condensers discharge to a hot well. Visible<br />
and odiferous emissions, principally fatty acid<br />
vapors, occur from the hot well. Uncondensable<br />
gases in the reactor recirculation system also are<br />
vented and cause odors and visible emissions.<br />
The flashed off water vapor from the product hold<br />
tank can also contain contaminants of an odorous<br />
nature. No other air pollutant emissions occur<br />
from this process.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
The same control equipment shown in Figure 584<br />
for HC1 acid mist serves to control the emissions<br />
from the first sulfoalkylation process producing<br />
the alkyltaurate.<br />
The hot well receiving condenser water and uncon-<br />
densed gas and vapor from the vacuum jet systems<br />
in the second process for sulfoesters must be<br />
closed and vented to control equipment to eliminate<br />
odors and visible acid mist emissions. Scrubbers<br />
with gas pressure drops ranging from 7 to 12 in-<br />
ches water column usually control these emissions.<br />
INTRODUCTION<br />
"Synthetic detergent products" applies broadly to<br />
cleaning and laundering compounds containing surfactants<br />
along with other ingredients formulated<br />
for use in aqueous solutions. These products are<br />
marketed as heavy- or light-duty granules or liquids,<br />
cleansers. - - and laundrv or toilet bars. The heavvduty<br />
granules representthemajor portionof allproducts<br />
manufactured, with the light- andheavy-duty<br />
liquid or light-duty granules in far lesser production.<br />
The manufacture of all detergentproducts incorporates<br />
equipment andprocesses similar to those for<br />
manufacturing soapproducts. Themanufacture of<br />
the granular products is ofparamountinterest, with<br />
more severe air pollutionproblems than those encountered<br />
with soap granule production. The manufacture<br />
of liquid detergent andhar products is oflesser<br />
importance, withlittle or no difference from similar<br />
soap products in either process equipment or air<br />
pollution potential, and will not be discussed.<br />
Raw Materials<br />
The surfactants used in formulating synthetic detergent<br />
products are either anionic or nonionic.<br />
The products also contain other chemical compounds<br />
which supplement the detergent of the surfactant.<br />
Each particular formulation depends upon<br />
the ultimate design for consumer use. Table 203<br />
illustrates the formulations commonly used in<br />
large-volume granule and liquid detergent manufacture.<br />
Sodium tripolyphosphate (STP) or tetrasodium<br />
pyrophosphate (TSPP) are incorporated in most<br />
granular formulations as "builders" or seques-<br />
tering agents. They serve to eliminate inter-<br />
ference with the detergent action by the calcium<br />
and magnesium ions (hardness) in the water used<br />
in the wash solution. STP and TSPP may be used<br />
in powder, prill, or granule form, and are re-<br />
ceived in carlots. These ingredients are most<br />
often blended into the slurry before spray drying.<br />
Nitrilotriacetic acid (NTA) and its sodium salts<br />
have recently been incorporated in some heavy-<br />
duty granule products to replace part of the STP.<br />
It is more expensive than STP, but is a better<br />
sequestrant. The growing public concern with the<br />
role phosphates in detergents may play in deteri-<br />
oration of water quality has generated manufac-<br />
turers' interest in this substitute. Indications<br />
are that it will be employed in the near future in<br />
more formulations and in larger quantities. The<br />
acid is a crystalline powder and the salts (disodi-<br />
um or trisodium) are powders. They are receiv-<br />
ed in carlots. NTA is added to the slurry mix be-<br />
fore drying.
760 CHEMICAL PROCESSING EQUIPMENT<br />
Surfactants<br />
Table 203. FORMULATIONS(1N PERCENT) FOR LARGE-VOLUME DETERGENT<br />
MANUFACTURE IN THE UNITED STATES<br />
Constituent<br />
Alcohol sulfate<br />
Alkyl sulfonate<br />
Ethoxulated<br />
fatty alcohol<br />
Alkyl amine oxide<br />
Soap<br />
Builders<br />
Fatty alcohol<br />
or amines<br />
STPINTA<br />
TS P<br />
Additives<br />
CMC<br />
Sodium silicate<br />
Sodium sulfate<br />
Enzyme<br />
Other<br />
Heavy duty Light duty<br />
p-<br />
High suds<br />
granules<br />
Low suds<br />
granules<br />
Brand A Brand B Brand C Brand D Liquid Granules Liquid<br />
Fillers, usually sodium sulfate or sodium car--<br />
bonate, are incorporated in granule products.<br />
They are either powders or crystalline powders<br />
and are added in bulk form to the slurry before<br />
drying.<br />
8<br />
8<br />
-<br />
-<br />
-<br />
1. 5-2<br />
60<br />
-<br />
0. 5-0. 9<br />
5-7<br />
10-20<br />
Amides of various types are used as supplemen-<br />
tary surfactants in many formulations. They im-<br />
prove detergency of the sulfonic and sulfate sur-<br />
factants and act as foam boosters or stabilizers.<br />
Amides used include the higher fatty amides<br />
(e. g. cocomonethanolamide), ethanolamides,<br />
dialkyl and alkylol (hydroxyalkyl) amides, mor-<br />
pholides, and nitriles, as well as the lower acyl<br />
derivatives of higher fatty amines. These ma-<br />
terials are handled as liquids and received in<br />
tank cars or barrels. In granule manufacture,<br />
they are either incorporated in the slurry before<br />
drying or blended with the detergent granules<br />
after drying.<br />
18<br />
-<br />
-<br />
-<br />
0~0. 5<br />
50-60<br />
0. 5-0. 9<br />
5-7<br />
10-20<br />
0-35<br />
0-35<br />
0-35<br />
0-15<br />
-<br />
0-12<br />
-<br />
0-20<br />
0. 5-0. 9<br />
5-7<br />
-<br />
0.2-0.730.2-0.750.2-0.740.2-0.750.2-0.75<br />
0-5 0-5 0-5 0-5 0-5<br />
-<br />
6<br />
6<br />
-<br />
2<br />
50-60<br />
.<br />
0. 5-0. 9<br />
7-9<br />
10-30<br />
17<br />
-<br />
-<br />
.<br />
50-60<br />
-<br />
0. 5-0. 9<br />
7-9<br />
10-30<br />
25-32<br />
25-32<br />
-<br />
-<br />
.<br />
-<br />
.<br />
-<br />
0-5<br />
0-4<br />
60-70<br />
-<br />
0-5<br />
Function<br />
Cleaning<br />
agent<br />
for oily<br />
and organic<br />
s 011<br />
Foam booster<br />
or stabilizer<br />
Overcome<br />
water hardness<br />
and clean<br />
inorganic stains<br />
Antiredepositlon<br />
Corrosion<br />
inhibitor<br />
Flller<br />
Clean protein<br />
stain<br />
Perfume, dye,<br />
bleach, etc.<br />
Trisodium phosphate (TSP) is used in detergent<br />
granule formulations such as dishwasher com-<br />
pounds and wall cleaners which are designed to<br />
clean hard surfaces. TSP is considered func-<br />
tionally as an alkali rather than a sequestering<br />
agent. It is usually handled as a crystalline<br />
powder and is received in carlots, drums, or<br />
bags.<br />
Carboxy methylcellulose (CMC; sodium cellulose<br />
glycolate) usually is added to heavy-duty granules<br />
and serves to prevent redeposition onto the fabric<br />
of the dirt removed by the detergent. This chemical<br />
is received in bags or drums as a powder or<br />
granule. It is added to the slurry mixture before<br />
drying.<br />
Sodium silicate is added in most synthetic deter-<br />
gent formulations to inhibit the surfactant's ten-<br />
dency to corrode metal. It also is used to over-<br />
-<br />
20-25<br />
or<br />
20-25<br />
-<br />
10-12<br />
5-12<br />
2-15<br />
0-20<br />
-<br />
0-4<br />
0-5
come production and packaging problems encoun-<br />
tered with detergent granules. It is functionally<br />
used as a primary detergent alkali in compounds<br />
designed for hard surface washing, e. g. , machine<br />
dishwashing comp~unds. It can serve to retain<br />
uniform viscosity in the mixing and pumping of<br />
the slurry before it is dried, and it reduces the<br />
"tackiness" of the dried granules, facilitating<br />
their handling and reducing caking of the product<br />
after packaging. The sodium silicates are re-<br />
ceived in tank cars as water solutions.<br />
Optical brightners are added to many formulations.<br />
These are usually fluorescent dyes which absorb<br />
ultraviolet rays and reflect them as visible light.<br />
The dyes are received as powders in bags or as<br />
liquids in drums, and they are usually blended in<br />
the slurry before drying.<br />
Perfumes are added to almost all detergent pro-<br />
ducts to overcome unpleasant odors and impart a<br />
pleasing scent to laundered fabrics. The per-<br />
fumes are added by spraying onto the dried gran-<br />
ules or mixing with the liquid detergents. They<br />
are handled as liquids in small-size containers<br />
or drums.<br />
Bleaches of various kinds are frequently incor-<br />
porated in heavy-duty detergents. Sodium per-<br />
borate, along with magnesium silicate as a sta-<br />
bilizer, is commonly employed. They are re-<br />
ceived as powders or crystals in boxes or bags<br />
and are added to the granules after drying.<br />
Synthetic Detergent Product Manufacturing Equipment<br />
Enzymes have recently been introduced as part<br />
of the formulation of heavy-duty detergent pro-<br />
ducts to assist in the removal of protein-based<br />
stains from fabrics. The enzymes are received<br />
as powders in bags or drums. The enzymes are<br />
heat sensitive and are destroyed if heated to212"F.<br />
Most manufacturers blend the enzymes into the<br />
detergent granules after drying.<br />
Many other compounds may be incorporated in<br />
various products. Preservatives, antioxidants,<br />
foam- suppressors and other types of additives<br />
are used. The scouring cleansers are composed<br />
principally of finely pulverized silica, active<br />
detergent, small amounts of phosphates, and fre-<br />
quently a bleach.<br />
Detergent surfactants include alkyl sulfonic,<br />
alkyl sulfate, and alcohol sulfates, discussed<br />
above, and almost the entire range of anionic<br />
and nonionic detergents, including soap. Plants<br />
manufacturing their own sulfonic and sulfate sur-<br />
factants also use other surfactants in some or all<br />
of their products. The detergents are received<br />
and handled mostly as liquid solutions of varying<br />
strength, but some surfactants are received as<br />
flake or powder. Surfactants are principally mix-<br />
ed with the slurry before drying.<br />
Processes<br />
The only manufacturing process to be discussed<br />
here will be the production of detergent granule<br />
formulations incorporating spray-drying pro-<br />
cesses. All other products are produced in pro.<br />
cesses, such as drum drying, similar to soap<br />
production, discussed in the previous section.<br />
Manufacture of detergent granules incorporates<br />
three separate steps: Slurry preparation, spray<br />
drying, and granule handling (including cooling,<br />
additive blending, and packaging). Figure 586<br />
illustrates the various operations.<br />
SLURRY PREPARATION<br />
The formulation of slurry for detergent granules<br />
requires the intimate mixing of various liquid,<br />
powdered, and granulated materials. The soap<br />
crutcher is almost universally used for this mix-<br />
ing operation. Premixing of various minor ingre-<br />
dients is performed in a variety of equipment prior<br />
to charging to the crutcher or final mixer. The<br />
slurry, mixed in batch operations, is then held<br />
in surge vessels for continuous pumping to the<br />
spray drier.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
The receiving, storage, and hatching of the vari-<br />
ous dry ingredients creates dust emissions.<br />
Pneumatic conveying of fine materials causes<br />
dust emissions when conveying air is separated<br />
from the bulk solids. Many detergent products re-<br />
quire raw materials with high percentages of<br />
fines, viz.. typical specifications for some raw<br />
materials include the followiug percentage of<br />
fine materials passing a 200 mesh screen: Sodium<br />
sulfate - 12 percent; sodium tetrapyrophosphate -<br />
74 percent; sodium tripolyphosphate - 53 percent.<br />
The storage and handling of the liquid ingredients,<br />
including the sulfonic acids, sulfonic salts, and<br />
sulfates, do not cause emission problems other<br />
than mild odors.<br />
In the hatching and mixing of fine dry ingredients<br />
to form slurry, dust emissions are generated at<br />
scale hoppers, mixers, and the crutcher. Liquid<br />
ingredient addition to the slurry creates no vis-<br />
ible emissions but may cause odors.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
Control of dusts generated from pneumatic or<br />
mechanical conveying or from discharge of fine<br />
materials into bins or vessels is described in<br />
Chapters 3 and 4. There are no unique problems<br />
in hoodihg or exhaust systems for controlling dust<br />
emissions from conveying and slurry preparation.
762 CHEMICAL PROCESSING EQUIPMENT<br />
Baghouses are employed not only to reduce and<br />
eliminate the dust emissions but for salvage of<br />
raw materials. None of the dusts causes any<br />
serious corrosion problems. Filter fabrics should<br />
be selected which have good resistance to alkalis.<br />
Filter ratios for baghouses with intermittent<br />
shaking cleaning mechanisms should be under 3<br />
cfm per square foot.<br />
SPRAY DRYING<br />
All spray drying equipment designed for detergent<br />
granule production incorporates the following com-<br />
ponents: Spray-drying tower, air heating and<br />
supply system, slurry atomizing equipment,<br />
slurry pumping equipment, product cooling equip-<br />
ment, and conveying equipment. The towers are<br />
cylindrical with cone bottoms and range in size<br />
from 12 to 24 feet in diameter and 40 to 125 feet<br />
in height. Single towers may be of varying dia-<br />
meter, being larger at the top and smaller at the<br />
bottom. <strong>Air</strong> is supplied to the towers from di-<br />
rect-heated furnaces fired with either natural gas<br />
or fuel oil. The products of combustion are tem-<br />
pered with outside air to lower temperatures and<br />
then are blown to the dryer under forced draft.<br />
The towers are usually maintained under slightly<br />
negativepressure, between 0.05 and 1.5 inches of<br />
water column, withexhaust blowers adjusted topro-<br />
vide this balance. Most towers designed for de-<br />
tergent production are of the countercurrent type,<br />
with the slurry introduced at the top and the heat-<br />
ed air introduced at the bottom. A few towers of<br />
the concurrent type are used for detergent spray<br />
drying, with both hot air and slurry introduced at<br />
the top. Some towers are equipped for either<br />
mode of operation as illustrated in Figure 586.<br />
In most towers today, the slurry is atomized by<br />
spraying through a number of nozzles, rather<br />
than by centrifugal action. The slurry is sprayed<br />
at pressures of 600 to 1000 psi in single-fluid<br />
nozzles, and at pressures of 50 to 100 psi in two-<br />
fluid nozzles. Steam or air is used as the atomiz-<br />
ing fluid in the two-fluid nozzles.<br />
Tower operations vary widely between manufac-<br />
turers and between products. Heated air supplied<br />
to the tower varies from 350' to 750°F. Temp-<br />
eratures of air supplied to countercurrent towers<br />
are generally lower, and most often range from<br />
500" to 650DF. Concurrent tower temperatures<br />
are somewhat higher. Solids content of slurrys<br />
for detergent spray drying varies from 50 to 65<br />
percent by weight, with some operations to as<br />
high as 70 percent. Moisture content of the dried<br />
product varies from 10 to 17 percent. Towers are<br />
designed for specific air-flow rates, and these<br />
rates are maintained throughout all phases of op-<br />
eration. Slurry temperatures may vary, but in<br />
most formulations they do not exceed 160°F.<br />
Frequently, they are as low as 8O0F. Exit gas<br />
temperatures range from 150" to 250°F with wet-<br />
bulb temperatures of 120" to 150°F. <strong>Air</strong> velocities<br />
in concurrent towers are usually higher than ve-<br />
locities in countercurrent towers. The concurrent<br />
towers produce granules which are mostly hollow<br />
beads of light specific gravity (0.05 to 0.20).<br />
Countercurrent towers produce granules which<br />
are multicellular and irreaularlv shaved and which<br />
have higher specific gravities ranging from 0.25<br />
to 0.45.<br />
In countercurrent towers, with lower air velocities<br />
and droplets descending against a rising column<br />
of air, most of the dried granules fall into the<br />
cone at the bottom of the tower. They are dis-<br />
charged through a star valve, or regulated open-<br />
ing, while still hot. Cooling of the granules is<br />
discussed below with other granule processing<br />
procedures. Unlike other product spray drying<br />
operations, e. g., powdered milk, the desired de-<br />
tergent granule product is comparatively large in<br />
size. The specifications for some well known<br />
granular products require 50 percent by weight<br />
or more to be retained on a 28-mesh screen. A<br />
certain amount of the product is dried to compar-<br />
atively small size. This amount is dependent on<br />
tower feed rates, the liquid droplet size in slurry<br />
atomization, the paste viscosity, the particular<br />
product, and other variables. Usually the exhaust<br />
air entrains 7 to 10 percent of that portion of the<br />
granular product which is too fine to settle out at<br />
the base of the tower.<br />
Concurrent towers, operate with higher air velocities<br />
than countercurrent towers. The air is vented<br />
just above the bottom of these towers through a<br />
baffle which causes violent changes of direction to<br />
the exhaust air to dynamically separate the dried<br />
granules, which then fall to the cone bottom for<br />
discharge. Concurrent towers producing very<br />
low-gravity granules vent air still conveying the<br />
~roduct to auxiliary equipment for separation.<br />
The loss of detergent fines entrained in the exhaust<br />
air stream will be somewhat higher from<br />
concurrent towers than from countercurrent<br />
towers.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
The exhaust air from detergent spray drying tow-<br />
ers contains two types of air contaminants. One<br />
is the fine detergent particles entrained in the<br />
exhaust air discussed above; the second consists<br />
of organic materials vaporized in the higher temp-<br />
erature zones of the tower.<br />
The detergent particles entrained in the exhaust<br />
air are relatively large in size. Over 50 percent<br />
by weight of these particles are over 40 microns.<br />
These particles constitute over 95 percent of the<br />
total weight of air contaminants in the exhaust air<br />
(Phelps, 1967). They consist principally of deter-<br />
-
Synthetic Detergent Product Manufacturing Equipment 763<br />
Figure 586. Detergent spray-drying with tower equ~pped for elther concurrent or countercurrent operation<br />
(dampers in countercurrent mode of operatlon).<br />
gent compounds, although some of the particles<br />
are uncombined phosphates, sulfates, and other<br />
mineral compounds.<br />
The second type of the air contaminant in the ex-<br />
haust air can create serious air pollution control<br />
problems. Various organic components in the<br />
slurry vaporize in the tower. The amount vapor-<br />
ized is dependent upon many variables, such as<br />
tower zone temperatures and volatility of the<br />
organics in the slurry mixture. The vaporized<br />
organic materials condense upon cooling in the<br />
tower exhaust air stream into micron- and sub-<br />
micron-size droplets or particles. Spray drying<br />
of one particular product resulted in the measured<br />
discharge of condensed organic particulates in the<br />
tower exhaust air ranging in size from 0.2 to 0.5<br />
micron.<br />
The variety of possible detergent compounds is<br />
almost infinite, and manufacturers are continually<br />
introducing new formulations or reformulating<br />
older ones. It is not always possible to predict<br />
how certain organic compounds in a slurry mix-<br />
ture will affect stack emission. If amides are<br />
present in the slurry in amounts greater than 0.5<br />
percent by weight, emission problems will occur.<br />
Source tests of exhaust air from an air pollution<br />
control scrubber with amides present in the slurry<br />
being dried in the spray tower revealed 0.08 grain<br />
of organic particulates per scf of exhaust gas. The<br />
presence of this relatively low concentration of<br />
submicron-size aerosols causes water vapor plumes<br />
to persist for long distances. Following the break<br />
or end of the water vapor plume, a highly visible<br />
contaminant plume persists for even greater dis-<br />
tances. The amide emission rate increases ex-<br />
ponentially with increases in tower operating tem-<br />
peratures. Many tower operating variables affect<br />
air contaminant emissions, but the temperature<br />
of the exhaust air probably is the most important.<br />
At one spray drier installation, opacities of organ-<br />
ic aerosol emissions were reduced by approximate-<br />
ly 30 percent when tower operation was altered to<br />
effect a reduction of exhaust gas temperature from<br />
2004 to 180°F.<br />
t<br />
i
764 CHEMICAL PROCESSING EQUIPMENT<br />
Slurry formulations containing ethoqlated alcohol<br />
surfactants cause similar aerosol emissions.<br />
When this nonionic detergent is used in the slurry,<br />
source tests of the aerosol leaving the scrubber<br />
indicate a particle size range of 0. 2 to 1 micron.<br />
Plumes persist for long distances following the<br />
disappearance of the water vapor plume.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
The collection of air contaminants not only pro-<br />
vides for the economic return of detergent fines<br />
to the process, but also provides for control of<br />
submicron particles to ensure compliance with<br />
air pollution prohibitory rules.<br />
Manufacturers producing detergent granu!es have<br />
developed two separate approaches for capturing<br />
the detergent fines in the spray drier effluent for<br />
return to process. One method utilizes centrifugal<br />
separators to capture most of the product<br />
dust. The exhaust gases then are vented from<br />
the separators to scrubbers for removal of micron-size<br />
particles. The cyclone separators remove<br />
approximately 90 percent by weight of the<br />
detergent product fines in the tower exhaust air.<br />
The detergent dust remaining in the effluent<br />
vented from the cyclones consists of particles<br />
almost all of which are over 2 microns in size.<br />
Size distribution of these particles by weight will<br />
peak in the range of 7 to 10 microns. Particulate<br />
concentrations vary from 0.1 to 1.0 grain per scf.<br />
The cyclones are designed for relatively high<br />
efficiencies and operate at pressure drops from<br />
8 to 10 inches of water column (Phelps, 1967).<br />
A venturi type scrubber is used downstream of the<br />
cyclone, using water at 8 to 10 psig distributed<br />
through nozzles in the throat. Throat velocities<br />
of the exhaust gases average 8,500 fpm. With<br />
water supplied . to the throat at a ratio of 4.5 to<br />
5.0 gallons per 1,000 cubic feet of effluent, scrubber<br />
exhaust gases have loadings of about 0.085 grain<br />
per scf when slurries with amides are spray dried.<br />
A highly visible plume persists after the condensed<br />
water vapor plume has dissipated.<br />
In the second method of recovery of detergent<br />
fines, the centrifugal collector is eliminated, and<br />
only a scrubber is used. However, the scrubber<br />
uses detergent slurry as a scrubbing medium<br />
rather than water. The scrubbing slurry is main-<br />
tained at a high enough concentration to prevent<br />
foam, but at a low enough concentration to permit<br />
pumping and spraying. No further control device<br />
is used to cleanse the exhaust gases from the<br />
scrubber. When slurries with volatile organic<br />
materials are spray dried, highly visible plumes<br />
persist after the condensed water vapor plume<br />
has dissipated. A plume was even observed from<br />
a pilot venturi scrubber operating at a high pres-<br />
sure drop of 50 inches of water column. A 40 per-<br />
cent solids slurry was used as the scrubbing fluid<br />
delivered at gas scrubbing ratios of 60 to 100 gal-<br />
lons per 1000 cubic feet of gas.<br />
An alternative method for controlling emissions<br />
from the drier caused by volatile organics in the<br />
slurry is to reformulate the slurry to eliminate<br />
these offending organic compounds. When amide<br />
compounds were identified as causing the emission<br />
problems, some manuiacturers developed other<br />
formulations or methods for adding the amides<br />
to the spray dried granules to achieve a compar-<br />
able product.<br />
When reformulation is not possible, the tower<br />
production rate may be reduced, permitting oper-<br />
ation at lower air inlet temperatures and lower<br />
exhaust gas temperatures. When tower tempera-<br />
tures are reduced, lesser amounts of organic<br />
compounds are vaporized in the spray drier, and<br />
the scrubber is able to collect these emissions.<br />
GRANULE HANDLING<br />
Many manufacturers discharge hot granules from<br />
the spray tower into mixers where dry or liquid<br />
ingredients are added. The granules are usually<br />
mechanically conveyed away from the tower or<br />
mixer discharge and then are air-conveyed to<br />
storage and packaging. <strong>Air</strong> conveying serves to<br />
cool the granules and to elevate them for gravity<br />
flow through further processing equipment to<br />
storage and packaging. <strong>Air</strong> conveying of lowdensity<br />
granules usually is designed for 50 to 75<br />
scfm air per ~ound of granules conveyed. At the<br />
end of the conveyor, centrifugal separators remove<br />
granule product from the conveying air.<br />
Some manufacturers mechanically lift the granules<br />
from the spray tower to aeration bins where the<br />
granules are cooled or aged by injecting air at<br />
the bottom of the bin. This air percolates upward<br />
through the scrubber.<br />
The cooled granules are screened to deagglomerate<br />
the large granules and to remove undersize or<br />
oversize particles. Further mixing or blending<br />
may be performed to add heat-sensitive compounds<br />
to the detergent ~roducts. Many manufacturers<br />
do not store the finished granules, but convey<br />
them directly to packaging equipment. Some de-<br />
tergent products are held in storage, either in<br />
large fixed bins or small-wheeled buggy bins, and<br />
then are charged to packaging equipment. Pack-<br />
aging is done with either scale or volumetric fill-<br />
ing machines.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
Conveying, mixing, packaging, and other equip-<br />
ment used for granules can cause dust emissions.<br />
The granule particles, which are hollow beads, are<br />
crushed during mixing and conveying, and generate<br />
fine dusts. Dusts emitted from screens, mixers,<br />
bins, mechanical-conveying equipment, and air-<br />
conveying equipment are quite irritating to eyes<br />
and nostrils with continuous exposure. Some of
the additive materials, such as enzymes also<br />
cause serious health problems. Equipment in-<br />
volving enzymes requires very efficient ventila-<br />
tion in addition to proper dust collection. Dust<br />
emissions in most cases represent a significant<br />
product loss, and their collection and return to<br />
process (usually as an ingredient of the slurry to<br />
be spray dried) is necessary for economic plant<br />
operation as well as for air pollution control.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
Dust generated by granule processing, convey-<br />
ing, and storage equipment does not create unique<br />
air pollution control problems. Usually, bag-<br />
houses provide the best control. Collection effi-<br />
ciencies for baghouses are high; in many cases,<br />
efficiencies exceed 99 percent. No extreme con-<br />
ditions of temperature or humidity have to be met,<br />
but filter fabrics selected must show good resis-<br />
tance to alkaline materials. Baghouses utilizing<br />
intermittent shaking mechanisms should not have<br />
filtering velocities exceeding 3 fpm. Baghouses<br />
with continuous cleaning mechanisms may have<br />
filtering velocities as high as 6 fpm.<br />
GLASS MANUFACTURE<br />
Glass has been made for over 3,500 years, but<br />
only in the last 75 years have engineering and<br />
science been able to exploit its basic properties<br />
of hardness, smoothness, and transparency so<br />
that it can now be made into thousands of diverse<br />
products.<br />
The economics and techniques connected with<br />
mass production of glass articles have led to<br />
the construction of glass-manufacturing plants<br />
near or within highly populated areas. Un-<br />
fortunately, airborne contaminants generated<br />
by these glass plants can contrihute substantial-<br />
ly to the air pollution problem of the surround-<br />
ing community. Control of dust and fumes has,<br />
therefore, been, and must continue to be, in-<br />
herent to the progress of this expanding industry.<br />
<strong>Air</strong> pollution control is necessary, not only to<br />
eliminate nuisances, but also to bring substan-<br />
tial savings by extending the service life of<br />
the equipment and by reducing operating ex-<br />
penses and down time for repair. Reduction<br />
in plant source emissions can be accomplished<br />
by several methods, including control of raw<br />
materials, batch formulation, efficient com-<br />
bustion of fuel, proper design of glass-melt-<br />
ing furnaces, and the installation of control<br />
equipment.<br />
ufacture 765<br />
TYPES OF GLASS<br />
Nearly all glass produced commercially is one<br />
of five basic and broad types: Soda-lime, lead,<br />
fused silica, borosilicate, and 96 percent silica.<br />
Of these, modern soda-lime glass is well suited<br />
for melting and shaping into window glass, plate<br />
glass, containers, inexpensive tableware, elec-<br />
tric light bulbs, and many other inexpensive,<br />
mass-produced articles. It presently consti-<br />
tutes 90 percent of the total production of com-<br />
mercial glass (Kirk and Othmer, 1947).<br />
Typical compositions of soda-lime glass and<br />
the four other major types of commercial glass<br />
are shown on Table 204. Major ingredients of<br />
soda-lime glass are sand, limestone, soda ash,<br />
and cullet. Minor ingredients include salt cake,<br />
aluminum oxide, barium oxide, and boron oxide.<br />
Minor ingredients may be included as impuri-<br />
ties in one or more of the major raw ingredients.<br />
Soda-lime glasses are colored by adding a<br />
small percentage of oxides of nickel, iron,<br />
manganese, copper, and cobalt, and elemen-<br />
tal carbon as solutions or colloidal particles<br />
(Tooley, 1953).<br />
Although glass production results in tens of<br />
thousands of different articles, it can be divid-<br />
ed into the following general types (Kirk and<br />
Othmer, 1947):<br />
70<br />
-<br />
Flat glass 2 5<br />
Containers 5 0<br />
Tableware 8<br />
Miscellaneous instruments, scientific<br />
equipment, and others 17<br />
GLASS-MANUFACTURING PROCESS<br />
Soda-lime glass is produced on a massive scale<br />
in large, direct-fired, continuous melting fur-<br />
naces. Other types of glass are melted in small<br />
batch furnaces having capacities ranging from<br />
only a few pounds to several tons per day. <strong>Air</strong><br />
pollution from the batch furnaces is minor, but<br />
the production of soda-lime glass creates major<br />
problems of air pollution control.<br />
A complete process flow diagram for the con-<br />
tinuous production of soda-lime glass is shown<br />
in Figure 587. Silica sand, dry powders,<br />
granular oxides, carbonates, cullet (broken<br />
glass), and other raw materials are transferred<br />
from railroad hopper cars and trucks to storage<br />
bins. These materials are withdraw from the<br />
storage bins, batch weighed, and blended in a<br />
mixer. The mixed batch is then conveyed to<br />
the feeders attached to the side of the furnace.<br />
Although dust emissions are created during
766 CHEMICAL PROCESSING EQUIPMENT<br />
Table 204. COMPOSITIONS OF COMMERCIAL GLASSES (Kirk and Othmer, 1947)<br />
Component<br />
Si02<br />
Na20<br />
K2O<br />
CaO<br />
PbO<br />
B2°3<br />
AlZ03<br />
MgO<br />
. Soda-lime<br />
70 to 75 (72)<br />
12 to 18 (15)<br />
0 to 1<br />
5 to 14 (9)<br />
-<br />
-<br />
0.5 to 2.5 (1)<br />
0 to 4 (3)<br />
Figure 587. Flow diagram for soda-lime glass manufacture (Kirk<br />
and Othmer, 1947).<br />
these operations, control can be accomplished<br />
by totally enclosing the equipment and install-<br />
ing filter vents, exhaust systems, and bag-<br />
houses.<br />
Screw- or reciprocating-type feeders contin-<br />
uously supply hatch-blended materials to the<br />
direct-fired, regenerative furnace. These dry<br />
materials float upon the molten glass within<br />
Composition, qoa<br />
a~he figures in parentheses give the approximate composition of a typical member.<br />
SILICA SAND SODI ASH LIMESTONE<br />
or burn, ,me<br />
~andlonrr (in ~s.,<br />
wva. I,,. MO., "D<br />
(rushed. wd$hzd.<br />
@pro*. 20.120 me*<br />
Oc8,a"Ylar<br />
M ~ atlo O relulfr<br />
,,raw mateldl<br />
ionIans MgCO,<br />
screenad to APProx. 2[il20 rn8.h<br />
8PP'o.. 20-100 mash<br />
CO"ti""0UI tan* turnsre ioo*mg Mdtlng<br />
doxnt"r.ugh top (crown,. about 2. 70o0i CYilPlcrYIhlng<br />
suhmera~ tnroat in aridaera,,<br />
Lead<br />
53 to 68 (68)<br />
5 to 10 (10)<br />
1 to 10 (6)<br />
0 to 6 (1)<br />
15 to 40 (15)<br />
-<br />
0 to 2<br />
-<br />
2<br />
Relining:<br />
fining and<br />
~orosilicate 96% silica<br />
73 to 82 (80)<br />
3 to 10 (4)<br />
0.4 to 1<br />
0 to 1<br />
0 to 10<br />
5 to 20 (14)<br />
2 to 3 (2)<br />
-<br />
96<br />
-<br />
-<br />
-<br />
-<br />
3<br />
-<br />
-<br />
Silica glass<br />
99. 8<br />
-<br />
-<br />
-<br />
-<br />
the furnace until they melt. Carbonates de-<br />
compose releasing carbon dioxide in the form<br />
of bubbles. Volatilized particulates, com-<br />
posedmostly of alkali oxides and sulfates,<br />
are captured by the flame and hot gases pass-<br />
ing across the molten surface. The particu-<br />
lates are either deposited & the checkers and<br />
refractory-lined passages or expelled to the<br />
atmosphere.
Glass Ma1<br />
The mixture of materials is held around 2, 700°F<br />
in a molten state until it acquires the homogeneous<br />
character of glass. Then it is gradually cooled<br />
to about 2,200aF to make it viscous enough to<br />
form. In a matter of seconds, while at a yellow-<br />
orange hot temperature, the glass is dram<br />
from the furnace and worked on forming machines<br />
by a variety of methods including pressing, blow-<br />
ing in molds, drawing, rolling, and casting.<br />
One source of air pollution is hydrocarbon greases<br />
and oils used to lubricate the hot delivery systems<br />
and molds of glass-forming machines. The smoke<br />
from these greases and oils creates a significant<br />
amount of air pollution separate from furnace<br />
emissions.<br />
Immediately after being shaped in the machines,<br />
the glass articles are conveyed to continuous<br />
annealing ovens, where they are heat treated<br />
to remove strains that have developed during<br />
the molding or shaping operations and then<br />
subjected to slow, controlled cooling. Gas-<br />
fired or electrically heated annealing ovens<br />
are not emitters of air contaminants in any<br />
significant quantity. After leaving the anneal-<br />
ing ovens, the glass articles are inspected and<br />
packed or subjected to further finishing opera-<br />
tions.<br />
Glass-forming machines for mass production<br />
of other articles such as rod, tube, and sheet<br />
usually do not emit contaminants in significant<br />
amounts .<br />
HANDLING, MIXING, AND STORAGE SYSTEMS<br />
FOR RAW MATERIALS<br />
Material-handling systems for batch mixing<br />
and conveying materials for making soda-<br />
lime glass normally use commercial equip-<br />
ment of standard design. This equipment is<br />
usually housed in a structure separate from<br />
the glass-melting furnace and is commonly<br />
referred to as a "batch plant. " A flow dia-<br />
gram of a typical batch plant is shown in Figure<br />
588. In most batch plants, the storage bins are<br />
located on top, and the weigh hoppers and mixers<br />
are below them to make use of the gravity flow.<br />
Major raw materials and cullet (broken scrap<br />
glass) are conveyed from railroad hopper cars<br />
or hopper trucks by a combination of screw<br />
conveyors, belt conveyors, and bucket eleva-<br />
tors, or by pneumatic conveyors (not shown in<br />
Figure 588) to the elevated storage bins. Minor<br />
ingredients are usually delivered to the plant<br />
in paper bags or cardboard drums and trans-<br />
ferred hy hand to small bins.<br />
Ingredients comprising a batch of glass are<br />
dropped by gravity from the storage bins into<br />
weigh hoppers and then released to fall into<br />
the mixer. Cullet is ground and then mixed<br />
with the dry ingredients in the mixer. Ground<br />
cullet may also bypass the mixer and be mixed<br />
instead with the other blended materials in the<br />
bottom of a bucket elevator. A typical batch<br />
charge for making soda-lime flint glass in a<br />
mixer with a capacity of 55 cubic feet consists<br />
of:<br />
Silica sand<br />
Cullet<br />
Soda ash<br />
Limestone<br />
Niter<br />
Salt cake<br />
Arsenic<br />
Raw materials are blended in the mixer for peri-<br />
ods of 3 to 5 minutes and then conveyed to a<br />
charge bin located alongside the melting furnace.<br />
At the bottom of the charge bin, rotary valves<br />
feed the blended materials into reciprocating- or<br />
screw-type furnace feeders.<br />
In a slightly different arrangement of equipment<br />
to permit closer control of batch composition,<br />
blended materials are discharged from the mixer<br />
into batch cans that have a capacity of one mixer<br />
load each. Loaded cans are then conveyed by<br />
monorail to the furnace feeders. Trends in<br />
batch plant design are toward single reinforced-<br />
concrete structures in which outer walls and<br />
partitions constitute the storage bins. Complete<br />
automation is provided so that the batch plant is<br />
under direct and instant control of the furnace<br />
foreman.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
The major raw materials for making soda-lime<br />
glass--sand, soda-ash, and limestone--usually con-<br />
tain particles averaging about 300 microns in size,<br />
Particles less than 50 microns constitute only a<br />
small portion of the materials, but are presentin<br />
sufficient quantities to cause dust emissions during<br />
conveying, mixing, and storage operations. More-<br />
over, minor raw materials such as salt cake and<br />
sulfur can create dust emissions during handling.<br />
Dust is the only air contaminant from batch plants.<br />
and the control of dust emissions poses problems<br />
similar to those in industrial plants handling simi-<br />
lar dusty powder or granular materials.<br />
Hooding and Ventilation Requirements<br />
Dust control equipment can be installed on con-<br />
veying systems that use open conveyor belts.<br />
A considerable reduction in the size of the dust
768 CHEMICAL PROCESSING EQUIPMENT<br />
CULLET<br />
- > - 2 - * - =<br />
MAGNETIC E<br />
SEPARATOR +<br />
<<br />
-<br />
CRUSHER = rn<br />
RAW MATERIALS<br />
RECEIVING<br />
HOPPER<br />
V<br />
SCREW<br />
CONVEYOR<br />
C<br />
-<br />
-1<br />
BELT CONVEYOR<br />
Figure 588. Process flow diagram of a hatch plant.<br />
control equipment can be realized by totally en- cfm per foot of belt width, with 200 fpm minimum<br />
closing all conveying equipment and sealing all<br />
covers and access ooenines with easkets of<br />
velocity through the hood openings.<br />
polyurethane foam. In fact, by totally enclos-<br />
-<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
ing all conveying ~. equipment, . - exhaust systems<br />
become unnecessary, and relatively small filter<br />
Because dust emissions contain particles only i<br />
vents or dust cabinets can be attached directly<br />
!<br />
a few microns in diameter, cyclones, and cento<br />
the conveying equipment and storage bins.<br />
I<br />
On the other hand, exhaust systems are re-<br />
quired for ventilating the weigh hoppers and<br />
mixers. For example, a 60-cubic-foot-capacity<br />
mixer and a 4,500-pound-capacity mixer each<br />
require about 600 cfm ventilation air. Seals of<br />
polyvinylchloride should be installed between<br />
the rotating body of the mixer and its frame td<br />
reduce ventilation to a minimum.<br />
Railroad hopper cars and hopper bottom trucks<br />
must be connected to sealed receiving hoppers<br />
by fabric sleeves so that dust generated in the<br />
hoppers during the loading operation is either<br />
filtered through the sleeves or exhausted tbrough<br />
a baghouse.<br />
Local exhaust systems for dust pickup are de-<br />
signed by using the recommended practice of the<br />
Committee on Industrial Ventilation (1960). For<br />
example, the ventilation rate at the transfer<br />
point between two open belt conveyors is 350<br />
- -<br />
trifueal scrubbers are not as effective as haehouses<br />
or filters are in collecting these small<br />
I<br />
;<br />
particles; consequently, simple cloth filters and I<br />
I<br />
baghouses are used almost exclusively in con- 1.<br />
trolling dust emissions from batch plants.<br />
Filter socks or simple baghouses with intermittent<br />
shaking mechanisms are usually de-<br />
I<br />
signed for a filter velocity of 3 fpm, but bag-<br />
i<br />
I<br />
houses with continuous cleaning devices such as i<br />
pulse jets or reverse air systems can be de-<br />
!<br />
signed for filter velocities as high as 10 fpm. i'<br />
Filtration cloths are usually cotton, though ny- i<br />
lon, orlon, and dacron are sometimes used.<br />
Dusts collected are generally noncorrosive.<br />
Filters or baghouses for storage bins are designed<br />
to accommodate not only displaced air<br />
from the filling operation but also air induced<br />
by falling materials. Filtration of air exhaust<br />
from pneumatic conveyors used in filling the<br />
bins must also be provided. Filters with at least<br />
a 1-square-foot area should be mounted on the<br />
hand-filled minor-ingredient bins.<br />
4<br />
I<br />
!<br />
i<br />
!<br />
~
Glass Man1<br />
Transfer chutes of special design are used for<br />
hand filling the minor ingredient bins. They are<br />
first attached securely with gaskets to the top of<br />
the bins. The bags are dropped into a chute<br />
containing knives across the bottom. The knives<br />
split the bag, and as the materials fall into the<br />
bin, the broken bag seals off the escape of dust<br />
from the top of the chute.<br />
CONTlNUOUS SODA-LIME GLASS-MELTING FURNACES<br />
While limited quantities of special glasses such<br />
as lead or horosilicate are melted in electrically<br />
heated pots or in small-batch, regenerative fur-<br />
naces with capacities up to 10 tons per day, the<br />
hulk of production, soda-lime glass, is melted<br />
in direct-fired, continuous, regenerative furnaces.<br />
Many of these furnaces have added electric induc-<br />
tion systems called "boosters" to increase capac-<br />
ity. Continuous, regenerative furnaces usually<br />
range in capacity from 50 to 300 tons of glass<br />
per day; 100 tons is the most common capac-<br />
ity found in the United States.<br />
Continuous, regenerative, tank furnaces differ<br />
in design according to the type of glass products<br />
manufactured. All have two compartments. In<br />
the first compartment, called the melter, the dry<br />
ingredients are mixed in correct prcportions and<br />
are continuously fed onto a molten mass of glass<br />
having a temperature near 2,700-F. The dry mate-<br />
rials melt after floating a third to one-half of the<br />
way across the compartment and disappearing into<br />
the surface of a clear, viscous-liquid glass. Glass<br />
flows from the melter into the second compartment,<br />
commonly referred to as the refiner, where it is<br />
mixed for homogeneity and heat conditioned to<br />
eliminate bubbles and stones. The temperature<br />
is gradually lowered to about 2,200-F. The<br />
amount of glass circulating within the melter<br />
and refiner is about 10 times the amount with-<br />
drawn for production (Sharp, 1954).<br />
Regenerative furnaces for container and tableware<br />
manufacture have a submerged opening or throat<br />
separating the refiner from the melter. The throat<br />
prevents undissolved materials and scum on the<br />
surface from entering the refiner. Glass flows<br />
from the semicircular refining compartment into<br />
long, refractory-lined chambers called forehearths.<br />
Oil or gas burners and ventilating dampers ac-<br />
curately control the temperature and viscosity of<br />
the glass that is fed from the end of the forehearth<br />
to glass -forming machines.<br />
Continuous furnaces for manufacturing rod, tube,<br />
and sheet glass differ from furnaces for container<br />
and tableware manufacture in that they have no<br />
throat between the melter and refiner. The com-<br />
partments are separated from each other by float-<br />
ing refractory beams riding in a drop arch across<br />
the entire width of the furnace. Glass flows from<br />
the rectangular-shaped refiner directly into the<br />
forming machines.<br />
Regenerative firing systems for continuous glass<br />
furnaces were first devised by Siemens in 1852,<br />
and since then, nearly all continuous glass fur-<br />
naces in the United States have used them. In<br />
Europe, continuous glass furnaces employ both<br />
recuperative and regenerative systems.<br />
Regenerative firing systems consist of dual<br />
chambers filled with brick checkerwork. While<br />
the products of combustion from the melter pass<br />
through and heat one chamber, combustion air<br />
is preheated in the opposite chamber. The func-<br />
tions of each chamber are interchanged during<br />
the reverse flow of air and combustion products.<br />
Reversals occur every 15 to 20 minutes as re-<br />
quired for maximum conservation of heat.<br />
Two basic configur~tions are used in designing<br />
continuous, regenerative furnaces - end port.<br />
Figure 589, and side port, Figure 590. Ir, the<br />
side port furnace, combustion products and<br />
flames pass in one direction across the melter<br />
during one-half of the cycle. The flow is reversed<br />
during the other half cycle. The side<br />
port design is commonly used in large furnaces<br />
with melter areas in excess of 300 square feet<br />
(Tooley, 1953).<br />
In the end port configuration, combustion products<br />
and flames travel in a horizontal U-shaped path<br />
across the surface of the glass within the melter.<br />
Fuel and air mix and ignite at one port and dis-<br />
charge through a second port adjacent to the first<br />
on the same end wall of the furnace. While the<br />
end port design has been used extensively in small-<br />
er furnaces with melter areas from 50 to 300<br />
square feet, it has also been used in furnaces with<br />
melter areas up to 800 square feet.<br />
Continuous furnaces are usually operated slightly<br />
above atmospheric pressure within the melter to<br />
prevent air induction at the feeders and an over-<br />
all loss in combustion efficiency. Furnace draft<br />
can be produced by several methods: Induced-<br />
draft fans, natural-draft stacks, and ejectors.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
Particulates expelled from the melter are the re-<br />
sult of complex physical and chemical reactions<br />
that occur during the melting process.<br />
Glass has properties akin to those of crystalline<br />
solids, including rigidity, cold flow, and hard-<br />
ness. At the same time, it behaves like a super-
. .<br />
. .<br />
. .~<br />
. . . ~.<br />
770 CHEMICAL PROCESSING EQUIPMENT<br />
Figure 589. Regeneratlve end port glass-melting furnace.<br />
cooled liquid. It has nondirectional properties,<br />
fracture characteristics of an amorphous solid,<br />
and no freezing or melting point. To account for<br />
the wide range of properties, glass is considered<br />
to be a configuration of atoms rather than an aggregate<br />
of molecules. Zachariasen (1932) proposed<br />
the theory that glass consists of an extended,<br />
continuous, three-dimensional network of ions with<br />
a cet.tain amount of short-distance-ordered arrangement<br />
similar to that of a polyhedral crystal.<br />
These dissimilar properties explaln in part why<br />
predictions of particulate losses from the melter<br />
based solely upon known temperatures and vapor<br />
pressures of purp compounds have been inaccurate.<br />
Other phenomena affect the generation of par-<br />
ticulates. During the melting process, carbon<br />
dioxide bubbles and propels particulates from the<br />
melting batch. Particulates are entrained by the<br />
fast-moving stream of flames and combustion<br />
gases. As consumption of fuel and refractory tem-<br />
peratures of the furnace increase with glass ton-<br />
nage, particulates also increase in quantity. Par-<br />
ticulates, swept from the melter, are either col-<br />
lected in the checkerwork and gas passages or<br />
exhausted to the atmosphere.<br />
Source test data<br />
In a recent study, many source tests of glass<br />
furnaces in Los Angeles County were used for<br />
determining the major variables influencing stack<br />
emissions. As summarized in Table 205, data<br />
include: Particulate emissions, opacities, pro-<br />
cess variables, and furnace design factors.<br />
Particle size distributions of two typical stack<br />
samples are shown in Table 206. These particu-<br />
late samples were obtalned from the catch of a<br />
pilot baghouse venting part of the effluent from<br />
a large soda-lime container furnace.
Chemical composition of the particulates was<br />
determined by microquantitative methods or by<br />
spectrographic analysis. Five separate samples,<br />
four from a pilot baghouse, and one from the<br />
stack of a soda-lime regenerative furnace, are<br />
given in Table 207. They were found to be com-<br />
posed mostly of alkali sulfates although alkalies<br />
are reported as oxides. The chemical composi-<br />
tion of sample 5 was also checked by X-ray<br />
crystallography. In this analysis, the only<br />
crystalline material present in identifiable<br />
amounts was two polymorphic forms of sodium<br />
sulfate.<br />
Opacity of stack emissions<br />
From the source test data available, particu-<br />
late emissions did not correlate with the opacity<br />
of the stack emissions. Some generalizations on<br />
opacity can, however, be made. Opacities usu-<br />
Glass Manufacture 771<br />
Figure 590. Regenerative side port glass-melting furnace.<br />
ally increase as particulate emissions increase.<br />
More often than not, furnaces burning U. S. Grade<br />
5 fuel oil have plumes exceeding 40 percent white<br />
opacity while operating at a maxim- pull rate,<br />
which is the glass industry's common term for<br />
production rate. Plumes from these same fur-<br />
naces were only 15 to 30 percent white opacity<br />
while burning natural gas or U. S. Grade 3<br />
(P.S. 200) fuel oil. Somewhat lower opacities<br />
may be expected from furnaces with ejector<br />
draft systems as compared with furnaces with<br />
natural-draft stacks or induced-draft fans.<br />
Hooding and Ventilation Requirements<br />
In order to determine the correct size of air<br />
pollution control equipment, the volume of dirty<br />
exhaust gas from a furnace must be known. Some<br />
of the more important factors affecting exhaust<br />
volumes include: Furnace size, pull rate, com-
772 CHEMICAL PROCESSING EQUIPMENT<br />
Test No.<br />
C-339b<br />
C-339<br />
C-382-1<br />
C-382-2<br />
C-536<br />
C-383<br />
Pri Lab<br />
Pri Lab<br />
Pri Lab<br />
Pri Lab<br />
Pri Lab<br />
Pri Lab<br />
Pri Lab<br />
C-101<br />
C-120<br />
C-577<br />
C-278-1<br />
C-278-2<br />
C-653<br />
C-244-1<br />
C-244-2<br />
C-420-1<br />
C-420-2<br />
C-743<br />
C-471<br />
Table 205. SOURCE TEST DATA FOR GLASS-MELTING FURNACES<br />
x1<br />
Type Type (particulate<br />
of of<br />
furnacea fuel<br />
x33<br />
wt fraction<br />
of cullet in<br />
chargeC<br />
0.300<br />
0.300<br />
0. 300<br />
0.300<br />
0.199<br />
0.300<br />
0. 094<br />
0. 094<br />
0. 157<br />
0. 094<br />
0. 365<br />
0.269<br />
0. 175<br />
0.300<br />
0. 320<br />
0. 134<br />
0.361<br />
0. 360<br />
0. 131<br />
0. 182<br />
0.100<br />
0. I00<br />
0.100<br />
0.047<br />
0.276<br />
x4<br />
(checker volumd,<br />
ft3/ft2 of melter<br />
a~~ = end port, regenerative furnace; SP = side port, regenerative furnace.<br />
bG = natural gas; 0-200 = U.S. Grade 3 fuel oil; 0-300 = U.S. Grade 5 fuel oil.<br />
CConstants: Sulfate content of charge 0. 18 to 0. 34 wt 70.<br />
Fines (-325 mesh) content of charge 0.2 to 0.3 wt %.<br />
bustion efficiency, checker volume, and furnace<br />
condition.<br />
Exhaust volumes can be determined from fuel<br />
requirements for container furnaces given by<br />
the formula of Cressey and Lyle (1956).<br />
where<br />
F = total beat, 10<br />
6<br />
Btulday<br />
A = melter area, ft2<br />
T = pull rate, tonsfday.<br />
This straight-line formula includes minimum<br />
heat to sustain an idle condition plus additional<br />
heat for a specified pull rate. Fuel require-<br />
ments for bridgewall-type, regenerative fur-<br />
naces are also given by Sharp (1955) and are<br />
Maximum<br />
opacity<br />
of stack<br />
emissions,<br />
5 0<br />
10<br />
10<br />
10<br />
10<br />
2 0<br />
2 5<br />
2 5<br />
25<br />
2 5<br />
--<br />
45<br />
20<br />
2 0<br />
2 0<br />
3 5<br />
2 0<br />
2 0<br />
40<br />
2 5<br />
25<br />
10<br />
5<br />
2 5<br />
30<br />
shown in Figure 591. The melter rating para-<br />
meter of 4 square feet of melter surface area<br />
per daily ton of glass should be used to estimate<br />
the fuel requirements of container furnaces at<br />
maximum pull rates, but 8 square feet per ton<br />
can be used for estimating fuel requirements<br />
for non-bridgewall furnaces supplying glass<br />
for tableware and for sheet, rod, and tube<br />
manufacture. Fuel requirements given are<br />
averages for furnaces constructed before 1955;<br />
consequently, these furnaces generally require<br />
more fuel per ton of glass than do furnaces con-<br />
structed since 1955. After the fuel require-<br />
ments are determined, exhaust volumes are com-<br />
puted on the basis of combustion with 40 percent<br />
excess combustion air. Forty percent excess<br />
combustion air is chosen as representing av-<br />
erage combustion conditions near the end of the<br />
campaign/ (a total period of operation without shut-<br />
ting down for repairs to the furnace).<br />
Exhaust volumes determined from fuel require-<br />
ments are for furnaces with induced-draft sys-
Glass Manufacture 773<br />
Table 206. SIZE DISTRIBUTION OF in temperature from 600" to 850°F, but exhaust<br />
PARTICULATE EMISSIONS gas temperatures from furnaces containing ejec-<br />
(MICROMEROGRAPH ANALYSES) tors are lower and vary from 400" to 600°F:<br />
- In Table 208 are found chemical analyses of gas-<br />
Furnace 1<br />
D~ameler (D),<br />
W<br />
36.60<br />
22.00<br />
18. 30<br />
16.50<br />
14.60<br />
12.80<br />
12.20<br />
11.60<br />
11.00<br />
10.40<br />
9. 80<br />
9.20<br />
8.5G<br />
7. 30<br />
6. 10<br />
4. 88<br />
3. 66<br />
3. 05<br />
2.44<br />
1. 83<br />
1. 52<br />
1.22<br />
Fhnt glass<br />
70 (by wt)<br />
Less than D<br />
100<br />
99. 5<br />
98. 6<br />
97.7<br />
94.0<br />
84. 6<br />
80.7<br />
76.6<br />
72. 7<br />
67. 7<br />
62.4<br />
58. 3<br />
51.8<br />
43.1<br />
34.4<br />
28. 0<br />
21.3<br />
18. 6<br />
14. 9<br />
11.0<br />
8. 3<br />
4. 1<br />
Furnace 2 Amber @ass<br />
Dlamcter (D), 70 (by wt)<br />
&.<br />
less than D<br />
17.40 100<br />
15.70<br />
99.8<br />
14. 00<br />
99.4<br />
12.20<br />
96. 8<br />
11.60<br />
92. 5<br />
11.00<br />
84. 5<br />
10.50<br />
87. 2<br />
9.90<br />
83.4<br />
9.30<br />
78. 7<br />
8.80<br />
75.0<br />
8. 10<br />
73.4<br />
7.00<br />
60. 3<br />
5.80<br />
47.6<br />
4. 65<br />
35. 6<br />
3.49<br />
25. 4<br />
2.91<br />
20. 5<br />
2.33<br />
16. 4<br />
1.74<br />
10.9<br />
1.45<br />
8.9<br />
1. 16<br />
5.3<br />
eous components of exhaust gases from large,<br />
regenerating, gas-fired furnaces melting three<br />
kinds of soda-lime glass.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Methods<br />
As the furnace chmpaign progresses, dust carry-<br />
over speeds destruction of the checkers. Upper<br />
courses of the firebrick checker glaze when sub-<br />
jected to high temperatures. Dust and condensate<br />
collect on the brick surface and form slag that<br />
drips downward into the lower courses where it<br />
solidifies at the lower temperature and plugs the<br />
checkers. Slag may also act somewhat like fly-<br />
paper, tenaciously clinging to the upper courses<br />
and eventually sealing off upper gas passages.<br />
Hot spots develop around clogged checkers and<br />
intensify the destructive forces, which are re-<br />
flected by a drop in regenerator efficiency and a<br />
rise in fuel consumption and horsepower required<br />
to overcome additional gas flow resistance through<br />
the checkers. Checker damage can finally reach<br />
tems or natural-draft stacks. Exhaust volumes a point where operation is no longer economical or<br />
for ejector systems can he estimated by increas- is physically impossible because of collapse. Thus,<br />
ing the exhaust volume by 30 to 40 percent to successful operation of modern regenerative fur-<br />
account for ejector air mixed with the furnace naces requires keeping dust carryover from the<br />
effluent. melter to an absolute minimum, which also coin-<br />
cides with air pollution control ohjectives by prevent-<br />
Exhaust gases from furnaces with natural-draft ing air contaminants from entering the atmosphere.<br />
stacks or induced-draft fan systems usually range Aside from reducing air contaminants, benefits de-<br />
Table 207. CHEMICAL COMPOSITION OF PARTICULATE EMISSIONS<br />
(QUANTITATIVE ANALYSES), METALLIC IONS REPORTED AS OXIDES<br />
Sample source<br />
Test<br />
type of glass<br />
components<br />
Baghouse<br />
catch<br />
No. 1<br />
amber,<br />
wt %<br />
Silica (Si02) (SiO,)<br />
0. 03<br />
Calcium oxide (CaO)<br />
1. 70<br />
Sulfuric anhydride (SO3) )<br />
Boric anhydride (B203)<br />
Arsenic oxide (As,O?) - -<br />
Chloride (Cl)<br />
46.92<br />
3. 67<br />
7. 71<br />
Lead oxide (PbO) 0. 39<br />
Fe203<br />
MgO<br />
ZnO<br />
Unknown metallic oxide (R203)<br />
Loss on ignition<br />
10.10<br />
Baghouse<br />
catch<br />
No. 2<br />
flint,<br />
wt Yo<br />
0.3<br />
2.3<br />
25. 1<br />
1.3<br />
30.8<br />
Baghouse<br />
catch<br />
No. 3<br />
amber,<br />
wt Yo<br />
0.1<br />
0.8<br />
46. 7<br />
0.1<br />
0.5<br />
25. 7<br />
Baghouse<br />
catch<br />
No. 4<br />
flint,<br />
wt %<br />
4.1<br />
19.2<br />
30.5<br />
0.6<br />
1.4<br />
7.5<br />
Millipore<br />
filter<br />
No. 5<br />
flint,<br />
wt %<br />
3.3<br />
39.4<br />
6. 5<br />
11. 6
774 CHEMICAL PROCESSING EQUIPMENT<br />
By the method of Brandon (1959), particulate emis-<br />
sions, the dependent variable were found to corre-<br />
late with the following independent variables and<br />
nonquantitative factors:<br />
1. Process weight, lb/hr-ft2;<br />
2. cullet, wt % of charge;<br />
3 2<br />
3. checker volume, ft /ft melter;<br />
4. type of furnace, side port or end port;<br />
5. type of fuel, U.S. Grade 5 (PS300) oil or<br />
natural gas;<br />
2<br />
6. melter area, ft .<br />
Several simplifying assumptions are made so that<br />
furnaces of different sizes can be compared. Pro-<br />
cess weight per square foot of melter describes a<br />
unit process occurring in each furnace regardless<br />
of size. Cubic feet of checkers per square foot<br />
of melter not only defines the unit's dust-collect-<br />
ing capability but is also a measure of fuel economy.<br />
Source tests C-382 and C-536 in Table 205 (and<br />
other source tests) show no appreciable difference<br />
in particulate emissions from burning natural gas<br />
or U. S. Grade 3 fuel oil.<br />
MELTER AREA. f t 2<br />
Correlation of particulate emissions with weight<br />
percent sulfate (SO3) and minus 325-mesh fines<br />
in the charee - was not nossible because of insuffi-<br />
Figure 591' gas for<br />
regenerative furnaces (Sharp, 1955).<br />
I-type<br />
cient test data. Limited data available indicate<br />
that particulate emissions may double when total<br />
sulfate (SO2) content of the batch charge is in-<br />
A<br />
creased from 0. 3 to 1. 0 weight percent. Total<br />
rived from reducing dust carryover are many and sulfates (SO3) include equivalent amounts of eleinclude<br />
longer furnace campaigns, lower mainte- mental sulfur and all compounds containing sulfur.<br />
nance costs, and savings on fuel.<br />
Sulfates usually comprise over 50 percent of the<br />
particulate emissions. They act as fluxing agents<br />
In order to determine which design and operating preventing the melting dry-hatch charge from<br />
variables have the greatest effect upon dust carry- forming a crust that interferes with heat transover<br />
and particulate emissions, statistical analysis fer and melting (Tooky, 1953). Compounds of<br />
was performed on the source test data given in arsenic, boron, fluorine, and metallic selenium<br />
Table 205.<br />
are also expected to be found alone with sodium<br />
sulfate in the particulate emissions because of<br />
their high vapor pressures.<br />
Data roughly indicate that particulate emissions<br />
increase severalfold when the quantity of minus<br />
325-mesh fines increases from 0. 3 weight per-<br />
cent to 1 or 2 weight percent.<br />
Statistical analysis using the method of curvilinear<br />
multiple correlation by Ezekiel (1941) results in the<br />
following equation, which describes particulate<br />
emissions, the dependent variable, as a function<br />
Table 208. CHEMICAL COMPOSITION OF GASEOUS EMISSIONS<br />
FROM GAS-FIRED, REGENERATIVE FURNACES<br />
Gaseous components<br />
Nitrogen, vol 7%<br />
Oxygen, vol 70<br />
Water vapor, vol 70<br />
Carbon dioxide, vol %<br />
Carbon monoxide, vol %<br />
Sulfur dioxide (SO2), ppm<br />
Sulfur trioxide (SO3), ppm<br />
Nitrogen oxides (NO,NOZ), ppm<br />
Organic acids, ppm<br />
Aldehydes, ppm<br />
aNA = not available.<br />
Flint glass<br />
71. 9<br />
9. 3<br />
12.4<br />
6. 4<br />
0<br />
0<br />
0<br />
724<br />
NA"<br />
N A<br />
Amber glass<br />
81. 8<br />
10.2<br />
7.7<br />
8. 0<br />
0. 007<br />
61<br />
12<br />
137<br />
5 0<br />
7<br />
Georgia green<br />
72.5<br />
8.0<br />
12.1<br />
7.4<br />
0<br />
14<br />
15<br />
N A<br />
N A<br />
N A
of four independent variables and two nonquanti-<br />
tative independent factors. This equation is<br />
valid only when two other independent variables--<br />
sulfate content and content of minus 325-mesh fines<br />
of the batch--lie between 0. 1 to 0. 3 weight percent<br />
and, also, when fluorine, boron, and lead com-<br />
pounds are either absent from the batch charge or<br />
present only in trace amounts.<br />
where<br />
XI = particulate emissions, lb/hr<br />
2<br />
X2 = process wt, lb/hr-ft melter<br />
X3 =<br />
wt fraction of cullet in charge<br />
X4 =<br />
3 2<br />
checker volume, ft /ft melter<br />
X5 =<br />
2<br />
melter area, ft 1100<br />
Glass Ma nufacture 77c<br />
(152)<br />
a = constant involving two nonquantitative<br />
independent factors relating the type<br />
of furnace (side port or end port) and<br />
the type of fuel (U. S. Grade 5 fuel or<br />
natural gas).<br />
a = -0.493 end port - U.S. Grade 5<br />
fuel oil<br />
a = -0. 623 side port - U.S. Grade 5<br />
fuel oil<br />
a = -1.286 end port - natural gas<br />
a = -1.416 side port - natural gas.<br />
Particulate emissions computed by this equation<br />
for 25 source tests show a standard deviation<br />
from measured particulate emissions of + 1.4<br />
pounds per hour. Further statistical refine-<br />
ment failed to yield a lower standard deviation.<br />
Emissions to the atmosphere can be predicted<br />
by using equation 152 or Figures 592 to 595, which<br />
are based upon this equation. The curves should<br />
be used only within the limits indicated for the<br />
variables. The curves should not be extrapolated<br />
in either direction with the expectation of any de-<br />
gree of accuracy, even though they appear as<br />
straight lines. Particulate emissions are first<br />
determined from Figure 592, then positive or<br />
negative corrections obtained from Figures 593<br />
to 595 are added to the emissions obtained from<br />
Figure 592.<br />
Design and operation of soda-lime, continuous,<br />
regenerative furnaces to alleviate dust carry-<br />
over and minimize particulate emissions are<br />
discussed in succeeding paragraphs. Advantages<br />
of all-electric, continuous furnaces for melting<br />
glass are also cited.<br />
Control of raw materials<br />
Although glassmakers have traditionally sought<br />
fine-particle materials for easier melting, these<br />
materials have intensified dust carryover in regenerative<br />
furnaces. A compromise must be<br />
reached. Major raw materials should be in the<br />
form of small particles, many of them passing<br />
U. S. 30-mesh screen, but not more than 0. 3<br />
weight percent passing U. S. 325-mesh screen.<br />
Because crystals of soda ash, limestone, and<br />
other materials may be friable and crush in the<br />
mixer, producing excessive amounts of fines,<br />
screen analyses of individual raw materials<br />
should not be combined for estimating the screen<br />
analyses of the batch charge. Crystalline shape<br />
and density of raw materials should be thoroughly<br />
investigated before raw material suppliers are<br />
selected.<br />
Since particulate emissions from soda-lime re -<br />
generative furnaces increase with an increase in<br />
equivalent sulfate (SO3) present in the batch<br />
charge, sulfate content should be reduced to an<br />
absolute minimum consistent with good glassmaking.<br />
Preferably, it should be below 0. 3<br />
weight percent. Equivalent sulfate (SO3) content<br />
of the batch includes all sulfur compounds and<br />
elemental sulfur. Compounds of fluorine, boron,<br />
lead, and arsenic are also known to promote dust<br />
carryover (Tooley, 1953), but the magnitude of<br />
their effect upon emissions is still unknown. In<br />
soda-lime glass manufacture, these materials<br />
should be eliminated or should be present in only<br />
trace amounts.<br />
From the standpoint of suppressing stack emis-<br />
sions, cullet content of the batch charge should<br />
be kept as high as possible. Plant economics may,<br />
nevertheless, dictate reduction in cullet where fuel<br />
or cullet is high in cost or where cullet is in short<br />
supply. Some manufacturing plants are able to<br />
supply all their cullet requirements from scrap and<br />
reject glassware.<br />
Batch preparation<br />
There are a number of ways to condition a batch<br />
charge and reduce dust carryover. Some sodalime<br />
glass manufacturers add moisture to the<br />
dry batch, but the relative merits of this process<br />
are debatable. Moisture is sprayed into the drybatch<br />
charge at the mixer as a solution containing<br />
1 gallon of surface-active wetting agent to 750<br />
gallons of water. Surface tension of the water is<br />
reduced by the wetting agent so that the water<br />
wets the finest particles and is evenly distributed
776 CHEMICAL PROCESSING EQUIPMENT<br />
Figure 592. Particulate emlsslons versus checker<br />
volume per ft2 of melter.<br />
- x<br />
z<br />
w,<br />
-<br />
B<br />
B<br />
7<br />
6<br />
E 3<br />
+<br />
c, u 2<br />
=<br />
-<br />
cz<br />
U I<br />
0<br />
I<br />
3<br />
0 B 12 16 20 24 28 31<br />
X2 PROCESS WEIGHT, Ib/hr Per 11' neller<br />
Figure 593. Correction to particulate emissions<br />
for process weight per ft2 melter.<br />
U.YI U,IU U. 13 U.IU 0.25 0.30 0.35<br />
X1. KEIGHT FRACTIOH OF CULLET IH CHIAGE<br />
Figure 594. Correction to particulate emissions for cullet content<br />
of the batch charge.<br />
Figure 595. Correction to particulate emissions<br />
for melter area.<br />
, Other<br />
throughout the batch (Wilson, 1960). Fluxing<br />
materials such as salt cake appear more effec-<br />
tive, since the unrnelted batch does not usually<br />
travel so far in the melter tank before it melts.<br />
Moisture content of the batch is normally in-<br />
creased to about 2 percent by weight. If the<br />
moisture content exceeds 3 percent, batch in-<br />
gredients adhere to materials-handling equip-<br />
ment and may cake in storage bins or batch cans.<br />
batch preparation methods have been em-<br />
ployed on a limited-production or experimental<br />
basis to reduce dust carryover from soda-ash<br />
glass manufacture. One method involves prer<br />
sintering the batch to form cullet and then charg-<br />
ing only this cullet to the furnace. Advantages
Glass Manx<br />
claimed are faster melting, better batch con-<br />
trol, less seed formation, reduced clogging in<br />
the checkers, and lower stack losses (Arrandale,<br />
1962). A Dutch oven doghouse cover also reduces<br />
dust carryover by sintering the top of the floating<br />
dry batch before it enters the melter. This meth-<br />
od is probably not as efficient as is complete pre-<br />
sintering in reducing dust carryover.<br />
Other methods include: (1) Charging briquets,<br />
which are made from regular batch ingredients<br />
by adding up to 10 percent by weight of water;<br />
(2) charging wet hatches containing 6 percent<br />
moisture, which are made by first dissolving<br />
soda-ash to form a saturated solution and mix-<br />
ing this solution with sand and the other dry<br />
materials: (3) charging the dry batch (Submerged)<br />
in the melter; (4) enclosing batch feeders (Fabri-<br />
anio, 1961); and (5) installing batch feeders on<br />
opposite sides of end port, regenerative furnaces<br />
and charging alternately on the side under fire.<br />
Checkers<br />
The design concept of modern regenerative fur-<br />
naces, with its emphasis on maximum use of<br />
fuel, is also indirectly committed to reducing<br />
dust carryover. All things being equal, less<br />
fuel burned per ton of glass means less dust<br />
entrainment by hot combustion gases and flames<br />
flowing across the surface of the melting glass.<br />
Although container furnaces constructed over<br />
15 years ago required over 7,000 cubic feet of<br />
natural gas per ton of glass at maximum pull<br />
rates, container furnaces built today can melt<br />
a ton of glass with less than 5,000 cubic feet<br />
of natural gas.<br />
While several design changes are responsible<br />
for this improvement, one of the most important<br />
is the increase in checker volume. The ratio of<br />
checker volume (cubic feet) to meter area (square<br />
feet) has been rising during the years from about<br />
5 in earlier furnaces to about 9 today. Enlarged<br />
checkers not only reduce fuel consumption and<br />
particulate formation but also present a more<br />
effective trap for dust particles that are expelled<br />
from the melter. Source tests conducted by a<br />
large glass-manufacturing company indicated<br />
that over 50 percent of the dust carryover from<br />
the melter is collected by the checkers and gas<br />
passages instead of entering the atmosphere.<br />
Of course, the economics connected with regen-<br />
erative furnace operation dictates the checker<br />
volume. The law of diminishing returns oper-<br />
ates where capital outlay for an added volume<br />
of checkers will no longer be paid within a spe-<br />
cified period by an incremental reduction in fuel<br />
costs. Checkers have been designed in douhle-<br />
pass arrangements to recover as much as 55 per-<br />
cent of the heat from the waste gases (Sharp, 1954).<br />
Although dust collects within checkers by mechan-<br />
isms of impingement and settling, the relationship<br />
among various factors influencing dust collection<br />
is unknown. These factors include: Gas velocity,<br />
brick size, flue spacing, brick setting, and brick<br />
composition. Checkers designed for maximum<br />
fuel economy may not necessarily have the high-<br />
est collection efficiency. Further testing will<br />
he necessary in order to evaluate checker de-<br />
signs. Checkers designed for maximum heat<br />
exchange contain maximum heat transfer surface<br />
per unit volwne, a condition met only by smaller<br />
refractories with tighter spacing. Heat transfer<br />
surfaces can be computed by the method given in<br />
Trinks (1955). Since gas velocities are also<br />
highest for maximum heat transfer, less dust<br />
collects by simple settling than by impingement.<br />
Dust collection is further complicated in that<br />
smaller brick increases the potential for clogging.<br />
To prevent clogging in the checkers and ensure<br />
a reasonable level of heat transfer, checkers<br />
should be cleaned once per month or more often;<br />
an adequate number of access doors should be<br />
provided for this purpose (Spain, 195613). Com-<br />
pressed air, water, or steam may be used to<br />
flush fine particles from the checkers. Virtual-<br />
ly nothing can be done to remove slag after it<br />
has formed. Checkers can be arranged in a<br />
double vertical pass to reduce overall furnace<br />
height and make cleaning easier. Access doors<br />
should also be provided for remqving dust de-<br />
posits from the flues.<br />
Preheaters<br />
Further reductions in fuel consumption to re-<br />
duce dust emissions may be realized by install-<br />
ing rotsry, regenerative air preheaters in series<br />
with the checkers. Additional benefits include<br />
less checker plugging, reduced maintenance, and<br />
increased checker life. Rotating elements of<br />
the preheater are constructed of mild steel, low-<br />
alloy steel, or ceramic materials. Preheaters<br />
raise the temperature of the air to over 1, 000°F.<br />
and the increased velocity of this preheated air<br />
aids in purging dust deposits that block gas pas-<br />
sages of the checkers. Exhaust gases passing<br />
through the opposite side of the preheater are<br />
cooled below 800°F before being exhausted to<br />
the atmosphere. A heat balance study of a plate<br />
glass, regenerative furnace shows a 9 percent<br />
increase in heat use by the installation of a<br />
rotary, regenerative air preheater (Waitkus,<br />
1962). To maintain heat transfer and prevent<br />
re-entrainment, dust deposits on the preheater<br />
elements must he removed by periodic cleaning.<br />
Ductwork and valves should he installed for by-<br />
passing rotary air preheaters during the cleaning<br />
stage.
778 CHEMICAL PROCESSING EQUIPMENT<br />
Refractories and insulation<br />
Slagging of the upper courses of checkerwork<br />
can be alleviated in most cases by installing<br />
basic (high alumina content) brick in place of<br />
superduty firebrick (Robertson et al., 1957).<br />
Basic brickcourses extend from the topdown-<br />
ward to positions where checker temperatures<br />
arebelow l,50O0F. Atthis temperature, fire-<br />
bricknolonger "wets" and forms slagwithdust<br />
particles. Dust usually collects in the lower<br />
courses of firebrick in the form of fine particles<br />
that are easily removed by cleaning. Although<br />
basic brick costs 3 or 4 times as much as super-<br />
duty firebrick, some glass manufacturers are<br />
constructing entire checkerworks of basic brick<br />
where slagging and clogging are most severe.<br />
In some instances, basic refractories are re-<br />
placing fireclay rider tiles and rider arches<br />
in checker supports (Van Dreser, 1962). A<br />
word of caution, basic brick is no panacea for<br />
all ills of checkers. Chemical composition of<br />
the dust should be known, to determine com-<br />
patibility with the checkers (Fabrianio, 1961).<br />
Regenerative furnaces can be designed to con-<br />
sume less fuel and emit less dust by proper<br />
selection and application of insulating refrac-<br />
tories. A heat balance study of a side port, re-<br />
generative furnace shows that, in the melting<br />
process, glass receives 10 percent of heat<br />
transfer from convection and 90 percent from<br />
radiation. Of the radiation portion of heat<br />
transferred, the crown accounts for 33 percent<br />
(Merritt, 1958). Since heat lossesthrough the un-<br />
insulated crown can run as high as 10 percent of<br />
the total heat input, there is need for insulation<br />
at this spot.<br />
Most crowns are constructed of silica brick<br />
with a maximum furnace capacity restricted to an<br />
operating temperature of 2,850"F (Sharp, 1955).<br />
Insulation usually cons is ts of insulating silica<br />
brick backed with high-duty plastic refractory.<br />
Furnaces are first operated without insulation,<br />
so that cracks can be observed. Then the cracks<br />
are sealed with silica cement, and the ~nsulation<br />
is applied.<br />
Insulation is needed on the melter sidewall and<br />
at the port necks to prevent glassy buildup caused<br />
by condensation of vapors. Condensate buildup<br />
flows across port sills into the melter and can<br />
become a major source of stones.<br />
While insulation of sidewalls shows negligible<br />
fuel reduction for flint glass manufacture, it<br />
does show substantial fuel reduction for colored<br />
glasses. The problem in manufacturing colored<br />
glass is to maintain a high enough temperature<br />
below the surface to speed the solution of stones<br />
and prevent stagnation. Insulation on sidewalls<br />
raises the mean temperature to a point where<br />
stones dissolve and glass circulates freely.<br />
Six inches or more of electrofusion cast block<br />
laid over a clay bottom in a bed of mortar (Baque,<br />
1954) not only saves fuel but is also less subject<br />
to erosion than is fireclay block.<br />
Insulation is seldom needed on the refining end<br />
of the furnace since refiners have become cool-<br />
ing chambers at today's high pull rates. Nose<br />
crowns, however, are insulated to minimize con-<br />
densation and drip (Bailey, 1957). Checkers are<br />
sometimes encased in steel to prevent air infiltra-<br />
tion through cracks and holes that develop in the<br />
refractory regenerator walls during the campaign.<br />
Combustion of fuel<br />
Furnace size also has an effect upon use of fuel,<br />
with a corresponding effect on the emissions of<br />
dust. Large furnaces are more economical than<br />
are small furnaces because the radiating surface<br />
or heat loss per unit volume of glass is greater<br />
for small furnaces.<br />
Slightly greater fuel economy may be expected<br />
from end port furnaces as compared with side<br />
port furnaces of equal capacity. Here again, the<br />
end port furnace has a heat loss advantage over<br />
the side port furnace because it has less exposed<br />
exterior surface area for radiating heat. Side<br />
port furnaces can, however, be operated at great-<br />
er percentages in excess of capacity since mixing<br />
of fuel with air is more efficient through several<br />
smaller inlet ports than it is through only one<br />
large inlet port. In fact, end port furnaces are<br />
limited in design to the amount of fuel that can<br />
be efficiently mixed with air and burned through<br />
this one inlet port (Spain, 1955). As far as dust<br />
losses are concerned, there are only negligible<br />
differences between end port and side port furnaces<br />
of equal size. Reduced fuel consumption to re-<br />
duce dust carryover can also be realized by in-<br />
creasing the depth of the melter to the maximum<br />
consistent with good-quality glass. Maximum<br />
depths for container furnaces are 42 inches for<br />
flint glass (Tooley, 1953) and about 36 inches for<br />
amber glass and emerald green glass.<br />
Dust emissions as well as fuel consumption can<br />
also be reduced by firing practice. Rapid changes<br />
in pull rates are wasteful of fuel and increase<br />
stack emissions. Hence, charge rates and glass<br />
pull rates for continuous furnaces should remain<br />
as constant as possible by balancing loads be-<br />
tween the glass -forming machines. If possible,<br />
furnaces should be fired on natural gas or U. S.<br />
Grade 3 or lighter fuel oil. Particulate emis -<br />
sions increase an average of about 1 pound per<br />
hour when U. S. Grade 5 fuel oil is used instead
of natural gas or U. S. Grade 3 fuel oil, and<br />
opacities may exceed 40 percent white.<br />
Combustion air should be thoroughly mixed with<br />
fuel with only enough excess air present to en-<br />
sure complete combustion without smoke. Ex-<br />
cess air robs the furnace of process heat by<br />
dilution, and this heat loss must be overcome<br />
by burning additional fuel. Volume of the melter<br />
should be designed for a maximum fuel heat re-<br />
lease of about 13.000 Btu per hour per cubic foot.<br />
Furnace reversals should be performed by an<br />
automatic control system to ensure optimum<br />
combustion. Only automatic systems can pro-<br />
vide the exact timing required for opening and<br />
closing the dampers and valves and for co-<br />
ordinating fuel and combustion airflow (Bulcraig<br />
and Haigh, 1961). For instance, fuel flow and<br />
ignition must be delayed until combustion air<br />
travels through the checkers after reversal to<br />
mix with fuel at the inlet port to the melter. Fur<br />
nace reversals are usually performed in fixed<br />
periods of 15 to 20 minutes, but an improvement<br />
in regenerator efficiency can be realized by pro-<br />
gramming reversal periods to checker tempera-<br />
tures measured optically. Reversals can then<br />
occur when checker temperatures reach preset<br />
values consistent with maximum heat transfer<br />
(Robertson et al., 1957).<br />
An excellent system for controlling air-to-fuel<br />
ratios incorporates continuous flue gas analyzers<br />
for oxygen and combustible hydrocarbons. With<br />
this system, the most efficient combustion and<br />
best flame shape and coverage occur at optimum<br />
oxygen with a trace of combustible hydrocarbons<br />
present in the flue gas. Sample gas is cleaned<br />
for the analyzers through water-cooled probes<br />
containing sprays. The system automatically<br />
adjusts to compensate for changes in ambient<br />
air density. Fuel savings of 6 to 8 percent<br />
can be accomplished on furnaces with analyzers<br />
over furnaces not so equipped (Gunsaulus, 1958).<br />
Combustion of natural gas in new furnaces occurs<br />
efficiently when the.oxygen content of the flue<br />
gases in the exhaust ports is less than 2 percent<br />
by volume. As the campaign progresses, air<br />
infiltration through cracks and pores in the<br />
brickwork, air leakage through valves and damp-<br />
ers, increased pressure drop through the regen-<br />
erators, and other effects combine to make<br />
combustion less efficient. To maintain maxi-<br />
mum combustion throughout the campaign, pres-<br />
sure checks with draft gages should be run peri-<br />
odically at specified locations (Spain, 1956a).<br />
Fuel savings can also be expedited by placing<br />
furnace operators on an incentive plan to keep<br />
combustion air to a minim-.<br />
Glass Manufacture 774<br />
Electric melting<br />
Although melting glass by electricity is a more<br />
costly process than melting glass by natural<br />
gas or fuel oil, melting electrically is a more<br />
thermally efficient process since heat can be<br />
applied directly to the body of the glass.<br />
Electric induction systems installed on regen-<br />
erative furnaces are designed to increase max-<br />
imum pull rates by as much as 50 percent. These<br />
systems are called boosters and consist of sev-<br />
eral water-cooled graphite or molybdenum elec-<br />
trodes equally spaced along the sides of the melt-<br />
er 18 to 32 inches below the surface of the glass.<br />
Source test results indicate that pull rates can<br />
be increased without any appreciable increase in<br />
dust carryover or particulate emissions. Fur-<br />
nace temperatures may also be reduced by<br />
boosters, preventing refractory damage at peak<br />
operations.<br />
Furnace capacity increase is nearly proportional<br />
to the amount of electrical energy expended. A<br />
56-ton-per-day regenerative furnace requires<br />
480 kilowatt-hours in the booster to melt an addi-<br />
tional ton of glass, which is close to the theoret-<br />
ical amount of heat needed to melt a ton of glass<br />
(Tooley, 1953).<br />
Electric induction can also be used exclusively<br />
for melting glass on a large scale. Design of<br />
this type of furnace is simplified since regen-<br />
erative checkerworks and large ductwork are no<br />
longer required (Tooley, 1953). One recently<br />
collstructed 10-ton-per-day, all-electric furnace<br />
consists of a simple tank with molybdenum elec-<br />
trodes. A small vent leads directly to the at-<br />
mosphere, and dust emissions through this vent<br />
are very small. The furnace operates with a<br />
crown temperature below 600°F and with a<br />
thermal efficiency of over 60 percent. Glass<br />
quality is excellent, with homogeneity nearly<br />
that of optical glass. After the first 11 months<br />
of operation, there was no apparent wear on the<br />
refractories (Peckham, 1962). First costs and<br />
maintenance expenses are substantially lower<br />
than for a comparable-size regenerative furnace.<br />
An electric furnace may prove competitive with<br />
regenerative furnaces in areas with low-cost<br />
electrical power.<br />
Baghouses and centrifugal scrubbers<br />
<strong>Air</strong> pollution control equipment can be installed<br />
on regenerative furnaces where particulate<br />
emissions or opacities cannot be reduced to<br />
required amounts through changes in furnace de-<br />
sign, control of raw materials, and operating<br />
procedures. Regenerative furnaces may be<br />
vented by two types of common industrial con-<br />
trol devices--wet centrifugal scrubbers and<br />
baghouses.
780 CHEMICAL PROCESSJI \iG EQUIPMENT<br />
Figure 596 shows a low-pressure, wet, centrifugal<br />
scrubber containing two separate contacting<br />
sections within a singk casing. Separate<br />
50-horsepower, circulating fans force<br />
dirty gas through each section containing two<br />
to three impingement elements similar to fixed<br />
blades of a turbine. Although the collection<br />
efficiency of this device is considered about the<br />
highest for its type, source tests show an over-<br />
.~ .<br />
. . all efficiency of only 52 percent. This low efficiency<br />
demonstrates the inherent inability of<br />
the low-pressure, wet, centrifugal scrubbers<br />
to collect particulates of submicron size.<br />
Figure 596.~et, centrifugal-type scrubber con-<br />
trolling emissions from a glass-melting furnace<br />
(Thatcher Glass Co., Saugus, Calif. ).<br />
On the other hand, baghouses show collection<br />
efficiencies of over 99 percent. Although<br />
baghouses have not as yet been installed on<br />
large continuous, regenerative furnaces, they<br />
have been installed on small regenerative fur-<br />
naces. One baghouse alternately vents a l, 800-<br />
pound- and a 5,000-pound-batch regenerative<br />
furnace used for melting optical and special<br />
glasses used in scientific instruments. Bags<br />
are made of silicone-treated glass fiber. Off-<br />
gases are tempered by ambient air to reduce the<br />
temperature to 40OSF, a safe operating temper-<br />
ature for this fabric.<br />
Another baghouse, although no longer m operation,<br />
venteda 10-ton-per-day regenerative furnace for<br />
melt~ng soda-lime fllnt glass. Stack gases were<br />
cooled to 250°F by rad~ation and convect~on from<br />
an uninsulated steel duct before entering the baghouse<br />
contaming orlon bags.<br />
To determine the feasibility of using a cloth fil-<br />
tering device on large continuous, regenerative<br />
furnaces, a pilot baghouse was used with bags<br />
made of various commercial fabrics. An air-<br />
to-gas heat exchanger containing 38 tubes, each<br />
1-112 inches in outer diameter by 120 inches in<br />
length, cooled furnace exhaust gases before the<br />
gases entered the pilot baghouse. The baghouse<br />
contained 36 bags, each 6 inches in diameter by<br />
111 inches in length, with a 432-net-square-foot<br />
filter area. A 3-horsepower exhaust fan was<br />
mounted on the discharge duct of the baghouse.<br />
When subjected to exhaust gases from amber<br />
glass manufacture, hags made of cotton, orlon,<br />
dynel, and dacron showed rapid deterioration<br />
and stiffening. Only orlon and dacron bags ap-<br />
peared in satisfactory condition when controlling<br />
dirty gas from flint glass manufacture and when the<br />
dirty gas was held well above its dew point. This<br />
difference in corrosion between amber and flint<br />
glass was found to be caused by the difference in<br />
concentrations of sulfur trioxide (SO3) present in<br />
the flue gas.<br />
To reduce the concentration of SO3 from amber<br />
glass manufacture, iron pyrites were substituted<br />
for elemental sulfur in the hatch, but this change<br />
met with no marked success. Stoichiometric<br />
amounts of ammonia gas were also injected to<br />
remove SO3 as ammonium sulfate. Ammonia in-<br />
jection not only failed to lessen bag deterioration<br />
but also caused the heat exchanger tubes to foul<br />
more rapidly.<br />
In all cases, the baghouse temperature had to be<br />
kept above the dew point of the furnace effluent<br />
to prevent condensation from blinding the bags<br />
and promoting rapid chemical attack. At times,<br />
the baghouse had to be operated with an inlet<br />
temperature as high as 280°F to stay above the<br />
elevated dew point caused by the presence of SO3.<br />
Additional pilot baghouse studies are needed to<br />
evaluate orlon and dacron properly for flint glass<br />
manufacture. Experiments are also required for<br />
evaluating silicone-treated glass fiber bags in con-<br />
trolling exhaust gases from regenerative furnaces<br />
melting all types of glass.<br />
Information now available indicates that glass<br />
fiber bags can perform at temperatures as<br />
high as 500 "F, well above the elevated dew<br />
points. They are virtually unaffected by rela-<br />
tively large concentrations of SOZ and SO3, and<br />
there is less danger from condensation. One<br />
advantage of glass fiber is that less precooling<br />
of exhaust gases is required because of the high-<br />
er allowable operating temperatures. Reverse<br />
air collapse is generally conceded to be the best<br />
method of cleaning glass fiber bags, since this
Glass Manu<br />
material is fragile and easily breaks when regu-<br />
lar shakers are installed.<br />
Furnace effluent can be cooled by several meth-<br />
ods: <strong>Air</strong> dilution, radiation coollng columns,<br />
air-gas heat exchangers, and water spray<br />
chambers. Regardless of the cooling method se-<br />
lected, automatic controls should be installed to<br />
ensure proper temperatures during the complete<br />
firing cycle. Each cooling method has its ad-<br />
vantages and disadvantages. Dilution of offgases<br />
with air is the simplest and most troublefree<br />
way to reduce temperature but requires the larg-<br />
est baghouse. <strong>Air</strong>-to-gas heat exchangers and<br />
radiation and convection ductwork are subject to<br />
rapid fouling from dust in the effluent. Automatic<br />
surface-cleaning devices should be provided, or<br />
access openings installed for frequent manual<br />
cleaning to maintain clean surfaces for adequate<br />
heat transfer. If spray chambers are used, se-<br />
vere problems in condensation and temperature<br />
control are anticipated.<br />
GLASS-FORMING MACHINES<br />
From ancient times, bottles and tableware were<br />
made by handblowing until mechanical production<br />
~. began in the decade preceding the turn of the cen-<br />
!<br />
tury with the discovery of the "press and blow"<br />
I<br />
and the "blow and blow" processes. At first,<br />
- machines were semiautomatic in operation.<br />
Machine feeding was done by hand. Fully automatic<br />
machines made their appearance during<br />
World War I and completely replaced the semiautomatic<br />
machines by 1925. Two types of automatic<br />
feeders were developed and are in use today.<br />
The first type consists of a device for dipping and<br />
evacuating the blank mold in a revolving pot of<br />
glass. The second type, called a gob feeder,<br />
consists of an orifice in the forehearth combined<br />
with shears and gathering chutes (Tooley, 1953).<br />
Glass container-forming machines are of two<br />
general types. The first type is a rotating ma-<br />
chine in which glass is processed through a<br />
sequence of stations involving pressing, blowing,<br />
or both. An example of this type of machine is<br />
a Lynch machine. A second type is used in con-<br />
junction with a gob feeder and consists of inde-<br />
pendent sections in which each section is a com-<br />
plete manufacturing unit. There is no rotation,<br />
and the molds have only to open and close. An<br />
example of this type is the Hartford-Empire<br />
Individual Section (I.S.) six-section machine<br />
shown in Figure 597. Mechanical details and<br />
operations of various glass-forming machines<br />
for manufacturing containers, flat glass, and<br />
tableware are found in the Handbook of Glass<br />
Manufacture (Tooley, 1953).<br />
Figure 597. Hartford-Empire I.S. six-section glass-<br />
forming machine. (Thatcher Glass Co., Sangus, Calif.).<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
Dense smoke is generated by flash vaporization<br />
of hydrocarbon greases and oils from contact<br />
lubrication of hot gob shears and gob delivery<br />
systems. This smoke emission can exceed 40<br />
percent white opacity.<br />
Molds are lubricated with mixtures of greases<br />
and oils and graphite applied to the hot internal<br />
surfaces once during 10- to 20-minute periods.<br />
This smoke is usually 100 percent white in<br />
opacity and exists for 1 or 2 seconds. It rapid-<br />
ly loses its opacity and is completely dissipated<br />
within several seconds.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Methods<br />
During the past decade, grease and oil lubri-<br />
cants for gob shears and gob delivery systems<br />
have been replaced by silicone emulsions and<br />
water-soluble oils at ratios of 90 to 150 parts<br />
of water to 1 part oil or silicone. The effect<br />
has been the virtual elimination of smoke. The<br />
emulsions and solutions are applied by intermittent<br />
sprays to the delivery system and shears only when<br />
the shears are in an opened position.
782 CHEMICAL PROCESSING EQUIPMENT<br />
Lubricating properties of silicone-based emul- when mounted at the point of transfer of gobs<br />
sions appear in some respects superior to those from the blank mold to the blow mold.<br />
of soluble oil solutions. Gob droo soeeds are<br />
a<br />
increased by 20 to 25 percent. Apparently, the<br />
gob rides down the deliverv chute on a cushion<br />
-<br />
of steam. Heat from the gob breaks the silicone<br />
emulsion, forming an extremely stable resin, a<br />
condensation product of siloxane, which acts as<br />
a smooth base for the cushioning effect of steam.<br />
This resin is degraded in a matter of seconds<br />
and must be reformed continuously hy reapply-<br />
ing the silidone emulsion.<br />
While graphite gives no apparent advantages to<br />
emulsions, a combination of water soluble oil and<br />
silicone emulsion appears to be most effective<br />
(Singer, 1956). Oil aids the wetting of metal<br />
surfaces with silicone and coats metal surfaces,<br />
retarding rust formation. Sodium nitrite is also<br />
helpful in inhibiting rust when added to silicone<br />
emulsion. Water for mixtures must be pure, and<br />
in most cases, requires treatment in ion ex-<br />
changers or demineralizers.<br />
Water treatment is most critical for soluble oil to<br />
prevent growth of algae and bacteria. Oil solutions<br />
form gelatinous, icicle-like deposits upon drying<br />
on the surfaces of pipes and arms of the I.S. ma-<br />
chine. These particles should not be allowed to<br />
fall into the mold. Optimum results are obtained<br />
by flood lubrication'of the delivery system to the<br />
maximum amount that can be handled by a runoff<br />
wire or blown off by air. Dry lubrication of<br />
delivery systems has been tried on an experi-<br />
mental basis by coating the metal contact sur-<br />
faces with molybdenum disulfide or graphite.<br />
Although future developments in the application<br />
of emulsions to molds look promising, present<br />
practice still relies upon mixtures of hydro-<br />
carbon greases, oils, and graphite. Silicone<br />
emulsions and soluble oils eliminate smoke,<br />
but several difficulties must be overcome be-<br />
fore they can be widely used for mold lubrica-<br />
tion. Water emulsions with their high specific<br />
heat cause excessive cooling if they are not ap-<br />
plied evenly to the mold surfaces by proper<br />
atomization. Fine sprays meet with wind re-<br />
sistance, and these sprays cannot be effectively<br />
directed to cover the shoulder sectipns of some<br />
molds. Because of the low viscosity of water<br />
emulsions, the emulsions are very difficult to<br />
meter through existing sight oil feeders. One<br />
company has equipped its machine with individual<br />
positive-displacement pumps for each nozzle.<br />
Invert-post cross-spraying is found to be most<br />
effective in giving a uniform coating to the molds<br />
of I. S. machines (Bailey, 1957).<br />
INTRODUCTION<br />
FRlT SMELTERS<br />
Ceramic coatings are generally divided into two<br />
classes, depending upon whether they are applied<br />
to metal or to glass and pottery. In the case of<br />
metal, the coating is widely referred to in this<br />
country as;porcelain enamel. The use of the<br />
term vitreous enamel seems to be preferred in<br />
Europe. Glass enamel is sometimes used inter-<br />
changeably with both terms. On the other hand,<br />
the coating applied to glass or pottery is known<br />
as ceramic glaze.<br />
Ceramic coatings are essentially water suspen-<br />
sions of ground frit and clay. Frit is prepared<br />
by fusing various minerals in a smelter. The<br />
molten material is then quenched with air or<br />
water. This quenching operation causes the<br />
melt to solidify rapidly and shatter into numerous<br />
small glass particles, called frit. After a drying<br />
process, the frit is finely ground in a ball mill,<br />
where other materials are added. When suspend-<br />
ed in a solution of water and clay, the resulting<br />
mixture is known as a ceramic slip. Enamel<br />
slip is applied to metals and fired at high tem-<br />
peratures in a furnace. Glaze slip is applied to<br />
pottery or glass and fired in a kiln.<br />
Raw Materials<br />
The raw materials that go into the manufacture<br />
of various frits are similar to each other whether , j<br />
the frit is for enameling on steel or aluminum or<br />
1.<br />
for glazing. The basic difference is in the chem- i.<br />
I<br />
ical composition.<br />
The raw materials used in enamels and glazes<br />
may he divided into the following six groups:<br />
i<br />
Refractories, fluxes, opacifiers, colors, floatl<br />
ing agents, and electrolytes (Andrews, 1961). ~<br />
The refractories include materials such as I !<br />
quartz, feldspar, and clay, which contribute to<br />
the acidic part of the melt and give body to the<br />
glass. The fluxes include minerals such as<br />
borax, soda ash, cryolite, fluorspar, and litharge,<br />
which are basic in character and react with the<br />
acidic refractories to form the glass and, more-<br />
over, tend to lower the fusion temperatures of<br />
the glasses. These refractory and flux materials<br />
chiefly comprise the ingredients that go into the<br />
raw batch that is charged to the smelter.<br />
Rotating machines are much easier to lubricate Materials falling into the other four groups are<br />
than are individual section machines. Emulsion introduced later as mill additions and rarely exsprays<br />
are most effective on rotating machines ceed 15 percent of the total frit composition.<br />
I<br />
i<br />
j
They include opacifiers, which are compounds<br />
added to the glass to give it an opaque appear-<br />
ance such as the characteristic white of porce-<br />
lain enamels. Examples are tin oxide, anti-<br />
mony oxide, sodium antimonate, and zirconium<br />
oxide. The color materials include compounds<br />
such as the oxides of cobalt, copper, iron, and<br />
nickel. The floating agents consist of clay and<br />
gums and are used to suspend the enamel or<br />
glaze in water. Electrolytes such as borax,<br />
soda ash, magnesium sulfate, and magnesium<br />
carbonate are added to flocculate the clay and<br />
further aid the clay in keeping the enamel or<br />
glaze in suspension (Parmelee, 1951).<br />
Types of Smelters<br />
Frit Smt<br />
Smelters used in frit making, whether for<br />
enamel or glaze, may be grouped into three<br />
classes: Rotary, hearth, and crucible. The<br />
rotary smelter is cylindrical and can be rotated<br />
in either direction to facilitate fusing, as shown<br />
in Figure 598. It can also be tilted vertically<br />
for the pouring operation, as demonstrated in<br />
Figure 599. The smelter is open at one end for<br />
the introduction of fuel and combustion air. It<br />
is similarly open at the opposite end for the dis-<br />
charge of flue gases and for charging raw mate-<br />
rials. Operated solely as a batch-type smelter,<br />
it is normally charged by means of a screw con-<br />
veyor, which is inserted through the opening.<br />
Rotary smelters are normally sized to take<br />
batches varying from approximately 100 to 3,000<br />
pounds. Fired with either gas or oil, the smelter<br />
is lined with high-alumina, refractory firebrick<br />
with an average life of from 400 to 600 melts.<br />
Firing cycles vary from 1 to 4 hours.<br />
The hearth smelter consists of a brick floor, on<br />
which the raw materials are melted, surrounded<br />
by a boxlike enclosure. This type of smelter<br />
can be either continuous, as illustrated in Figure<br />
600, or batch type, as shown in Figure 601. In<br />
either case, the hearth (or bottom) is sloped<br />
from one side to a point on the opposite side<br />
where the molten material is tapped. The con-<br />
tinuous type is usually screw fed. A flue stack<br />
is located on the opposite end. Oil or gas is<br />
normally used as fuel for the one or more burn-<br />
ers. The walls and floor are lined with a first-<br />
quality, refractory firebrick. The batch type is<br />
sized to take batches ranging from 100 to several<br />
thousand pounds. About 30 pounds of batch can<br />
be smelted for each square foot of hearth area.<br />
The typical continuous-hearth smelter can pro-<br />
cess 1, 000 to 1, 500 pounds of raw materials<br />
per hour.<br />
The crucible smelter consists of a high-refrac-<br />
tory, fireclay, removable crucible mounted<br />
within a circular, insulated, steel shell lined<br />
with high-grade firebrick, as shown in Figure<br />
602. Heating is usually accomplished with oil<br />
or gas burners, though electricity can be used.<br />
The combustion chamber surrounds the crucible,<br />
occupying the space between the crucible and the<br />
shell lining. Because the heat must be trans-<br />
mitted through the crucible to the batch, re-<br />
fractory and fuel costs are high. Crucibles can<br />
be sized to smelt a 5-pound batch for laboratory<br />
purposes, but the commercial crucibles are<br />
sized to take batches from 100 pounds to 3, 000<br />
pounds. Smelting cycles vary from 2 to 3 hours<br />
at temperatures around 2,200mF, depending<br />
upm the size of the batch and its composition.<br />
The steel shell is supported by trunnions so<br />
that the crucible can be tilted for the pouring<br />
operation.<br />
Frit Manufacturing<br />
Since the raw materials that comprise the<br />
smelter batch consist of refractories and<br />
fluxes, thorough and uniform mixing of these<br />
ingredients before the charging operation is<br />
essential for efficient smelting. Smelting<br />
involves the heating of raw materials until a<br />
fairly homogeneous glass is formed. The<br />
fundamental changes that occur are inter-<br />
action of acids and bases, decomposition,<br />
fusion, and solution. A considerable quanti-<br />
ty of steam is evolved as the borax begins to<br />
melt. The order of melting for some of the<br />
materials is: Sodium nitrate at 586"F, borax<br />
at 1, 366"F, soda ash at 1,564"F, litharge at<br />
1, 630°F, feldspar at 2,138"F, and quartz at<br />
3,llO"F.<br />
If white or light-colored frits are being smelted,<br />
smelter refractory linings must be high in alumi-<br />
na and low in iron to prevent discoloration and<br />
dark specks in the frit. The batch is protected.<br />
from contact with fuel gases during the-early<br />
stages of smelting by the evolution of gases<br />
within the smelter. To illustrate, a batch<br />
containing 35 percent borax and 10 percent<br />
soda ash loses about 165 pounds of water and<br />
42 pounds of carbon dioxide for a 1,000-pound<br />
batch of frit. This is equivalent to 483 cfm<br />
water vapor and 60 cfm C02 at a smelter tem-<br />
perature of 1,700°F and for a smelting period<br />
of 30 minutes.<br />
The rate and period of heat application is criti-<br />
cal in smelting enamels and glazes. A temper-<br />
ature too low may be sufficient only to vaporize<br />
the more fusible materials instead of volatilizing<br />
them, and thereby result in a very slow reaction<br />
with the more refractory ingredients. A higher<br />
operating temperature eliminates this low pro-<br />
duction rate. If the batch is heated too rapidly,<br />
however, the more fusible elements are melted
784 CHEMICAL PROCESSING EQUIPMENT<br />
Figure 598. Rotary-type frit smelter (Ferro Corp.,<br />
Los Angeles, Calif.).<br />
Figure 599. Rotary-type frit smelter in pouringposition (Ferro Corp.,<br />
Los Angeles, Calif.).
Frit Smelters 785<br />
Figure 600. Continuous-hearth-type frit smelter '(~erro Corporation,<br />
Las Angeles, Calif.).<br />
Flgure 601. Batch-hearth-type frlt smelter (Ferro Corporatlon.<br />
Los Angeles, Cal~f.).
786 CHEMICAL PROCESS11 VG EQUIPMENT<br />
Flgure 602. Crucible-type f r ~ smelter t<br />
(Californla<br />
Metal Enamel lng Company, Los Angeles, Cal lf.).<br />
and volatilized hefore they have a chance to react<br />
with the more refractory materials. Driving off<br />
the fluxes in this manner results in a harder (less<br />
fusible) final batch. Excessive smelting, after the<br />
melt is ready to pour, results in a similar condi-<br />
tion and, if permitted to continue, necessitates a<br />
further increase in temperature to facilitate<br />
pouring. Oversmelting also causes loss of opac-<br />
ity, poor gloss, and discoloration in the frit.<br />
Insufficient smelting, on the other hand, causes<br />
blistering, loss of acid resistance, and poor<br />
texture in the finished coating. Batch composi-<br />
tion is the determining factor in selecting op-<br />
timum smelter temperatures and cycles.<br />
After the smelting operation, the molten material<br />
is quenched. Rapid cooling can be accomplished<br />
with either air, water, or a combination of the<br />
two. <strong>Air</strong> quenching produces a better product<br />
but is not normally practiced, owing to the quench-<br />
ing Znd storage space required. Water quench-<br />
ing is commonly practiced in the industry by<br />
pouring the molten material from the tilted<br />
smelter into a large pan of water. Water quench-<br />
ing is also frequently done by pouring molten mate-<br />
rial into a metal trough in which a continuous<br />
stream of water is flowing. The trough empties<br />
into a large wire basket suspended in a well,<br />
which holds the shattered frit but permits the<br />
water to flow out through an overflow. Rapid<br />
cooling is somewhat impeded by this method<br />
owing to a layer of steam that forms over the<br />
glass. <strong>Air</strong>-water quenching appears to be the<br />
most economical and effective method since a<br />
more thorough shattering of the glass results.<br />
In this method the molten material is poured<br />
from the smelter and passed through a blast of<br />
air and water. Quenching causes the molten ma-<br />
terial to solidify and shatter into numerous small<br />
glass particles (called frit) ranging from 114 inch<br />
in diameter down to submicron sizes. Its main<br />
purpose is to facilitate grinding.<br />
After draining, the frit contains 5 to 15 percent<br />
water and may be milled in this condition or<br />
may first he dried. Three types of dryers are<br />
employed: The drying table, the stationary<br />
dryer, and the rotary dryer. The drying table<br />
is a flat hearth on which the frit is placed. Heat<br />
is applied beneath the hearth, and the frit is<br />
raked manually. The stationary dryer consists<br />
of a sheet iron chamber in which a basket of<br />
frit is placed. Heated air from an exchanger on<br />
the smelter flue is passed through the basket of<br />
frit. The rotary dryer consists of a porcelain-<br />
lined rotating cylinder that is inclined slightly,<br />
causing the frit to move through continuously.<br />
The typical size is approximately 2 feet in di-<br />
ameter and 20 feet long, though larger cylinders<br />
are used. The rotary dryer, which is economical<br />
and efficient, can he heated by waste heat or by<br />
oil or gas. The frit can he further refined by<br />
using magnetic separation to remove small iron<br />
particles, which would otherwise cause black<br />
specks in the enamel.<br />
The final step in frit making is size reduction,<br />
which is normally done with a ball mill. Frits<br />
used in porcelain enamel are required to pass<br />
a No. 100 seive (150 microns), though a certain<br />
percent of fines must remain as residue on a<br />
finer sieve. In the case of ceramic glaze frits, a<br />
finer grind is necessary. About one-half of a<br />
batch must he less than 2. 5 microns with the<br />
remainder no greater than 10 microns. Effi-<br />
cient milling is best obtained when the speed of<br />
rotation is such that the balls ride three-fourths<br />
of the way up one side of the cylinder, and the inner-<br />
most balls slide back down over the outermost<br />
balls. This is achieved, for example, at a speed<br />
of 25 rpm for a 4-foot-diameter cylinder. Porce-<br />
lain balls or flint pebbles are used in the mill. The<br />
diameter of the balls ranges from 1 to 3 inches,<br />
and the charge should be maintained at about 55<br />
percent of the mill volume. Ball wear amounts to<br />
5 to 10 pounds in milling 1,000 pounds of frit.<br />
Colors, opacifiers, floating agents, and electro-<br />
lytes are mixed with the frit before it is charged<br />
to the ball mill. After the milling operation be-<br />
gins, water is added at a constant rate to keep<br />
the specific gravity of the slurry (referred to as<br />
slip) at the correct value at all times. After the<br />
milling operation, the ceramic slip is screened<br />
to remove large particles. A 1- to 2-day aging<br />
process then takes place at a temperature close
to that at which the enamel or glaze is to be ah-<br />
plied. Aging is necessary to set up an equilibrium<br />
among the clay, frit, and solution. The enamel<br />
or glazs slip is now ready for application.<br />
Application, Firing, and Uses of Enamels<br />
Enamels and glazes may be applied to ware<br />
blanks by immersion or spraying (Hansen,<br />
1932). The pouring and brushing methods are<br />
seldom employed today. In the dipping opera-<br />
tion, the blank is immersed in the slip and then<br />
withdrawn and allowed to drain. If the slip is<br />
thick, the excess enamel must he shaken from<br />
the ware, a process called slushing. Spraying<br />
is the application of enamel or glaze slip to<br />
ware by atomizing it through an air gun.<br />
After the enamel or glaze has been applied, it<br />
must then be burned or fired on the ware to<br />
fuse the coating to a smooth, continuous, glassy<br />
layer. The firing temperatures and cycles for<br />
porcelain enamel on steel and aluminum are<br />
approximately 1, 500°F (Shreve, 1945) for 5<br />
minutes and 1,000"F for 5 minutes, respective-<br />
ly. Ceramic glaze, however, is fired on pottery<br />
at about 2, 300°F for several hours or even days.<br />
The firing is accomplished in what is called a<br />
furnace in the porcelain enamel industry, and a<br />
kiln in the ceramic glaze industry.<br />
Porcelain enamel is used as a protective coating<br />
for metals--primarily steel, cast iron, and<br />
aluminum. Familiar items are bathtubs, water<br />
heater tanks, refrigerators, washing machines,<br />
and cooking ranges. Coated aluminum is being .<br />
used more and more in recent times for signs<br />
such as those installed on highways. Ceramic<br />
elazes are used as a decorative or orotective<br />
"<br />
coating on a wide variety of pottery and glass<br />
articles. Examples are lavatory basins, water<br />
closets, closet bowls, chinaware, and figurines.<br />
THE AIR POLLUTION PROBLEM<br />
Significant dust and fume emissions are created<br />
by the frit-smelting operation. These emissions<br />
consist primarily of condensed metallic oxide<br />
fumes that have volatilized from the molten charge.<br />
They also contain mineral dust carryover and<br />
sometimes contain noxious gases such as hydro-<br />
gen fluoride. In addition, products of combus-<br />
tion and glass fibers are released. The quanti-<br />
ty of these air contaminants can be reduced by<br />
following good smelter-operating procedures.<br />
This can be accomplished by not rotating the<br />
smelter too rapidly, to prevent excessive dust<br />
carryover, and by not heating the batch too rapid-<br />
ly or too long, to prevent volatilizing the more<br />
fusible elements before they react with the more<br />
refractory materials. A typical rotary smelter,<br />
Frit Smelters 7 87<br />
for example, discharges 10 to 15 pounds of dust<br />
and fumes to the atmosphere per hour per ton of<br />
material charged. In some cases, where ingredi-<br />
ents require,high melting temperatures (1, 500°F<br />
or higher), emissions as great as 50 pounds per<br />
hour per ton of material have been observed,<br />
Depending upon the composition of the batch, a<br />
significant visible plume may or may not be<br />
present. Tables 209 to 212 indicate the extent of<br />
emissions from uncontrolled, rotary frit smelters<br />
for various-sized batches and compositions.<br />
Table 209. DUST AND FUME DISCHARGE FROM<br />
A 1,000-POUND, ROTARY FRIT SMELTER<br />
process wt, lh/hr<br />
Stack vol, scfm 1,390 1,540 1,630<br />
Stack gas temp, .F<br />
Concentration, grlacf 0.118 0.387 0.381<br />
Stack emissions. Ihlhr 1.41 5.11 5. 32<br />
CO, vol % (stack condition) 0.002 0.001 0.002<br />
NZ. "01 % (stack condition) 76.9<br />
Process wf, lblhr<br />
Stack vol, scfm 1,310 1,400 1,480<br />
Stack gas temp, OF<br />
Concentration. . erlscf 0.111 0. 141 0. I24<br />
Stack emissions, iblhr 1. 25 1.79 1.57<br />
to. "01 % (stack condition) 0<br />
N. 1 % k d o 1 7 1 0 1 73. 30<br />
I I I<br />
aThese three tests represent approximately the 1st. 2d, and 3d<br />
hour. of a 248-minute smelting cycle. The total charge amounted<br />
to 717 pounds of material con$isting of borax. feldspar, sodium<br />
fluoride, soda ash, and zinc oxide.<br />
b~hese three fesis represent approximately the 1st. Zd, and 3d<br />
hours of a 195-minute smelting cycle. The total charge amounted<br />
to 949 pounds of material consi3ting of litharge, silica, boric<br />
acid, feldspar, fluorspar. borax, and aircon.<br />
Table 210. DUST AND FUME DISCHARGE FROM<br />
A 3,000-POUND, ROTARY FRIT SMELTER<br />
c<br />
Test data<br />
Process wt. Iblhr<br />
47Za<br />
Stack vol, scfm<br />
Stack - nas , temo. .. 'F<br />
. -<br />
2.240<br />
630<br />
Stack emissions, iblhr<br />
CO. "01 70 (stack condition)<br />
N ~ "01 , 70 (stack condition)<br />
2.70<br />
0.02<br />
75. 30<br />
7 1 8 I 9<br />
I I I<br />
472'<br />
2,270<br />
800<br />
Concentration. erlscf 1 0. 143 1 0. 114 1 0.172<br />
2.20<br />
0.02<br />
75.60<br />
=These three tests repreoent approximately the 1st. 2d, and 3d<br />
hours of a 248-minute smelting cycle. The total charge<br />
amounted to 1,951 pounds of material consisting of litharge,<br />
silicb, boric acid, feldspar, whiting. borax, and zircon.<br />
HOODING AND VENTILATION REQUIREMENTS<br />
472'<br />
2,260<br />
Rotary smelters require a detached canopy-type<br />
hood suspended from the lower end of a vertical<br />
stack as shown in Figures 598 and 599. It is<br />
suspended far enough above the floor to trap the<br />
discharge gases from the smelter when in the<br />
horizontal position. Refractory-lined, it is of<br />
sufficient size to prevent gases from escaping<br />
840<br />
3.30<br />
0.02<br />
76.30
788 CHEMICAL PROCESSING EQUIPMENT<br />
Table 211. FLUORIDE DISCHARGE FROM owing to the occasional presence of fluorides in<br />
A ROTARY FRIT SMELTER the effluent. The discharge gases must be cooled<br />
by heat exchangers, quench chambers, cooling<br />
Test data<br />
Process wt. lblhr<br />
stark "01, scrm<br />
Stack gas temp, .B<br />
Concentration, grlscl<br />
Stack emiasionr. . lbihr .<br />
174=<br />
1.400<br />
130<br />
0. 061<br />
0. 73<br />
~ert NO.<br />
'There two tests were of 90 minutesm dvration each and represented<br />
approximately the iirrt half and the second haU of a 248-minute<br />
smelting cycle. The total charge amounted fa 717 pounds of mntcrial<br />
~"nrirfing oi borax, feldspar, SOdivm fluoride, soda ash, and zinc<br />
oxide.<br />
b~hela two 60-minute t~ais reprerented approximately the 1st and the<br />
4th hours of s 450-mhufe am~iting cycle. Thm total charge amounted<br />
to i.211 pounds of malcrial consistin8 ol sodium carbonatm, calcium<br />
carbonate, pyrobar, and silica. The test was specifically conducted<br />
lorn batch containing maximum carbonaten (19%) and no liiharge.<br />
Table 212. DUST AND FUME DISCHARGE FROM<br />
A 2,000-POUND ROTARY FRIT SMELTER<br />
Test data<br />
14'<br />
174=<br />
1,600<br />
840<br />
0. 035<br />
0. 48<br />
162~<br />
1.000<br />
480<br />
0. 196<br />
1.68<br />
Process wt, lblhr<br />
857' 890~ 890b<br />
Stack vol. scim<br />
3<br />
2,430 4,347<br />
4,347<br />
Stack gas temp, 'P 600 600 340<br />
340<br />
Canrcnlration. crizcf 0.130 0.112 0.111 0. 103<br />
Stack emissions, lblhr 2.710 2.340 4. 110 3. 320<br />
"T~EJF two b0-minute testa rrprcaent the 1st and LC! hours of a 140minvtr<br />
rmclfing cycle. The total char~e amountad to 2.000 poimr,.<br />
oi rnatrria, containini: lilica, lifharge. an
Food Processing Equipment 789<br />
Figure 603. Venturi water scrubber venting<br />
three frit'smelters (Ferro Corporation, Los<br />
Angeles, Calif.).<br />
Table 213. EFFICIENCY OF VENTURI WATER SCRUBBER ON PARTICULATE<br />
MATTER AND FLUORIDES WHEN VENTING THREE FRIT SMELTERS<br />
Test data<br />
Process wt, lb/hr<br />
Stack "01, scim<br />
Stack gas temp, "F<br />
Dust concentration, gr/scf<br />
' Inlet<br />
Outlet<br />
Dust emissions, lb/hr<br />
Inlet<br />
Outlet<br />
Control efficiency, 7'0<br />
18<br />
1,360<br />
4,280<br />
570<br />
0.228<br />
0. 074<br />
8. 37<br />
2.72<br />
67.50<br />
19<br />
Dust and fumes<br />
Test No. a<br />
Fluorides<br />
1, 360 1, 360 1,360 1,360<br />
4.280 1 4.280 4.280 4.280<br />
552 564 570 552<br />
0.234<br />
0.077<br />
8.60<br />
2.85<br />
67.20<br />
a~ests No. 18 and 21 represent the first 54 minutes of the 107-minute smelting cycle, tests No. 19<br />
and 22 represent the last 54 minutes, and tests No. 20 and 23 represent the 23-minute tapping<br />
period. Total process weight was 3, 000 pounds of material consisting of borax, potassium carbonate,<br />
potassium nitrate, zinc oxide, titanium oxide, ammonium phosphate, lithium carbonate,<br />
sodium silico-fluoride, fluorspar, silica, and talc. Pressure drop across throat was 21 in. WC.<br />
Water flow rate to throat was 50 gpm.<br />
2 0<br />
0.127<br />
0.088<br />
1. 78<br />
1. 35<br />
30.70<br />
21<br />
0.092<br />
0.006<br />
3. 38<br />
0.22<br />
93.20<br />
2 2<br />
0.137<br />
0.008<br />
5. 03<br />
0.29<br />
94<br />
2 3<br />
1,360<br />
4.280 ,<br />
564<br />
0.034<br />
0.017<br />
0. 48<br />
0. 26<br />
5 0
. .<br />
790 CHEMICAL PROCESSING EQUIPMENT<br />
various preserving processes. More recently<br />
we find food purveyors increasingly concerned<br />
with processes that render foods more flavorful<br />
and easier to prepare. The trend toward greater<br />
presale food preparation has possibly caused<br />
a shift of at least some alr pollutants from many<br />
domest~c kitchens to a significantly smaller number<br />
of food-processing plants.<br />
Food processing includes operations such as<br />
. . .. . slaughtering, smoking, drying, cooking, baking,<br />
frying, boiling, dehydrating, hydrogenating,<br />
fermenting, distilling, curing, ripening, roasting,<br />
broiling, barbecuing, canning, freezing,<br />
enriching, and packaging. Some produce large<br />
volumes of air contaminants; others, only insignificant<br />
amounts. Equipment used to process<br />
food is legion. Some of the unit operations in-<br />
. .<br />
. . volved are the following (Kirk and Othrner, 1947):<br />
Material handling. Conveying, elevating, pump-<br />
ing, packing, and shipping.<br />
Figure 604. Baghouse with radiant cooling<br />
columns venting four rotary frit smelters<br />
(Glostex Chemicals, Inc., Vernon: Calif.).<br />
Separating. Centrifuging, draining, evacuating,<br />
filtering, percolating, fitting, pressing, skimming,<br />
sorting, and trimming. (Drying, screening, sifting,<br />
and washing fall into this category. )<br />
Heat exchanzing. Chilling, freezing, and refrig-<br />
erating; heating, cooking, broiling, roasting, bak-<br />
ing, and so forth.<br />
Mixing. Agitating, beating, blending, diffusing,<br />
dispersing, emulsifying, homogenizing, kneading,<br />
stirring, whipping, working, and so forth.<br />
Disintegrating. Breaking, chipping, chopping,<br />
crushing, cutting, grinding, milling, maturating,<br />
pulverizing, refining (as by punching, rolling,<br />
and so forth), shredding, slicing, and spraying.<br />
Forming. Casting, extruding, flaking, molding,<br />
pelletizing, rolling, shaping, stamping, and die<br />
casting.<br />
Coating. Dipping, enrobing, glazing, icing, pan-<br />
ning, and so forth.<br />
Decorating. Embossing, imprinting, sugaring,<br />
topping, and so forth.<br />
Controlling. Controlling air humidity, temperature,<br />
pressure, and velocity; inspecting, measuring, tem-<br />
pering, weighing, and so forth.<br />
Packaging. Capping, closing, filling, labeling,<br />
packing, wrapping, and so forth.<br />
Storing.<br />
forth.<br />
Piling, stacking, warehousing, and so<br />
A description and discussion of each type of equip-<br />
ment used for food processing is not within the
scope of this manual. The following discussion<br />
will be limited to food processes in which air pollu-<br />
tion problems are inherent and in which typical<br />
food-processing air contaminants are encountered.<br />
This section is not concerned with the production<br />
of pet foods or livestock feeds, though in some<br />
instances, these materials are byproducts of food<br />
processes.<br />
COFFEE PROCESSING<br />
Most coffee is grown in Central and South America.<br />
After harvesting and drying at or near the coffee<br />
plantation, most "green" coffee beans are exported<br />
and further processed before sale to the consumer.<br />
Coffee processing in the United States consists es-<br />
sentially of cleaning, roasting, grinding, and packag<br />
ing.<br />
Roasting is the key operation and produces most<br />
of the air contaminants associated with the indus-<br />
try. Roasting reduces the sugar and moisture<br />
contents of green coffee and also renders the bulk<br />
density of the beans about 50 percent lighter. An<br />
apparently desired result is the production of<br />
water-soluble degradation products that impart<br />
most of the flavor to the brewed coffee. Roasting<br />
also causes the beans to expand and split into<br />
halves, releasing small quantities of chaff.<br />
Botch Roosting<br />
The oldest and simplest coffee roasters are directfired<br />
(usually by natural gas), rotary, cylindrical<br />
chambers. These units are designed to handle<br />
from 200 to 500 pounds of green beans per 15- to<br />
20-minute cycle and are normally operated at<br />
about 400DF. A calculated quantity of water is<br />
added at the completion of the roast to quench the<br />
beans before discharge from the roaster. After<br />
they are dumped, the beans are further cooled<br />
with air and run through a "stoner" air classifier<br />
to remove metal and other heavy objects before<br />
the grinding and packaging. The roaster and<br />
cooler and all air-cleaning devices are normally<br />
equipped with cyclone separators to remove dust<br />
and chaff from exhaust gases. Most present-day<br />
Food Processing Equipment 791<br />
Flgure 605. A recirculating-batch coffee<br />
roaster (Jabez Burns - Gump Division,Blaw<br />
Knox Company, New York, N.Y.).<br />
An Integrated Coffee Plant<br />
A process flow diagram of a typical large integra-<br />
ted coffee plant is shown in Figure 606. Green<br />
beans are first run through mechanical cleaning<br />
equipment to remove any remaining hulls and<br />
foreign matter before the roasting. This system<br />
includes a dump tank, scalper, weigh hopper,<br />
mixer, and several bins, elevators, and convey-<br />
ors. Cleaning systems such as this commonly<br />
include one or more centrifugal separators from<br />
which process air is exhausted.<br />
The direct, gas-fired roasters depicted in Figures<br />
606 and 607 are of continuous rather than<br />
batch design. Temperatures of 4000F to 5000F<br />
are maintained in the roaster, and the residence<br />
time ~- --- is -~ adinrted bv controlline ~ - the drum soeed.<br />
~~ ~ ~~2 --- ~ - ~~ 3<br />
~-<br />
Roaster exhaust products are drawn off through<br />
a cyclone separator and afterburner, with some<br />
recirculation from the cyclone to the roaster.<br />
coffee roasters are of batch design, though the<br />
Chaff and other particulates from the cyclone<br />
newer and larger installations tend to favor con- are fed to a chaff collection system. Hot beans<br />
tinuous roasters. are continuously conveyed through the air cooler<br />
and stoner sections. Both the cooler and the<br />
In the batch roaster shown in Figure 605, some stoner are equipped with cyclones to collect parof<br />
the gases are recirculated. A portion of the ticulates.<br />
gases is bled off at a point between the burner<br />
and the roaster. Thus, the burner incinerates The equipment following the stoner is used only<br />
combustible contaminants and becomes both an to blend, grind, and package roasted coffee.<br />
air pollution control device and a heat source for Normally, there are no points in these systems<br />
the roaster.<br />
where process air is emitted to the atmosphere.<br />
1
792 CHEMICAL PROCESSING EQUIPMENT<br />
Fiaure 607. A continuous coffee roaster and cooler:(left) continuous<br />
roister. showine course of the heated eases as thev are drawn throueh<br />
the coffee beans in the perforated, heiical-flanged cylinder and then<br />
into the recirculation svstem; (right) left-side elevation of contin-<br />
uous roaster, showing relationship-of reci rculating and cooler fans<br />
and the respective collectors on the roof (Jabez Burns - Gump Division,<br />
Blaw-Knox Company, New York, N.Y.).
At the plant shown on the flow diagram, chaff is<br />
collected from several points and run to a hold-<br />
ing bin from which it is fed at a uniform rate to<br />
an incinerator. Conveyors in the chaff system<br />
may be of almost any type, though pneumatic con-<br />
veyors are most common. The design of the in-<br />
cinerator depicted is similar to that of the saw-<br />
dust burners described in Chapter 8 but the<br />
incinerator is much smaller.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
Dust, chaff, coffke bean oils (as mists), smoke,<br />
and odors are the principal air contaminants<br />
emitted from coffee processing. Ln addition,<br />
combustion contaminants are discharged if chaff<br />
is incinerated. Dust is exhausted from several<br />
points in the process, while smoke and odors are<br />
confined to the roaster, chaff incinerator, and, in<br />
some cases, to the cooler.<br />
Coffee chaff is the main source of particulates,<br />
but green beans, as received, also contain ap-<br />
preciable quantities of sand and miscellaneous<br />
dirt. The major portion of this dirt is removed<br />
by air washing in the green coffee-cleaning sys-<br />
tem. Some chaff (about 1 percent of the green<br />
weight) is released from the bean on roasting and<br />
is removed with roaster exhaust gases. A small<br />
amount of chaff carries through to the cooler and<br />
stoner. After the roasting, coffee chaff is light<br />
and flaky, partlcle sizes usually exceeding 100<br />
microns. As shown in Table 214, particulate-<br />
matter emissions from coffee processing are well<br />
below the limits permitted by typical dust and<br />
fume prohibitions.<br />
Table 214. ANALYSIS OF COFFEE<br />
ROASTER EXHAUST GASES<br />
I contaminant concentration<br />
Collt~nnous roaster Batch roaster<br />
-- 1 I75<br />
Particulate matter, grlscf 0. 1891 0.006 I 0.160<br />
/ 1<br />
Aldehydes<br />
(as formaldehyde), ppm 139 .- 42<br />
Organic acids<br />
(as acetic acid), ppm . . 213<br />
Oxides of nitrogen<br />
(as NOZ). P P ~<br />
26. 8 -- 21.4<br />
Coffee roaster odors are attributed to alcohols,<br />
aldehydes, organic acids, and nitrogen and sulfur<br />
compounds, which are all probably breakdown<br />
products of sugars and oils. Roasted coffee odors<br />
are considered pleasant by many people, and in-<br />
deed, they may often be pleasant under certain<br />
conditions. Nevertheless, continual exposure to<br />
uncontrolled roaster exhaust gases usually elicits<br />
widespread complaints from adjacent residents.<br />
The pleasant aroma of a short sniff apparently<br />
develops into an annoyance upon long exposure.<br />
Food Proces~ ;ing Equipment 793<br />
Visible bluish-white smoke emissions from coffee<br />
roasters are caused by distilled oils and organic<br />
breakdown products. The moisture content of<br />
green coffee is only 6 to 14 percent, and thus<br />
there is not sufficient water vapor in the 400"<br />
to 500°F exhaust gases to form a visible steam<br />
plume. From uncontrolled, continuous roasters,<br />
the opacity of exhaust gases exceeds 40 percent<br />
almost continuously. From batch roasters, ex-<br />
haust opacities normally exceed 40 percent only<br />
during the last 10 to 15 minutes of a 20-minute<br />
roast. Smoke opacity appears to be a function<br />
of the oil content, the more oily coffee producing<br />
the heavier smoke. The water quenching of<br />
batch-roasted coffee causes visible steam emis-<br />
sions that seldom persist longer than 30 seconds<br />
per batch.<br />
Hooding ond Ventilotion Requirements<br />
Exhaust volumes from coffee-processing sys -<br />
tems do not vary greatly from one plant to<br />
another insofar as roasting, cooling, and stoning<br />
are concerned. Roasters equipped with gas re-<br />
circulation systems exhaust about 24 scf per<br />
pound of finished coffee. Volumes from nonre-<br />
circulation roasters average ahout 40 scf per<br />
pound. A 10,000-pound-per-hour, continuous<br />
roaster with a recirculation system exhausts<br />
about 4, 000 scfm. A 500-pound-per-batch, non-<br />
recirculation roaster exhausts about 1, 000 scfm.<br />
Each hatch cycle lasts about 20 minutes.<br />
Coolers of the continuous type exhaust about<br />
120 scf per pound of coffee. Batch-type cool-<br />
ers are operated at ratios of about 10 scfm per<br />
pound. The time required for batch cooling<br />
varies somewhat with the operator. Batch-cool-<br />
ing requirements are inversely related to the<br />
degree of water quenching employed.<br />
Continuous-type stoners use about 40 scf air per<br />
pound of coffee. Batch-stoning processes require<br />
from 4 to 10 scfm per pound, depending upon duct-<br />
work size and batch time.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
<strong>Air</strong> contaminants from coffee-processing plants<br />
have been successfully controlled with afterburn-<br />
ers and cyclone separators, and combinations<br />
thereof. Incineration is necessary only with roaster<br />
exhaust gases. There is little smoke in other coffee<br />
plant exit gas streams where only dust collectors<br />
are required to comply with air pollution control<br />
regulations.<br />
Separate afterburners are preferable to the com-<br />
bination heater-incinerator of the batch roaster<br />
shown in Figure 605. When the afterburner serves<br />
as the roaster's heat source, its maximum operat-
794 CHEMICAL PROCESSING EQUIPMENT<br />
ing temperature is limited to about 1,00O0F. A<br />
temperature of 1,200°F or greater is necessary<br />
to provide good particulate incineration and odor<br />
removal.<br />
A roaster afterburner should always be preceded<br />
by an efficient cyclone separator in which most<br />
of the particulates are removed. A residence<br />
time of 0. 3 second is sufficient to incinerate<br />
most vapors and small-diameter particles at<br />
1,200°F. Higher temperatures and longer resi-<br />
dences are, however, required to burn large-<br />
diameter, solid particles. Afterburner design<br />
is discussed in Chapter 5.<br />
Properly designed centrifugal separators are<br />
required on essentially all process airstreams<br />
up to and including the stoner and chaff collec-<br />
tion system. With the plant shown, cyclones<br />
are required at the roaster, cooler, stoner,<br />
chaff storage bin, and chaff incinerator. In<br />
addition, the scalper is a centrifugal classiiier<br />
venting process air. Some plants also vent the<br />
green coffee dump tank and several conveyors and<br />
elevators to centrifugal dust collectors.<br />
For best results. the chaff incinerator should be<br />
of the design discussed in Chapter 8 in which<br />
combustible material is fed at a uniform rate.<br />
It is, however, considerably smaller and has<br />
burning rates usually below 100 pounds per hour.<br />
The inorganic ash content of the chaff, at approxi-<br />
mately 5 percent by weight, is considerably great-<br />
er than that of most combustible refuse fed to<br />
incinerators. Provisions should be made in the<br />
incinerator design so that this material does<br />
not become entrained in the exhaust gases. If<br />
most of the noncombustible material is dis-<br />
charged with products of combustion from the<br />
incinerator, the combustion contaminants then<br />
exceed 0.3 grain per cubic foot calculated to<br />
12 percent carion dioxide.<br />
SMOKEHOUSES<br />
Smoking has been used for centuries to preserve<br />
meat and fish products. Modern smoking opera-<br />
tions do not differ greatly from those used by our<br />
forefathers, though the prime purposes of smoking<br />
today appear to be the imparting of flavor, color,<br />
and "customer appeal" to the food product. Cur-<br />
ing and storage processes have been improved<br />
to the point where ,preservation is no longer the<br />
principal objective.<br />
The vast majority of smoked products are meats<br />
of porcine and bovine origin. Some fish and<br />
poultry and, in rare instances, vegetable prod-<br />
ucts are also smoked as gourmet items.<br />
The Smoking Process<br />
Smoking is a diffusion process in which food<br />
products are exposed to an atmosphere of hard-<br />
wood smoke. Table 215 is an analysis of smoke<br />
produced through the destructive distillation of<br />
a hardwood. As smoke is circulated over the<br />
food, aldehydes, organic acids, and other<br />
organics are adsorbed onto its outer surface.<br />
Smoking usually darkens the food's natural color,<br />
and in some cases, glazes the outer surface.<br />
Table 215. ANALYSIS OF WOOD SMOKE<br />
USED IN MEAT SMOKEHOUSES<br />
(Jensen, 1945)<br />
Contaminant Concentration, ppm<br />
Formaldehyde 20 to 40<br />
Higher aldehydes 140 to 180<br />
Formic acid 90 to 125<br />
Acetic and higher acids 460 to 500<br />
Phenols 20 to 30<br />
Ketones 190 to 200<br />
Resins 1,000<br />
Regardless of smokehouse design, some spent<br />
gases are always exhausted to the atmosphere.<br />
These contain odorous, eye-irritating gases and<br />
finely divided, organic particulates, often in<br />
sufficient concentration to exceed local opacity<br />
restrictions.<br />
Smokehouses are also used to cook and dry food<br />
products either before or after smoking. <strong>Air</strong><br />
contaminants emitted during cooking and drying<br />
are normally well below allowable control limits.<br />
Atmospheric Smokehouses<br />
The oldest smokehouses are of atmospheric or<br />
natural-draft design. These boxlike structures<br />
are usually heated directly with natural gas or<br />
wood. Smoke is often generated by heating<br />
sawdust on a steel plate. These smoke gener-<br />
ators are normally heated with natural gas pipe<br />
burners located in the bottom of the house. Hot,<br />
smoky gases are allowed to rise by natural con-<br />
vection through racks of meat. Large atmospheric<br />
houses are often built with two or three levels of<br />
meat racks. One or more stacks are provided<br />
to exhaust spent gases at the top of the house. In<br />
some instances the vents are equipped with ex-<br />
haust fans. During the smoking and drying cy-<br />
cles, exhaust gas temperatures range from<br />
120" to 150°F. Slightly higher temperatures<br />
are sometimes encountered during the cooking<br />
cycle.
Food Processing Equipment 795<br />
Recirculating Smokehouses Smoking by Immersion<br />
Most large, modern, production meat smokehouses<br />
are of the recirculating type (Figure 608)<br />
wherein smoke is circulated at reasonably high<br />
velocities over the surface of the product. The<br />
purpose is to provide faster and more nearly<br />
uniform diffusion of organics onto the product,<br />
and nlore uniform temperatures throughout the<br />
house. These units are usually of stainless<br />
steel construction and are heated by steam or<br />
gas. Smoke is piped to the house from external<br />
smoke generators. Each unit is equipped with<br />
a large circulating fan and, in some instances, a<br />
smaller exhaust fan. During smoking and cooking,<br />
exhaust volumes of 1 to 4 cfm per square foot of<br />
floor area are maintained. The exhaust rate is<br />
increased to 5 to 10 cfm per square foot during .<br />
the drying cycle. Recirculating smokehouses<br />
are usually equipped with temperature and humidity<br />
controls, and the opacity and makeup of<br />
exhaust gas are usually more constant than those<br />
from atmospheric units.<br />
/-ALTERNATING<br />
AUTOMATIC<br />
Figure 608. A modern recirculating smokehouse<br />
(Atmos Corp., Chicago, II I.).<br />
Corlventional smoking operations can.be seen<br />
as an extremely devious method of coating food<br />
products with a myriad of hardwood distillation<br />
products. One might wonder why this coating<br />
is not applied by simple immersion. Unfortu-<br />
nately, many of the compounds present in smoke<br />
are highly toxic. If these were deposited heavily<br />
on the food product, results could be fatal. Smok-<br />
ing, therefore, provides a reasonably foolproof,<br />
if quaint, means of assuring that these toxic com-<br />
pounds do not accumulate in lethal concentrations.<br />
Many states have laws prohibiting the smoking of<br />
meats by liquid immersion.<br />
Smoking by Electrical Precipitation<br />
Some attempts have been made to precipitate<br />
smoke particles electrically onto food products<br />
in the smokehouse, and a few smokehouses so<br />
designed are in operation today. From the opera-<br />
tors' point of view, this arrangement offers the<br />
advantages of faster smoking and greater use<br />
of generated smoke. From the standpoint of air<br />
pollution control, it is desirable inasmuch as<br />
considerably lesser quantities of air contami-<br />
nants are vented to the atmosphere than are<br />
vented from a conventional, uncontrolled smoke-<br />
house.<br />
These units normally consist of a conveyorized<br />
enclosure equipped with an ionizer section similar<br />
to those used with two-stage precipitators.<br />
The foo'd product is usually passed 2 to 3 inches<br />
below tlie ionizing wires, which are charged with<br />
about 15, 000 volts. No electrical charge is aplied<br />
to the food products or the conveyor. These<br />
smokers are operated at ambient temperatures<br />
and do not lend themselves to use for either cooking<br />
or drying food products. As would be expected,<br />
spacing is a critical factor.<br />
There are very few precipitation smokehouses<br />
in the Unites States today, and for this reason,<br />
little reliable data about the operating characteristics<br />
or the air pollutants emitted are available.<br />
Smokehouses of this design have been<br />
reported to operate with visible emissions of<br />
only 5 to 10 percent opacity. Concentrations of<br />
air contaminants in gases from precipitationtype<br />
smokehouses would, under optimum conditions,<br />
be expected to be approximately equivalent<br />
to those from conventional smokehouses<br />
equipped with two-stage electrical precipitators.<br />
These units offer the potential of markedly reduced<br />
smoking times. Indeed, the few operating<br />
units have residence times of less than 5 minutes.<br />
If equipment such as this were perfected for a<br />
wider range of operation, residence times would<br />
not be expected to exceed 10 minutes.
796 CHEMICAL PROCESS1 LNG EQUIPMENT<br />
The application of precipitation smokehouses<br />
is today limited by a number of inherent problems,<br />
the foremost of which is the irregular shape of<br />
many smoked products, that is, hams, ham hooks,<br />
and salami. The degree of smoke deposition in<br />
a unit such as this is governed by the distance :<br />
between the ionizer and the food product. Irregu-<br />
lar spacing results, therefore, in irregular smok-<br />
ing of round and odd-shaped products that cannot<br />
be positioned so that all surfaces are equidistant<br />
from ionizer wires. The few existing installa-<br />
tions are used to impart a light smoke to regular-<br />
shaped, flat items such as fish fillets and sliced<br />
meat products.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
Smokehouse exhaust products include organic<br />
gases, liquids, and solids, all of which must<br />
be considered air contaminants. Many of the<br />
gaseous compounds are irritating to the eyes<br />
and relatively odorous. A large portion of the<br />
particulates is in the submicron size range where<br />
light scattering is maximum. These air con-<br />
taminants are attributable to smoke, that is, to<br />
smoke generated from hardwood, rather than<br />
from the cooked product itself.<br />
Exhaust gases from both atmospheric and re-<br />
circulating smokehouses can be periodically<br />
expected to exceed 40 percent opacity, the<br />
maximum allowable under many local air pollu-<br />
tion control regulations. With the possible ex-<br />
ception of public nuisance, smokehouse exhaust<br />
gases are not likely to exceed other local air<br />
quality standards. As shown in Table 215, con-<br />
centrations of particulate matter average only<br />
0. 14 grain per scf.<br />
Hooding and Ventilation Requirements<br />
Atmospheric smokehouses are designed with ex-<br />
haust volumes of about 3 cubic feet per square<br />
foot of floor area. Somewhat higher volumes are<br />
used with atmospheric houses of two or more<br />
stories. Inasmuch as there are no air recircula-<br />
tion and normally little provision for forced draft,<br />
the exhaust rate for an atmospheric house is es-<br />
sentially constant over the drying, cooking, and<br />
smoking cycles. Moreover, there is often some<br />
smoke in the house even during the cooking and<br />
drying cycles. This is particularly true where<br />
smoke is generated in the house rather than in<br />
an external smoke generator. If gases are to be<br />
ducted to air pollution control equipment, an ex-<br />
haust fan should be employed to offset the added<br />
pressure drop. When an afterburner is used, it<br />
can often be positioned to provide additional nat-<br />
ural draft.<br />
Recirculation smokehouses have a considerably<br />
wider range of exhaust rates. During smoking<br />
and cooking cycles, volumes of 1 to 4 cubic<br />
feet per square foot of floor area are exhausted.<br />
The rate increases to 5 to 10 cubic feet per square<br />
foot during the drying cycle. Recirculation houses<br />
are almost always equipped with external smoke<br />
generators, and a control of smoke flow is much<br />
more positive. There is essentially no smoke in<br />
the houses during the cooking and drying cycles.<br />
Most smokehouses do not require hooding. Ex-<br />
haust gases are normally ducted directly to the<br />
atmosphere or to control equipment. Some at-<br />
mospheric houses are, however, equipped with<br />
hoods over the loading doors to gather smoke that<br />
might escape during the shifting of meat racks. The<br />
latter situation is due to the inherently poor dis-<br />
tribution of smoke and heat in an atmospheric house.<br />
To maintain product uniformity, the meat racks<br />
must often be shifted while there is smoke in the<br />
house. Most atmospheric houses do not have ex-<br />
haust systems adequate to prevent appreciable<br />
smoke emissions from the door during these in-<br />
stances. Hoods and exhaust systems are some-<br />
times installed principally for worker comfort. The<br />
hoods or fans, or both, may be located in corridor<br />
ceilings immediately above the doors. These ven-<br />
tilators are often operated automatically whenever<br />
the doors are opened. Volumes can be appreciable,<br />
in some instances exceeding the smokehouse's ex-<br />
haust rate.<br />
There are normally no appreciable smoke emis-<br />
sions from doors of recirculation-type smoke-<br />
houses. Temperature and smoke distribution<br />
are sufficient so that there is no need to shift<br />
meat in the houses. Moreover, the doors are<br />
designed to provide tighter closures. Recircula-<br />
tion houses are operated under positive pressure,<br />
and any small opening causes large emissions of<br />
smoke.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
Afterburners<br />
Smoke, odors, eye irritants, and organic partic-<br />
ulate matter can be controlled with afterburners,<br />
provided temperature and design are adequate.<br />
Most of these contaminants can be eliminated at<br />
temperatures of 1, 000°F to 1,200"F in well-de-<br />
signed units. Larger diameter particulate matter<br />
is somewhat more difficult to burn at these tem-<br />
peratures; however, since concentrations of par-<br />
ticulate matter from smokehouses are reasonably<br />
small, this limitation is not critical.
Electrical precipitators<br />
Low-voltage, two-stage electrical precipitators<br />
were installed in the Los Angeles area as early<br />
as 1957 to control visible smokehouse air con-<br />
tamirlants. They have since been used at many<br />
other locations in the United States. Before<br />
1957, their use had been confined principally to<br />
. . . . air-conditioning applications.<br />
Electrical precipitators are, of course, effective<br />
only in the collection of particulate matter. They<br />
cannot he used to control gases or vapors. At<br />
smokehouse installations, their purpose is to<br />
collect the suhmicron smoke particles responsible<br />
for visible opacity. Two-stage precipitators<br />
.. ~ . . have been shown capable of reducing smoke opaci-<br />
~ ~<br />
. . . . ~ .. ties to less than 10 percent under ideal conditions.<br />
A typical two-stage precipitator control system<br />
... .<br />
. . with a wet, centrifugal collector is shown in Figure<br />
609. The wet collector is used to control<br />
temperature and humidity and also remove a<br />
small amount of particulates. This is followed<br />
by a heater in which gas temperatures are regulated<br />
before the gases enter the ionizer. Voltages<br />
of 6, 000 to 15, 000 volts are applied to the ionizer<br />
I 1 and plate sections. Particulate matter collects<br />
on the plates and drains, as a gummy liquid, to<br />
1 the collection pan below.<br />
For sat~sfactory control of visihle emissions, it<br />
has been found that superficial gas velocities<br />
through the plate collector section should not exceed<br />
100 fpm. Some difficulty has been experience<br />
Food Processing Equipment 797 1<br />
owing to channeling in the collector. For best oper-<br />
ation, vanes or other means of ensuring uniform<br />
flow should bk used ahead of the plate section.<br />
Even under optimum conditions, a slight trace of<br />
smoke can he expected from the precipitator's<br />
outlet. At the discharge of the unit, eye irrita-<br />
tion is usually severe, and odors are strong<br />
though not overpowering. These odors and eye<br />
irritants can constitute a public nuisance, de-<br />
pending upon plant location.<br />
Electrical precipitation versus incineration<br />
Both electrical precipitation and incineration<br />
offer the classical choice of high initial cost<br />
versus high operating cost, but in addition, they<br />
differ markedly from the standpoint of air pollu-<br />
tion control.<br />
Electrical precipitators are capable of collect-<br />
ing particulate matter and thereby reducing<br />
visihle emissions to tolerable amounts. They<br />
have no effect on nitrogen oxides and little<br />
effect, if any, on gaseous eye irritants and<br />
odors. If arcing occurs, some small and proh-<br />
ably insignificant quantity of ozone is also pro-<br />
duced. The initial cost of precipitators is high,<br />
and their operating cost low in comparison with<br />
that of afterburners. Smokehouse precipita-<br />
tors do, however, require a relatively high<br />
degree of maintenance. If they are not proper-<br />
ly maintained, poor control efficiency and fire<br />
damage are probable. Fire damage can result<br />
d in extended outage periods during which uncon-<br />
Figure 609. A two-stage precipitator and wet centrifugal<br />
collector venting smokehouses (The Rath Pa-king Co., Vernon, Calif.).
798 CHEMICAL PROCESSING EQUIPMENT<br />
trolled exhaust gases may vent directly to the<br />
atmosphere.<br />
Incineration is much more effective than elec-<br />
trical precipitation is in controlling gaseous<br />
organics and finely divided particulates. Large<br />
particles are, however, relatively difficult to<br />
burn at the normal operating temperatures and<br />
residence times of smokehouse afterburners.<br />
Under average conditionsl collection efficiency<br />
for particulate matter (about 65 percent) is<br />
roughly the same as that of a two-stage elec-<br />
trical precipitator. Fuel costs make the oper-<br />
ation of an incineration device more expensive<br />
than that of a precipitator. Nevertheless,<br />
maintenance is much less a problem. There<br />
is no buildup of tars and resins in the afterburner<br />
or stack to impede its operation. As with any<br />
smokehouse control device, tars accumulate in<br />
the ductwork between the house and afterburner,<br />
necessitating periodic cleaning. As shown in<br />
Table 216, incineration creates additional nitro-<br />
gen oxides, increasing concentrations from about<br />
4 ppm to approximately 12 ppm on the average.<br />
Comparative test data on smokehouse afterburners<br />
and electrical precipitators, as shown in Tables<br />
216 and 217, indicate that collection efficiencies<br />
for particulate matter, aldehydes, and organic<br />
acids are of the same magnitude for both types<br />
of control dequipment. These data fail to re-<br />
flect larger concentrations of odors and eye<br />
irritants from electrical precipitators that are<br />
readily apparent upon personal inspection of the<br />
devices.<br />
Bypassing control devices during nonsmoking<br />
periods<br />
Many operators of recirculation smokehouses<br />
find it desirable to bypass air pollution control<br />
devices during nonsmoking periods. From the "<br />
standpoint of air pollution control, this practice<br />
is not unreasonable. The major smokehouse air<br />
contaminant is smoke. Concentrations of air con-<br />
taminants during cooking and drying are relative-<br />
ly small, comparable to those of ordinary meat-<br />
cooking ovens. Drying-cycle exhaust gases are<br />
2 to 4 times more voluminous than those vented<br />
Table 216. ANALYSES OF MEAT SMOKEHOUSE EXHAUST GASES BEFORE AND<br />
AFTER INCINERATION IN NATURAL GAS-FIRED AFTERBURNERS<br />
Particulate matter,<br />
grlscf<br />
Aldehydes (as form<br />
aldehyde), pprn . Organic acids (as<br />
acetic acid)<br />
Oxides of nitrogen<br />
(as NO2), ppm<br />
Contaminant concentration<br />
Smokehouse<br />
Afterburner<br />
Range Average Range Average<br />
0.016 to 0.234<br />
8 to 74<br />
30 to 156<br />
1.2 to 7.2<br />
40<br />
0.141<br />
87<br />
3.9<br />
0.011 to 0.070<br />
5 to 61<br />
0 to 76<br />
3.7 to 33.8<br />
0.048<br />
Control<br />
efficiency,<br />
%<br />
Table 217. ANALYSES OF MEAT SMOKEHOUSE EXHAUST GASES BEFORE AND AFTER<br />
CONTROL IN TWO-STAGE ELECTRICAL PRECIPITATION SYSTEMS<br />
Contaminant concentration<br />
. Control<br />
Smokehouse<br />
Control systema efficiency, %<br />
Range Average Range Average<br />
Particulate matter,<br />
grlscf<br />
0.33 to 0.181 0.090 ' 0. 016 to 0. 051 0. 032 6 5<br />
Aldehydes (as formaldehyde),<br />
ppm<br />
--- 7 4 --- 47<br />
3 7<br />
Organic acids (as acetic<br />
acid), ppm<br />
--- 91<br />
>.- 48<br />
47<br />
aEach control system is equipped with a wet centrifugal collector upstream from the<br />
precipitator.<br />
2 5<br />
33.5<br />
11.7<br />
6 6<br />
3 8<br />
62<br />
Negative
during the smoking cycle. The size of control<br />
equipment is materially increased if drying<br />
gases are ducted to it. The initial cost and oper-<br />
ating cost of a smokehouse's air pollution control<br />
system can, therefore, be considerably reduced<br />
if exhaust gases are bypassed during drying and<br />
cooking cycles when no smoke is introduced into<br />
the house.<br />
If control devices are to be bypassed duringnonsmok-<br />
ing periods, the ductwork and valving should be de-<br />
signed to provide automatic or nearly automatic<br />
operation. Water seal dampers (Figure 610) are<br />
preferable. Mechanical dampers demand optimum<br />
maintenance for satisfactory closure. They are<br />
considerably more likely to malfunction owing to<br />
corrosion and contamination with greases and tars.<br />
Moreover, mechanical dampers are more suscepti-<br />
ble to physical damage than water dampers are.<br />
Ideally, damper operation should be keyed to other<br />
smokehouse auxiliaries such as fans and smoke<br />
generators. Where controls are manually oper-<br />
ated, there is a strong possibility that dampers<br />
will not be opened or closed at proper times,<br />
causing either overloading of the control device<br />
or the discharge of untreated air contaminants<br />
directly to the atmosphere.<br />
CASES FROM<br />
SMObEHOUSE<br />
10 CONTROL<br />
C-<br />
OtYlCt AIMOSPHERE<br />
F~gure 610. Diagram of a water-operated damper<br />
used to bypass the a1 r pol lut~on control dev~ce<br />
dur~ng nonsmoking per~ods.<br />
DEEP FAT FRYING<br />
Deep fat or "French" frying involves the cooking<br />
of foods in hot oils or greases. Deep-fried prod-<br />
ucts include doughnuts, fritters, croquettes, vari-<br />
ous potato shapes, and breaded and batter-dipped<br />
fish and meat. Most of these foods contain some<br />
moisture, a large portion of which is volatilized<br />
out as steam during frying. Some cooking oils,<br />
as well as animal or vegetable oils from tb". prod-<br />
uct, are usually steam distilled during the pro-<br />
cess.<br />
Food Processing Equipment 799<br />
Deep fat frying is in common usage in homes,<br />
restaurants, and frozen food plants. In the home<br />
and in smaller commercial establishments, batch-<br />
type operation is more common. The principal<br />
equipment is an externally heated cooking oil vat.<br />
Oil temperatures are usually controlled to be-<br />
tween 325" and 400°F. Almost any type-of<br />
heating is possible. Where combustion fuels<br />
are used, burner gases are vented separately.<br />
The product to be fried is either manually or<br />
mechanically inserted into the hot grease and<br />
removed after a definite time interval.<br />
In large commercial establishments, highly<br />
mechanized, conveyorized fryers, such as that<br />
shown in Figure 611, are used. The raw food<br />
product is loaded onto an endless conveyor belt<br />
and passed through hot grease at a rate adjusted<br />
to provide the proper cook time. Almost all<br />
fryers are of one-pass design. Frequently, cook-<br />
ing units are followed by product coolers and pack-<br />
aging and freezing equipment.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
In a typical large industrial operation of this<br />
type, the cooking vat constitutes the principal<br />
source of air contaminants. Uncooked materials<br />
are usually wet or pasty, and the feed system<br />
produces little or no air pollution. Most cooked-<br />
product-handling systems are also innocuous,<br />
except in rare instances where fine, dusty mate-<br />
rials are encountered.<br />
TO - -+ Odors, visible smoke, and entrained fat particles<br />
are emitted from the cooking vats. Depending<br />
upon operating conditions and the surrounding<br />
area, these contaminants may or may not be m<br />
sufficient concentration to exceed the limits of<br />
local opacity or nuisance regulations.<br />
From the standpoint of air pollution control, the<br />
most objectionable operations involve foods con-<br />
taining appreciable fats and oils. Light ends of<br />
these oils are distilled during cooking. In gen-<br />
eral, the deep frying of vegetable products is<br />
less troublesome than that of fish and meat prod-<br />
ucts, which contain higher percentages of fats<br />
and oils.<br />
Most food products cooked in this manner con-<br />
tain between 30 and 75 percent moisture before<br />
the cooking. Almost all moisture is driven off<br />
in the cooking vat and appears as steam in ex-<br />
haust gases. Moisture concentrations in stack<br />
gases are usually between 5 and 20 percent, de-<br />
pending upon the volume of air drawn into the<br />
cooker hood and exhaust system. In highl!-<br />
mechanized installations, very little air enters<br />
under the cooker hood. As a result, the warm<br />
air-stream from a fryer such as this is often
800 CHEMICAL PROCESSING EQUIPMENT<br />
Figure 611. A continuous deep fat fryer: (left) Interior view,<br />
(right) end view (J.C. Pitman & Sons, Inc., Concord, N.H.).<br />
saturated, and downstream cooling causes visi-<br />
ble condensation at or near the stack exit.<br />
Moisture has two effects: (1) It causes fats and<br />
oils to be steam distilled from the cooking vat,<br />
and (2) it masks visible stack emissions. Smoke<br />
observations of equipment such as this must be<br />
made at the point in the stack plume where water<br />
vapor has disappeared. This is best accomplished<br />
when the weather is warm and dry. On a cold,<br />
moist day, the vapor plume may extend as far<br />
as the smoke.<br />
Excessive smoking is most often due either to<br />
overheating or to the characteristics of the<br />
material being cooked. When, for instance,<br />
potato chip or corn chip fryers are operated<br />
in normal temperature ranges, there is usually<br />
no more than a trace of smoke in exhaust gases.<br />
On the other hand, several meat product fryers<br />
have been found to exhaust gases of high opacity,<br />
and control equipment was needed to bring them<br />
into compliance with local regulations. These<br />
visible emissions appear to be finely divided<br />
fat and oil particles distilled either from the<br />
product or the cooking oil. Cooking oils are<br />
usually compounded within reasonably narrow<br />
boiling ranges, and when fresh, very little of<br />
the oils is steam distilled. Most objectionable<br />
air contaminants probably originate, therefore,<br />
in the product or in spent cooking oil.<br />
The carryover of oil droplets can also cause a<br />
nuisance by spotting fabrics, painted surfaces,<br />
and other property in the surrounding area.<br />
1<br />
j<br />
This problem is most likely to occur when the<br />
I<br />
raw food contains relatively large concentrations j<br />
of moisture, a situation in which steam distillation<br />
is proportionally higher. 1 i<br />
I<br />
Hooding and Ventilation Requirements<br />
Deep fat fryers should always be hooded and<br />
vented through a fan. Axial-flow fans are pre-<br />
ferred. Exhaust volumes are governed by the<br />
open area under the hood. Where there is open<br />
area around the full hood periphery, the indraft<br />
velocity should be at least 100 fpm. In many<br />
modern units, the dryer sides are completely<br />
enclosed, and the only open areas are at the con-<br />
veyor's inlet and outlet. At these installations,<br />
exhaust volumes are considerably less, even<br />
though indraft velocities are well above 100 fpm.<br />
If control equipment is to be employed, exhaust<br />
volumes become an important factor. In these<br />
instances, redesigning the existing hoods to low-<br />
er the exhaust rates is often desirable.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
Incineration, low-voltage electrical precipita-<br />
tion, and entrainment separation have been used<br />
to control air contaminants from deep fat fryers.<br />
Since practically all air contaminants from fry-<br />
ers are combustible, a well-designed afterburn-<br />
er provides adequate control if the cperating tem-<br />
perature is sufficiently high. Temperatures from
1, 000' to 1, 200°F are often sufficient to eliminate<br />
smoke-causing particulates and to incinerate odors<br />
and eye irritants. The combustion of larger par-<br />
ticles usually requires higher temperatures, some-<br />
times as high as 1,600-F. The concentration of<br />
particulates in fryer exit gases is, however, nor-<br />
mally less than 0. 1 grain per scf, which is well<br />
below common limits for particulate emissions.<br />
Two-stage, low-voltage electrical precipitators<br />
(6, 000 to 15, 000 volts) can be used to collect a<br />
substantial portion of the particulates responsible<br />
for visible air contamination. These devices,<br />
unfortunately, do not remove the gaseous con-<br />
taminants that are usually responsible for odors<br />
and eye irritation. As would be expected, the<br />
effectiveness of a precipitator depends upon the<br />
particular fryer it is serving. If particulates<br />
are the only significant contaminants in the ex-<br />
haust gases, a precipitator can provide an ade-<br />
quate means of control. If, on the other hand, the<br />
problem is due to odors of overheated oil or prod-<br />
uct, a device such as this is of little benefit. For<br />
optimum performance, the temperature, humidity,<br />
and volume of gases vented to a two-stage pre-<br />
cipitator must be controlled within reasonably<br />
narrow limits. The oils collected are usually<br />
free flowing and readily drain from collector<br />
plates. A collection trough should be provided<br />
to prevent plate fouling and damage to the roof<br />
or other supporting structure on which the pre-<br />
cipitator is located.<br />
Entrainment separators have been employed<br />
with varying success to remove entrained oils<br />
in fryer exhaust stacks. These are most use-<br />
ful where the concentration of oils is relatively<br />
large. The material collected can represent a<br />
savings in oil and can prevent damage to ad-<br />
jacent roofing. Because of the inherently low<br />
collection efficiency of these devices, their<br />
use is not recommended where smoke or<br />
odors constitute the major air pollution prob-<br />
lem. Some cooking oils usually collect on the<br />
inner surfaces of uninsulated exhaust stacks<br />
and drain back towards the cooker. Most com-<br />
mercial fryers are .equipped with pans to col-<br />
lect this drainage at the bottom of the stack.<br />
LIVESTOCK SLAUGHTERING<br />
Slaughtering operations have traditionally<br />
been associated with odorous air contaminants,<br />
though much of these odors is due to byproduct<br />
operations rather than to slaughtering and meat<br />
dressing itself. Slaughtering is considered to<br />
include only the killing of the animal and the<br />
separation of the carcass into humanly edible<br />
meat and inedible byproducts. The smoking<br />
of edible meat products, and reduction of edi-<br />
ble materials are discussed in this subsection,<br />
Food Processii ng Equipment 801<br />
while the reduction of inedible materials is<br />
covered in another part of this chapter.<br />
Cattle-, sheep-, and hog-killing operations are<br />
necessarily more extensive than those concerned<br />
with poultry, though poultry houses usually han-<br />
dle appreciably larger numbers of animals.<br />
A flow diagram of a typical cattle-slaughtering ,<br />
operation is shown in Figure 612. The animal<br />
is stunned, bled, skinned, eviscerated, and<br />
trimmed as shown. Blood is drained and col-<br />
lected in a holding tank. After removal, en-<br />
trails are sliced in a "gut hasher, " then washed<br />
to separate the partially digested food termed<br />
"paunch manure. " Many slaughterers have<br />
heated reduction facilities in which blood, in-<br />
testines, bones, and other inedible materials<br />
are processed to recover tallow, fertilizer,<br />
and animal feeds. The firms that do not oper-<br />
ate this equipment usually sell their offal to<br />
scavenger plants that deal exclusively in by-<br />
products. Hides are almost always shipped to<br />
leather-processing firms. Dressed beef, nor-<br />
mally about 56 percent of the live weight, is<br />
refrigerated before it is shipped.<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
Odors represent the only air contaminants<br />
emitted from slaughtering operations. The<br />
odors could be differentiated as (1) those<br />
released from the animal upon the killing and<br />
cutting, and upon the exposure of blood and<br />
flesh to air; and (2) those resulting from the<br />
decay of animal matter spilled on exposed sur-<br />
faces or otherwise exposed to the atmosphere,<br />
Odors from the first source are not appreciable<br />
when healthy livestock is used. Where nuisance-<br />
causing odors are encountered from slaughter-<br />
ing, they are almost always attributable to in-<br />
adequate sanitary measures. These odors are<br />
probably breakdown products of proteins. Amines<br />
and sulfur compounds are considered to be the<br />
most disagreeably odorous breakdown products.<br />
In addition to these sources, there are odors<br />
at slaughterhouse stockyards and from the stor-<br />
age of blood, intestines, hides, and paunch<br />
manure before their shipping or further process-<br />
ing.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
As has been explained, odorous air contami-<br />
nants are emitted from several points in a<br />
slaughtering operation. Installing control equip-<br />
ment at each source would be difficult if not im-<br />
possible. Methods of odor control available in-<br />
clude: (1) Rigid sanitation measures to prevent
802 CHEMICAL PROCESSING EQUIPMENT<br />
STORAGE<br />
RENDERING<br />
STORAGE<br />
Figure 612. Typical livestock-slaughtering and processing area<br />
(The Globe Company, Chicago, Ill.).<br />
RENDERING<br />
the decomposition of animal matter, and (2) com- EDIBLE-LARD AND TALLOW RENDERING<br />
plete enclosure of the operation to capture the<br />
effluent and exhaust it through a control device. Methods used to produce edible lard and tallow<br />
Where slaughtering is government inspected,<br />
the operators are required to wash their kill<br />
rooms constantly, clean manure from stock<br />
pens, and dispose of all byproducts as rapidly<br />
as possible. These measures normally hold<br />
plant odors to a tolerable minimum.<br />
When a slaughterer is located in a residential<br />
area, the odor reduction afforded by strict<br />
sanitation may not be sufficient. In these in-<br />
stances, full-plant air conditioning might be<br />
necessary. Filtration with activated carbon<br />
would appear to be the only practical means<br />
of controlling the large volume of exhaust<br />
gases from a plant of this type. The latter<br />
method has not yet been employed at slaughter<br />
houses in the United States. Nevertheless,<br />
activated-carbon filtration of the entire plant<br />
has been employed to control similar odors<br />
at animal matter byproduct plants. With in-<br />
creasing urbanization, this method of control<br />
may, conceivably, be used in the near future.<br />
are similar to those described later in this<br />
chapter for rendering of inedibles. As with<br />
processes for inedibles, feedstocks are heated<br />
either directly or indirectly with steam to effect<br />
a phase separation yielding fats, water,<br />
and solids. Moisture is removed either by<br />
vaporization or by mechanical means. Tallow<br />
and solids are mechanically separated from<br />
one another in presses, centrifuges, and filters.<br />
The only major process differences between<br />
rendering edibles and rendering inedibles are<br />
due to the composition and freshness of the<br />
materials handled. Edible feedstocks contain<br />
80 to 90 percent lard or tallow, 10 to 20 per-<br />
cent moisture, and less than 5 percent muscle<br />
tissue. Inedible feedstocks contain appreciably<br />
higher precentages of both moisture and solids.<br />
Edible feedstocks, in addition to being more<br />
select portions of the animal, are generally<br />
much fresher than inedible cooker materials<br />
are.
Food Processing Equipment 803<br />
Whenever its products are intended for human material in direct contact with live steam.<br />
consumption, the process is much more stringent- -<br />
ly supervised and regulated by Federal and local<br />
The principal advantage of this type of renderagencies.<br />
There are numerous government regulations<br />
concerning the freshness of edi,,le-rendering<br />
is that large quantities of lar* Or tallow<br />
ing feedstocks, the cleanliness of processing<br />
can be produced without finely grinding the feed<br />
equipment, and the handling of rendered fats. material. Low-cost equipment and labor can<br />
For instance, paragraph 15. 1 of the United States<br />
be used.<br />
- -<br />
Department of Agriculture's Meat Inspection<br />
Regulations specifies that inspected feed mate-<br />
Wet rendering, however, necessarily requires<br />
higher temperatures (280" to 300°F) and inrial<br />
must be heated to a temperature not lower<br />
than 170°F for a period of not less than 30<br />
ternal pressures of 38 to 40 psig, The quality<br />
minutes when edible lard or tallow is being proof<br />
the lard or tallow produced is relatively low,<br />
duced.<br />
owing to the high temperatures to which it is<br />
subjected.<br />
Dry Rendering<br />
The <strong>Air</strong> <strong>Pollution</strong> Problem<br />
Batchwise renderine - The only noteworthy air contaminants generated<br />
Most of the high-quality edible lard and tallow<br />
from edible-rendering processes are odors. In<br />
are produced in indirectly steam-heated cookers.<br />
comparison with odors generated from inedible-<br />
These processes are frequently carried out at<br />
rendering processes, however, those from<br />
temperatures of less than 212°F. The lower<br />
edible-rendering processes are relatively minor.<br />
operating temperatures are afforded either by<br />
vacuum cooking or by finely grinding the feed-<br />
In Los Angeles County, rendering of edibles acstocks.<br />
The vacuum process is usually percounts<br />
for only about 10 percent of the total aniformed<br />
batchwise in a horizontal, steam-jacmal<br />
matter rendered. Rendering of inedibles<br />
keted cooker very similar to those used for<br />
at packing houses constitutes approximately 32<br />
rendering of inedibles. The vacuum is usually<br />
percent and that at scavenger plants accounts<br />
created through the use of steam- or waterfor<br />
the remaining 58 percent of the tonnage.<br />
operated ejectors. Variations in dry, ediblerendering<br />
processes are usually concerned<br />
In addition, rates of odor emissions from renwith<br />
temperatures and the degree of comminu- dering of edibles are low compared with those<br />
tion of fats. Where raw materials are ground from inedible-rendering processes. Inasmuch<br />
into fine particles, operation at lower tem-<br />
as edible feedstocks contain relatively low perperatures<br />
is usually possible, even without<br />
centages of water, the resultant steam generated<br />
a vacuum-producing device.<br />
from cookers, 6,300 scf per ton, is not appre-<br />
Continuous low-temperature rendering<br />
ciable. Feedstocks contain approximately 15 percent<br />
moisture, as compared with 50 percent<br />
from inedible cooker materials. Odor concentrations<br />
in cxhaust gases from the rendering<br />
of edibles are significant at 3, 000 odor units<br />
per scf but not excessive. Equipment at plants<br />
rendering edibles is kept scrupulously clean,<br />
which substantially reduces odors from inplant<br />
handling operations.<br />
Dry rendering processes have been developed<br />
to produce edible lard and tallow on a continu-<br />
ous basis from high-fat feedstocks. A typical<br />
process is shown in Figure 613. Feedstocks<br />
are first introduced to a grinder, where they<br />
are finely shredded at 120°F. and then heated<br />
to approximately 185°F before being passed<br />
through a desludging centrifuge in which solids<br />
are removed from the water and tallow. Lia-<br />
uids are then reheated to about 200°F in a<br />
steam jet heater. The remaining moisture is<br />
removed from the hot tallow in a second cen-<br />
trifuge from which edible lard or tallow is<br />
run to storage. The separated water is piped<br />
to a skimming pond where it is cooled before<br />
being sewered. Vapors from the several<br />
vessels are vented to a fume scrubber (con-<br />
tact condenser).<br />
Hooding and Ventilation Requirements<br />
Almost always, cooker gases from rendering of<br />
edibles can be piped directly to air pollution con-<br />
trol devices. Where condenser odor control de-<br />
vices are used, there is usually enough vacuum,<br />
that is, pressure differential, in the ductwork<br />
to cause vapors to flow from the cooker at a<br />
sufficiently high rate. Steam or water ejectors<br />
are sometimes employed to lower operating<br />
temperatures or to remove water vapor more<br />
Wet Rendering<br />
rapidly. Uncondensible gases do not exceed<br />
5 percent of cooker gases unless there is ap-<br />
The wet rendering process involves rendering preciable leakage into the system, as through<br />
of fats in a vertical, klosed tank with the feed seals on shafts, doors, and so forth.
804 CHEMICAL PROCESSING EQUIPMENT<br />
HEADER SLOPE TO SCRUBBER. NO POCKETS OR TRAPS<br />
r'&..p AZZ-F-7<br />
PUMP PURIFIER TO STICKWATER PLANT<br />
OR CATCH BASIN<br />
1<br />
\scE!kEy<br />
1<br />
I<br />
i<br />
J<br />
---<br />
VAPOR VENT LINES<br />
COLD WATER<br />
1 i,L<br />
CATCH BASIN.<br />
B C<br />
TO SEWER<br />
80' TO 190°F 1185" TO 215OF<br />
Figure 613. DeLaval continuous centriflow process for edible protein recovery<br />
and edible fat rendering (The DeLaval Separator Co., Millbrae, Calif.).<br />
Where cooking is performed at pressures<br />
greater than 1 atmosphere, piping must usually<br />
be arranged in a manner that prevents surging<br />
when high-pressure gases are released. If<br />
the main valve is released quickly, the high-<br />
pressure vapors usually cause slugs of grease<br />
and solids to be carried over into the control<br />
system. Severe surging can cause siphoning<br />
of all the material from cooker to the control<br />
system. To prevent this, the piping is often<br />
arranged with a small pipe, 1 to 2 inches in<br />
diameter, that bypasses the main cooker's<br />
exhaust line. High pressures are reduced by<br />
venting first through the small pipe to the con-<br />
trol device. Once the high pressure is relieved,<br />
the large valve can be opened to provide great-<br />
er flow.<br />
<strong>Air</strong> <strong>Pollution</strong> Control Equipment<br />
lower volume of steam exhausted from the cook-<br />
er. Exit water temperatures should be held<br />
below 140°F to prevent the release of volatile,<br />
odorous materials from downstream piping<br />
and sewers.<br />
Surface condensers are also satisfactory con-<br />
trol devices for edible-rendering processes.<br />
At the same condensate volume and tempera-<br />
ture, however, surface condensers by them-<br />
selves are not as effective as contact con-<br />
densers. This is due to the inherently lower<br />
condensate volume and larger concentration<br />
of odorous materials in the condensate of sur-<br />
face condensers.<br />
That an edible-rendering process would require<br />
more extensive odor control than would be afforded<br />
by an adequate condenser is unlikely.<br />
Nevertheless, uncondensed offgases from condensers<br />
could be further controlled by incineration<br />
or carbon adsorption, as outlined for<br />
processing of inedibles later in this chapter.<br />
Water spray contact condensers are the simplest<br />
devices used for controlling odorous air contaminants<br />
from rendering . of edibles. These condense<br />
a major portion of the steam-laden effluent<br />
vapors and dissolve much of the odorous materi-<br />
FlSH CANNERIES AND FlSH<br />
als. Water requirements of the contact condenser<br />
for edible-rendering operations are considerably<br />
REDUCTION PLANTS<br />
lower than those for the contact condenser used Canning is the principal method of preserving<br />
to control cooker gases from rendering of in- highly perishable fish foodstuffs. Canneries<br />
edibles. This is due primarily to the lower fcr this purpose are usually located near harmoisture<br />
content of feedstocks and the resultant bors where fish can be unloaded directly from
oats. Byproduct reduction plants are operated<br />
at or near fish canneries to process scrap ma-<br />
terials, and much of the odorous air contami-<br />
nants generally attributed to canneries emanate<br />
from byproduct processes. Only choice por-<br />
tions of sound fish are canned for human con-<br />
sumption. The remainder is converted into by-<br />
products, notably fish oil and high-protein<br />
animal feed supplements.<br />
Basically there are two types of fish-canning<br />
operations in use today. In the older, so-called<br />
"wet-fish" method, trimmed fish are cooked<br />
directly in the can. The more popular "pre-<br />
cooked" process is used primarily to can tuna.<br />
The latter method is characterized by the cook-<br />
ing of whole, eviscerated fish, and the hand<br />
sorting of choice parts before canning.<br />
WET-FISH CANNING<br />
Wet-fish canning is used to preserve salmon,<br />
anchovies, mackerel, sardines, and similar<br />
species that can be obtained locally and brought<br />
to the cannery quickly. The distinctive feature<br />
of the wet-fish process is the complete removal<br />
of heads, tails, and entrails before the cooking.<br />
Fish Canneries and Fish Reduction Plants 805<br />
Trimmed and eviscerated raw fish is packed<br />
into open cans that are conveyed through a 100-<br />
to 200-foot-long hot-exhaust box. Here live<br />
steam is employed to cook the fish. Hot-ex-<br />
haust boxes are vented through several stacks<br />
located along their lengths (Figure 614). At<br />
the discharge end, cans may he mechanically<br />
upended so that "stick water" is decanted from<br />
the cans while the cooked fish remains. Stick<br />
water consists of condensed steam, juices, and<br />
oils that have cooked out of the fish. This liq-<br />
uid is collected and retained for byproduct<br />
processing as described later in this section.<br />
The cans of drained fish are filled with tomato<br />
sauce, olive oil, or other suitable liquid before<br />
being sealed. Sealed cans are pressure cooked<br />
before their labeling, packing, and shipping.<br />
TUNA CANNING<br />
The precooked canning method was developed<br />
to improve the physical appearance of canned<br />
fish. It is confined to the commercial canning<br />
of larger fishes, principally tuna. Whole,<br />
cviscerated fish are placed in wire baskets and<br />
charged to live-steam-heated cookers such as<br />
those of Figure 615. The cookers are operated<br />
Figure 614. Unsealed cans of cooked mackerel beina conveved from
806 CHEMICAL PROCE :SSING EQUIPMENT<br />
Flgure 615. A bank of 11ve-steam-heated cookers<br />
used to process raw, whole tuna (Star-Klst Foods,<br />
Inc., Termlnal Island, Calif.).<br />
at about 5 psig pressure, condensate being dis-<br />
charged through steam traps. <strong>Air</strong>, steam, and<br />
any uncondensed, odorous gases are bled from<br />
the cookers through one or more small vents in<br />
'the ceiling.<br />
As the fish are cooked, juices, condensed steam,<br />
and oils are collected, centrifuged, and pumped<br />
to stick water and oil storage tanks. Cooking<br />
reduces the weight of a fish by about one-third.<br />
After the cooking, the flesh is cooled so that it<br />
becomes firm before it is handled. It is then<br />
placed on a conveyorized picking line. Operators<br />
stationed along the conveyor select the portions<br />
to be canned for human consumption. After being<br />
packed and sealed in cans, the fisb is pressure<br />
cooked for sterilization before its labeling, pack-<br />
ing, and shipping. Much of the dark meat is<br />
canned for pet food. Only about one-third of the<br />
raw tuna weight is canned as food for humans and<br />
pets. The remaining skin, bone, and other scrap,<br />
roughly amounting to one-third of the raw weight,<br />
is fed to the fish meal reduction system.<br />
CANNERY BYPRODUCTS<br />
A large fraction of the fish received in a cannery<br />
is processed into byproducts. In the precook<br />
process, about two-thirds of the raw fish weight<br />
is directed to byproduct reduction systems as<br />
stick water or solid scrap. The wet-fish process<br />
usually produces somewhat less offal, depending<br />
principally upon the size of fish. Typical head-<br />
and-tail mackerel scrap is pictured in Figure 616.<br />
In addition, whole fish may be rejected at the can-<br />
ning line because of spoilage, freezer burns, bad<br />
color, and so forth. Any fish or portions of fish<br />
Figure 616. Typical raw head-and-tail mackerel j<br />
scrap awaiting processing in a fish meal reduction 1<br />
system (Star-Kist Foods, Inc., Terminal Island,<br />
Calif.). i<br />
not suitable for human consumption or for pet<br />
food are handled in the reduction plant. In order<br />
of volume and relative importance, the byproducts<br />
are: Fish meal, used almost exclusively as an<br />
animal feed supplement; fish oil, used in the<br />
paint industry and in vitamin manufacture; and<br />
"liquid fish" and "fish solubles, " high-protein<br />
concentrates. The latter are manufactured<br />
somewhat differently, but both are used as ani-<br />
mal feed supplements and as fertilizers.<br />
.FISH MEAL PRODUCTION<br />
Fish scrap from the canning lines, including<br />
any rejected whole fisb, is charged to continuous<br />
live-steam cookers in the meal plant.<br />
Flow through a typical fish meal plant is diagrammed<br />
in Figure 617. Cookers of the type<br />
shown in Figure 618 are operated at between 10<br />
and 25 psig steam pressure. Material charged<br />
to the cookers normally contains 20 to 30 percent<br />
solids. Cooked scrap has a slightly smaller<br />
solids content owing to the condensed steam<br />
picked up in cooking. After the material<br />
leaves the cooker, it is pressed to remove<br />
oil and water, and this pressing lowers the<br />
moisture content of the press cake to approximately<br />
50 percent. The press cake is broken Up,<br />
usually in a hammer mill, and dried in a directfired<br />
rotary drier or in a steam-tube rotary<br />
drier. Typical fish meal driers ~ield 2 to 10<br />
tons of meal per hour with a moisture content<br />
of 4 to 10 percent. Both types of driers em-
Fish Canneries and Fish Reduction Plants an7<br />
1<br />
FISH SCRAP VAPORS TO CONDENSER<br />
t t<br />
1 WATER<br />
LIVE STEAM COOKER CENTRIFUGE<br />
AN0 SOLUBLES<br />
STEAM 5 PSlg DRIER GASES TO<br />
GRINDER<br />
SOLIDS<br />
SEPARATOR<br />
SOLlOS LlQUlOS rv<br />
ROTARY FISH MEAL DRIER<br />
t<br />
, TO STORAGE MEAL +<br />
I I FINES I<br />
GRINDER<br />
Flgure 617. Flow d~agram of a fish meal reduct~on system includ~ng<br />
011-separat~ng and 011-clar~fy~ng equ~pment.<br />
Figure 618. A live-steam reduction cooker and a<br />
continuous press (Standard Steel Corp., Los Angeles,<br />
Calif.).<br />
ploy air as the drying medium. Moisture is<br />
removed with exhaust gases, which are volu-<br />
minous.<br />
Direct-fired driers include stationary fireboxes<br />
ahead of the rotating section, as shown in Fig-<br />
ure 619. They are normally fired with natural<br />
gas or fuel oil. Combustion is completed in<br />
the firebox. Hot products of combustion are<br />
mixed with air to provide a temperature of 400"<br />
to 1,000"F at the point where wet meal is initial-<br />
ly contacted. Hot, moist exhaust gases from<br />
the drier contain appreciable fine meal, which<br />
is commonly collected in a cyclone separator.<br />
The essential feature of steamtube driers is<br />
a bank of longitudinal, rotating steamtubes<br />
arranged in a cylindrical pattern, as shown in<br />
Figure 620. Steam pressures range from 50<br />
to 100 psig in the tubes. Heat is transferred<br />
both to the meal and air. As with direct-fired<br />
units, gases pass parallel to meal along the<br />
axis of the drier and are vented through a cy-<br />
clone separator. Meal produced in steamtube<br />
driers is less likely to be over-heated and is<br />
generally of higher quality than that from direct-<br />
fired units.<br />
FISH SOLUBLES AND FlSH OIL PRODUCTION<br />
Fish solubles is the term used to designate the<br />
molasses-like concentrate containing soluble<br />
proteins and vitamins that have been extracted<br />
from fish flesh by cooking processes. The<br />
flow diagram of Figure 617 includes the sep-<br />
aration of press water and fish oil. The sources<br />
of solubles and oils are the juices and conden-<br />
sate collected as press water and stick water.
808 CHEMICAL PROCESSLNG EQUIPMENT<br />
AUTOMATIC OIL<br />
OR GAS BURNER<br />
VAPOR LADEN GASES -<br />
VAPOR AND PRODUCT EXHAUST<br />
TEMPERATURE LIMIT<br />
SAFETY CONTROL \<br />
THERMOSTATIC<br />
TEMPERATURE<br />
CONTROL<br />
\ / I<br />
CONTINUOUS BED FRAME ROLLER BEARING MOTORIZED VAPOR AND<br />
PRODUCT<br />
OPTIONAL TRUNNIONS REDUCER<br />
PRODUCTFAN DISCHARGE<br />
Figure 619. A parallel-flow, direct-fired, rotary, fish meal drier<br />
(Standard Steel Corp., Los Angeles. Gal if.).<br />
Figure 620. A stearntube, rotary, fish meal drier (Standard Steel Corp.,<br />
Los Angeles, Calif.).<br />
!<br />
I<br />
I<br />
i
Thesetwo liquids may be processed separately<br />
or blended before their processing. The liq-<br />
uids are first acidified to prevent bacterial de-<br />
composition. Some protein is flocculatdd up-<br />
on the addition of acid. The floc and other<br />
suspended solids are removed in a centrifuge<br />
and recycled to the fish meal reduction process.<br />
Liquids pass through a second centrifuge, where<br />
the fish oils are removed. The water layer is<br />
pumped to multiple-effect evaporators where<br />
the solids content is increased from approxi-<br />
mately 6 to 50 percent by weight. Uncondensed<br />
gases are removed from the process at one of<br />
the evaporator effects, which is operated under<br />
high vacuum. The vacuum is held by a water<br />
or steam ejector. Where steam ejectors are<br />
used they are equipped with barometric-leg<br />
aftercondensers.<br />
DIGESTION PROCESSES<br />
Fish viscera are usually digested by enzymatic<br />
and bacterial action rather than by thermal reduction.<br />
The product is a liquid that is concentrated<br />
by evaporation and marketed as a highproteln<br />
livestock feed supplement very similar<br />
to fish solubles.<br />
Most cannery-operated digestion processes are<br />
of the enzymatic type and are used only to pro-<br />
cess viscera. Stomach enzymes, under con-<br />
trolled pH and temperature, reduce the viscera<br />
to a liquid. The process is u'sually carried out<br />
, in a simple tank at atmospheric pressure, near-<br />
ambient temperature, and an acid pH. Essen-<br />
tially no moisture is evaporated during digestion.<br />
Before concentration, the digested liquid is fil-<br />
tered and centrifuged to remove small quantities<br />
of scales, bones, and oil. The evaporation pro-<br />
cess is identical to that used for fish solubles,<br />
yielding a liquid of 50 percent solids.<br />
Bacterial digestion is used to reduce all types<br />
of fish flesh. It is carried out at an alkaline<br />
pH in equipment similar to that used for en-<br />
zymatic processes. Again, there is no appre-<br />
ciable moisture evaporation, but odors evolved<br />
are considerably stronger and more likely to<br />
elicit nuisance complaints.<br />
THE AIR POLLUTION PROBLEM<br />
<strong>Air</strong> contaminants emanate from a number of<br />
sources in fish canneries and fish reduction<br />
plants, including both edible-rendering and<br />
byproduct processes. Odors are the most ob-<br />
jectionable of these contaminants, though dust<br />
and smoke can be a major problem. In a fish<br />
cannery, some odor is unavoidable owing to<br />
the nature of the species. Heavy odor emis-<br />
Fish Canneries and Fish Reduction Plants<br />
sions that cause nuisance complaints can usu-<br />
ally, however, be traced to poor sanitation or<br />
inadequate control of air contaminants. Tri-<br />
methyl amine, (CH3)3N, is the principal com-<br />
pound identified with fish odors.<br />
Reduction processes produce more odors than<br />
camlery operations do. Materials fed to re-<br />
duction processes are generally in a greater<br />
state of decay than the fish are that are pro-<br />
cessed for human consumption. Edible por-<br />
tions of the fish are always handled first, and<br />
great care is maintained to guarantee the qual-<br />
ity of edible products. The portions that are<br />
unsuitable for human consumption have much<br />
less value, and it is not uncommon for opera-<br />
tors to allow reduction plant feedstocks to<br />
decompose markedly before the processing.<br />
The largest sources of reduction plant odors<br />
are fish meal driers. Lesser quantities of<br />
odors are emitted from cookers preceding<br />
meal driers, from digestion processes, oilwater<br />
separators, and evaporators. Dust<br />
emissions are lxmited to driers and the pneu-<br />
matic conveyors and grinders following them.<br />
Smoke can be created by overheating or burn-<br />
ing meal m the drier.<br />
Odors From Meal Driers<br />
809<br />
Fish meal driers exhaust large vollunes of gases<br />
at significantly large odor concentrations. Dur-<br />
ing the processing of fresh fish scrap, odor con-<br />
centrations in exhaust gases range from 1,000 to<br />
5,000 odor units per scf (see Appendix B for defi-<br />
nition of odor units and method of measuring odor<br />
concentrations). If the feedstocks are hlghly de-<br />
cayed, much greater odor concentrations can<br />
be expected. The result is an extremely heavy<br />
rate of odor emission, even when fresh fish<br />
scrap is processed. For example, a direct-fired<br />
drier operating under "low-temperature " condi-<br />
tions and producing 4 or 5 tons of dried fish meal<br />
per hour exhausts about 30 to 40 million odor<br />
units per minute if the concentration is about<br />
2,000 odor units per scf and the exhaust rate is<br />
15,000 to 20.000 scfm. Drier exit temperatures<br />
average about 200" F, and the moisture content<br />
normally ranges between 15 and 25 percent by<br />
volume.<br />
A more startling example is that of the direct-<br />
fired rotary drier operating under "high-tempera-<br />
ture" conditions. It produces 10 tons of dried<br />
fish meal per hour and exhausts 600 to 800 million<br />
odor units per minute. The odor concentration is<br />
about 40,000 odor units per scf with an exhaust<br />
rate of 15,000 to 20,000 scfm. Drier exit tem-<br />
peratures average above 300" F during high-<br />
temperature operation.
810 CHEMICAL PROCESSING EQUIPMENT<br />
Emissions from steamtube driers are less<br />
water aggravates the problem by lowering the<br />
voluminous and can he less odorous than those temperature, which increases condensation and,<br />
from direct-fired units. With steamtube driers,<br />
there is less likelihood of burning or overheatthereby,<br />
the opacity.<br />
ing the meal and, therefore, excessively heavy<br />
odor concentrations are encountered less often.<br />
Dust From Driers and Conveyors<br />
Moisture contents are comparatively greater<br />
in gases from steamtube driers. Typical gases<br />
from emitted steamtube driers during tuna scrap<br />
processing contain about 25 percent moisture as<br />
compared with approximately 15 percent from<br />
a direct-fired unit processing the same material.<br />
As a result, volumes from steamtuhe driers are<br />
30 to 45 oercent lower than those from com-oar-<br />
The only major points of dust emission in canneries<br />
and reduction plants are the driers themselves<br />
and the grinders and conveyors used to<br />
handle dried fish meal. Driers and pneumatic<br />
conveyors are equipped with cyclone separators,<br />
and emissions are functions of collection efficiencies.<br />
able direct-fired units. Odor concentrations<br />
from steamtuhe driers are generally in the same<br />
range as those from direct-fired units when<br />
fresh fish scrap is being processed under proper<br />
operating conditions, that is, when meal is<br />
not overheated.<br />
Fish meal does not usually contain a large fraction<br />
of fines. A particle size analysis of a typical<br />
meal is provided in Table 218. This meal<br />
sample was collected in a pneumatic conveyor<br />
handling ground fish meal. It can he seen that<br />
the sample contains only 0.6 percent by weight<br />
less than 5 microns in diameter, and 1. 4 per-<br />
Smoke From Driers<br />
Excessive visible air contaminants can be created<br />
in fish meal driers by the overheating of<br />
meal and volatilization of low-boiling oils and<br />
cent less than 10 microns in diameter. Ninetysix<br />
percent is larger than 20 microns.<br />
other organic compounds. Smoke is more likely Table 218. PARTICLE SIZE ANALYSIS OF<br />
to be emitted from direct-fired driers than from<br />
A TYPICAL GROUND,<br />
steam-tube units, particularly if the drier is<br />
operated under high-temperature conditions.<br />
DRIED FISH MEALa<br />
Range of particle diameter, I<br />
IL<br />
,t qo<br />
b<br />
All driers have limits for gas discharge tempera-<br />
tures above which excessive visible contaminants<br />
appear in the elrit gas steam. The smoking lim-<br />
it is a function of drier design as well as of feed-<br />
stocks and varies somewhat from unit to unit.<br />
For direct-fired units, this limit is about 300" F.<br />
Direct-fired driers operated under low-tempera-<br />
ture conditions process fish meal at about one-<br />
half the rate for high-temperature conditions,<br />
and the limit for gas discharge temperature is<br />
about 200" F. Only low-temperature operation<br />
is suitable for control by chlorinator-scrubbers.<br />
Chlorination of a drier exit gas steam which is<br />
in excess of 200' F will predictably produce an<br />
opacity coupled with a corresponding rise in odor<br />
level.<br />
The addition of certain low-boiling materials to<br />
drier feedstocks can also create visible emis-<br />
sions when there is essentially no overheating<br />
of meal in the drier. One such material is di-<br />
gested fish concentrate. Some operators add<br />
this high-protein liquid to drier feedstocks to<br />
upgrade the protein content of meal. Digested<br />
fish concentrate can contain low-boiling com-<br />
pounds that are vaporized into exhaust gases and<br />
condense upon discharge to the atmosphere.<br />
These finely divided, organic, liquid particulates<br />
can impart greater than 40 percent opacities to<br />
drier gases. Scrubbing the drier gases with<br />
0 to 5<br />
5 to 10<br />
10 to 20<br />
20 to 44<br />
44 to 74<br />
74 to 149<br />
149 to 246<br />
246 to 590<br />
590 to 1,651<br />
1, 651 to 2,450<br />
more than 2,450<br />
0.6<br />
0.8<br />
2.6<br />
7.5<br />
11. 5<br />
29. 9<br />
16.4<br />
22.8<br />
7.4<br />
0.4<br />
0.1<br />
asample drawn from a pneumatic conveyor<br />
following a direct-fired drier and hammer<br />
mill.<br />
b~ize determination by micromerograph.<br />
Concentrations of fines in exit gases are usually<br />
less than 0.4 grain per scf. The pneumatic con-<br />
veyor cyclone handling the meal of Table 218 was<br />
found to be better than 99.9 percent efficient,<br />
with an exit dust concentration of less than 0. 01<br />
grain per scf. This efficiency is much greater<br />
than would be predicted on the basis of cyclone<br />
design and particle size. It indicates that ap-<br />
preciable agglomeration probably takes place<br />
in the cyclone.
. .<br />
. .<br />
Odors From Reduction Cookers<br />
Fish Canneries and Fish Reduction Plants 811<br />
condensers, are used to produce the vacuum,<br />
odor emissions to the atmosphere are much<br />
The cookers preceding fish meal driers exhaust greater. Contact condensers (water ejectors)<br />
gases of heavy odor concentration. Nevertheless, provide a dilution of condensate 10 to 20 times<br />
: the volumes of these offgases are appreciablyless greater than that produced by surface-type con-<br />
than those from driers. Cooker gases are simi-. densers used with steam ejectors or vacuum<br />
lar to those from indirectly heated rendering Pumps.<br />
cookers. They consist almost entirely of water<br />
vapor but contain significant quantities of extremely<br />
odorous organic gases and vapors. Odor<br />
Odors From Edibles Cookers<br />
concentrations from live-steam-heated cookers<br />
range from 53 000 to over loo, 000 odor units Per<br />
scf, depending to a large degree upon the state<br />
of feedstocks. Any malodorous gases contained<br />
While most odorous air contaminants are considered<br />
to emanate from fish reduction processes,<br />
the handling and cooking of edible fish also produce<br />
measurable odors. The largest single sources<br />
in the cellular flesh structure are usually liberated<br />
when the material is first heated in the cooker.<br />
are the cookers described earlier in this section.<br />
...<br />
. .~ . .~<br />
~ . . . . .<br />
. . ... . .<br />
Essentially no solids are in the effluent from the<br />
cookers, though some entrained oil particulates<br />
are usually present. The volumes of exhaust<br />
vapors depend upon the degree of sealing provided<br />
in the cooker. All the steam can be contained in<br />
the cooker with no leakage. Most cookers, however.<br />
are desiened - to bleed off 100 to 1. 000 cfm<br />
through one or more stacks. The latter arrangement<br />
is recommended, since it provides a positive<br />
exhaust point at which air contaminants can<br />
be controlled. Otherwise the malodorous gases<br />
would be liberated at the press and grinder where<br />
they are difficult to contain.<br />
The precooked process is less productive of odors<br />
than the wet-fish orocess L - ~ is -~. ~ When - . --....-..-..<br />
~ tvna ~ iq cnnkod<br />
in the live-steam cookers of Figure 615, much of<br />
the odorous gases and vapors is condensed in the<br />
cooker and the steam trap. Only the volatile,<br />
albeit highly odorous, compounds are vented<br />
through the steamtrap.<br />
The hot-exhaust boxes of wet-fish production<br />
systems are commonly vented directly to the<br />
atmosphere. These offgases consist mostly of<br />
steam with some noncondensihle air and malodorous<br />
gases entrained. Hot-exhaust boxes<br />
are the points of initial cooking of wet fish, and<br />
are, therefore, origins of large quantities of<br />
Odors From Digesters<br />
gases and vapors.<br />
The digestion of fish scrap produces only small<br />
volumes of exhaust gases, though these gases<br />
can have a large odor concentration. The en-<br />
zymatic, acid-pH decomposition of viscera<br />
does not normally produce odor concentrations<br />
greater than 20,000 odor units per scf, depend-<br />
ing again upon the quality of feedstocks. Alka-<br />
line digestion of fish scrap, on the other hand,<br />
is productive of strong odors that are likely to<br />
create a public nuisance.<br />
Odors From Evoporotors<br />
The evaporation of the water-soluble extracts--<br />
stick water and press water--does not generally<br />
result in heavy odor emissions. This is pri-<br />
marily due to the use of water ejector-condensers.<br />
Odors could be considerably heavier if different<br />
types of vacuum-producing equipment were em-<br />
ployed. Most fish canneries are located near<br />
large bodies of water,and it is common to use<br />
water jet ejectors to maintain a vacuum on the<br />
evaporator system. All uncondensed gases and<br />
vapors from the evaporators are vented to the<br />
ejectors, which act as contact condensers. Most<br />
of the odorous compounds are condensed or dis-<br />
solved in the effluent water. If steam ejectors<br />
and surface condensers, rather than contact<br />
HOODING AND VENTILATION REQUIREMENTS<br />
When air pollution control is employed, most<br />
fish cannerv and reduction orocesses are vented<br />
directly to the control device. Cookers, presses,<br />
grinders, and the hot press-water auxiliary<br />
equipment require hooding. Hot material from<br />
the cooker evolves appreciable steam and odors<br />
when the oil and water are pressed from it<br />
and when the resultant press cake is broken<br />
up before the drying. The vapors liberated at<br />
these points consist principally of steam. When<br />
the gases are vented to a condenser, hooding<br />
should be as tight as possible to prevent dilu-<br />
tion with air. Indraft velocities of 100 fpm<br />
across the open area under the hood are nor-<br />
mally satisfactory. Where possible, the source<br />
itself should be totally enclosed and ducted to<br />
control equipment. Unfortunately, the designs<br />
of many presses and grinders are not conducive<br />
to complete enclosure, and hoods must be em-<br />
ployed.<br />
The largest contaminated gas streams are ex-<br />
hausted from fish meal driers. As shown in<br />
Table 219, volume rates are lower from steam-<br />
tube driers than from direct-fired units. For<br />
the hypothetical comparison made in this table,
812 CHEMICAL PROCESSING EQUIPMENT<br />
Table 219. CHARACTERISTICS OF EXHAUST tion is preferable if it can be adapted to the pro-<br />
GASES FROM TYPICAL DIRECT-FIRED AND cess. Incineration provides the most positive<br />
STEAMTUBE FISH MEAL DRIERSa control of nuisance-causing - odorous comoounds.<br />
Steamtube<br />
drier .....<br />
~ o ~ evapora~ed s t ~ from ~ meal. ~ scfmb 320 320<br />
Natural gas fuel, sctmC<br />
Moisture in product. of combustion. scfm<br />
Total moisture in exhaust gases, scfm<br />
I6<br />
3 1<br />
ory exhaust gases, scfm<br />
Total exhaust gases, ostm<br />
Moisture content, %by volume<br />
Direct-fir,?d<br />
drier<br />
Temperature of exhaust gases, 'F 180 I 205<br />
a~asis: 1 ton of feed per hour to drier. ~oist~re content of press<br />
cake to drier. 50% by weight.<br />
b~oisture content of dried meal, 8% by weight.<br />
C~atural gas o* I. LOO Bt" per scf gross heating value<br />
the fired drier exhausts 70 percent more gases<br />
than the steamtube drier does and the moisture<br />
content is comparatively less, 15 percent com-<br />
pared with 24 percent. A 10-ton-per-hour fired<br />
drier would exhaust 22,830 scfm at about 200aF,<br />
while a steamtube unit of the same size would<br />
exhaust only 13, 500 scfm at about 180°F.<br />
Exhaust volumes from live-steam-heated cook-<br />
ers range from 100 to 1,000 cfm and depend<br />
to a large degree upon cooker design. Inlet<br />
and exit seals should be tight to prevent leakage.<br />
Most cookers are vented through a single stack.<br />
Digestion tanks with a capacity of 2,000 gallons<br />
or less seldom exhaust more than 50 scfm. Ex-<br />
haust volumes from digesters vary appreciably<br />
during the processing of a batch, exit rates being<br />
negligible much of the time.<br />
Where water ejector contact condensers are em-<br />
ployed on evaporators, exhaust rates are well<br />
below 50 scfm. If surface condensers or vacuum<br />
pumps are employed instead of contact condensers,<br />
exhaust volumes can exceed 100 cfm.<br />
Fish meal pneumatic conveyors are designed to<br />
provide from 45 to 70 cubic feet of air per pound<br />
of meal conveyed. A pneumatic conveyor handling<br />
5 tons of dried meal per hour exhausts about<br />
10,000 cfm.<br />
Exhaust'gases from cookers used in the precooked<br />
tuna process are relatively small in volume and<br />
include only those gases that are not condensed or<br />
dissolved at the steamtrap or the cooker itself.<br />
Gases evolved from the hot-exhaust boxes of the<br />
wet-fish lines are considerably more voluminous.<br />
AIR POLLUTION CONTROL EQUIPMENT<br />
Fish cannery and fish reduction equipment are<br />
controlled principally with condensers, scrubbers.<br />
afterburners. and centrifueal - dust collectors.<br />
Where odors are concerned, incinera-<br />
Condensers are effective where exhaust gases<br />
contain appreciable moisture, while centrifugal .<br />
collectors are usually satisfactory to prevent<br />
excessive dust emissions. Scrubber-chlorina- - --<br />
tors find particular use in the control of odors<br />
from fish meal driers.<br />
Controlling Fish Meal Driers<br />
Because of the exceedingly large volume of mal-<br />
odorous exhaust products from driers, they<br />
cohstitute the most costly air pollution control<br />
problem in a reduction plant. Drier gases nor-<br />
mally contain only 15 to 25 percent moisture.<br />
Thus, even after condensation, the volume is<br />
great. Moreover, there are enough entrained<br />
solids in drier exit gases to make incineration<br />
difficult.<br />
Incinerating Drier Gases<br />
Incineration of odorous air contaminants from<br />
fish meal driers is possible, though costly. It<br />
is, however, the only feasible method presently<br />
available to control driers operated under high-<br />
temperature conditions. This occurs when the<br />
operator processes fish meal at such a rate that<br />
the drier gases emerge at a temperature above<br />
300" F, and the feed scrap is subjected to hot<br />
gases between 1,200" and 1,700" F.<br />
A properly designed afterburner control system<br />
requires a dust collector ahead of the afterburner<br />
to remove solids that cannot readily be burned.<br />
The incineration of solid particulates at 1, 2OO'F<br />
or lower can result in partial oxidation of partic-<br />
ulates, which tends to increase rather than de-<br />
crease odor concentrations. A contact condenser-<br />
scrubber removes much of the difficult-to-burn<br />
particulates and materially reduces the volume<br />
rate by condensing the moisture. If the partic-<br />
ulate matter concentration in gases to the after-<br />
burner is sufficiently small, incineration at<br />
1,200°F reduces odor concentrations to about<br />
50 odor units per scf. Owing to the high cost<br />
of fuel in such an arrangement, few large in-<br />
stallations of afterburners serve fish meal<br />
driers. To make incineration economically at-<br />
tractive, heat from the afterburner should be<br />
reclaimed in some manner. The most likely<br />
arrangement is the preheating of air to the drier.<br />
An afterburner operating at 1,200°F provides<br />
all the heat necessarv to oaerate the drier, which<br />
thus eliminates the need for a firebox.<br />
Chlorinoting and Scrubbing Drier Gores<br />
A unique scrubber-chlorinator design has been<br />
developed to control the odors from fish meal
driers. Units of this design have proved to be<br />
satisfactory, provided the driers are operated<br />
under low-temperature conditions. During lowtemperature<br />
operation, the drier exit gas temperature<br />
is not allowed to exceed 200" F. The<br />
driving force for drying air and products of combustion<br />
is maintained below 6 Btu per scf, and<br />
the temperature at the zone where the fish scrap<br />
enters is maintained below 600" F. Such stringent<br />
conditions placed upon the operation of the<br />
drier cause the production rate to be reduced to<br />
approximately one-half of design if the moisture<br />
content of the dried meal is to be maintained at<br />
an acceptable level. This unit is demonstrated<br />
in the flow diagram - of Figure - 621 and pictured in<br />
Figure 622.<br />
The process depends largely upon the reaction of<br />
chlorine gas with odorous compounds at drier exit<br />
temperatures. As shown in Figure 621, gases<br />
from the drier are first directed through a cyclone<br />
separator to remove fine particulates. Chlorine is<br />
then added at a rate calculated to provide a concentration<br />
of 20 ppm by volume in the gas stream.<br />
The reactionis allowed to ~roceedat about 200" F-the<br />
drier exit temperature--in the ductwork for<br />
approximately 0.6 second before the stream is<br />
chilled and scrubbed with sea water in a packed<br />
tower. Gases pass up through the packing countercurrently<br />
to the sea water.<br />
In Figure 623, odor concentrations from the<br />
scrubber exit are plotted against the chlorine<br />
addition rate at constant gas and sea water<br />
throughput. As can be seen from the curve,<br />
odors reach a minimum at about 20 ppm chlo-<br />
rine. When more than 20 ppm is added, chlo-<br />
Fish Canneries and Fish Reduction Plants 813<br />
rine odors become readily detectable in treated<br />
gases, and odor concentrations tend to increase.<br />
All the odor measurements used to draw this<br />
curve were made on drier gas.samples taken !<br />
between 170" and 205"F, when there was es-<br />
sentially no overheating of meal in the drier.<br />
This method provides an overall odor reduction<br />
of 95 to 99 percent when fresh fish scrap is being<br />
processed in the drier. Chlorination itself pro-<br />
vides a 50 to 80 percent reduction in odor con-<br />
centration. Scrubbing reduces the remaining I<br />
odor concentration by another 50 to 80 percent.<br />
Condensation provides a 12 to 22 percent re-<br />
duction in volume, depending upon the original<br />
moisture content of the gases. i<br />
The exact mechanism of the chlorination reac-<br />
tion is uncertain, but it is assumed that chlorine<br />
reacts with odorous compounds, probably amines,<br />
to form additional products that are less odorous<br />
than the original compounds. Chlorine is not<br />
considered to be a sufficiently strong oxidizing<br />
agent to oxidize fully the odorous organic mate-<br />
rials present in drier gases.<br />
Controlling Reduction Cookers and Auxiliary<br />
Equipment<br />
There is a tendency for the operator to use more<br />
live steam to cook fish scrap before it is fed into<br />
the rotary drier if low-temperature operation of<br />
the drier itself is employed. The entire prepara-<br />
tion of the drier feed is carried out at tempera-<br />
tures above 170nF, and large volumes of contam-<br />
inated steam are released to the atmosphere.<br />
These occur at the cooker, press, grinder, and<br />
press-water screen and sump. They can be con-<br />
-<br />
CHLORINE GbS<br />
Figure 621. A chlorinator-scrubber odor control system venting a fish<br />
meal dr~er.<br />
. ,
814 CHEMICAL PROCESSING EQUIPMENT<br />
Figure 622. A chlorinator-scrubber odor control<br />
system venting a fish meal drier (Star-Kist<br />
Foods, Inc., Terminal Island, Calif.).<br />
trolled by hooding the component parts and ex-<br />
hausting to a condenser followed by an after-<br />
burner. The firebox of the rotary drier may<br />
provide a satisfactory substitute for the after-<br />
burner if adequate control is maintained on the<br />
drier exit gases.<br />
Controlling Digesters<br />
Digester gases are most easily controlled with<br />
afterburners. These gases are small in volume<br />
and require only minimal fuel for incineration.<br />
Digester offgases contain no appreciable moisture<br />
or particulates. Odor concentrations can normal-<br />
ly be reduced by 99 percent or more at 1,200-F<br />
in a properly designed afterburner.<br />
Controlling Evoporotors<br />
Evaporators for stick water, press water, and<br />
digested liquor can be controlled with condensers<br />
and afterburners and combinations thereof. Most<br />
Figure 623. Exit odor concentrations from a chlo-<br />
rinator-scrubber as a function of the chlorine eas<br />
addition rate. Temperatures of gas discharged from<br />
drier are less than 205O~.<br />
evaporators are equipped with water ejector con-<br />
tact condensers to provide the necessary vacuum<br />
in the one effect of the multiple evaporator effects.<br />
Condensate temperatures from these ejectors are<br />
usually less than 80°F. As a result, they con-<br />
dense and dissolve most of the odorous compounds<br />
that would otherwise be discharged to the atmo-<br />
sphere. Condensate cannot be circulated through<br />
cooling towers without causing the emission of<br />
strong odors. Ideally, sea water or harbor<br />
water is used for this purpose, with no recircu-<br />
lation. The entrained air contaminants do not<br />
add enough material to tail waters to create a<br />
water pollution problem.<br />
If water ejectors are not used, odorous air con-<br />
taminants are emitted in much heavier concen-<br />
tration. The most likely alternative is a steam<br />
ejector and surface-type aftercondenser, possi-<br />
bly with multiple ejector stages. Noxious odors<br />
from an operation such as this are stronger and<br />
more voluminous than those emitted from contact<br />
condensers. Hooding of the condenser hot-well<br />
and exhausting to an afterburner operating at<br />
1.200" F or greater is usually the most feasible<br />
means of controlling these processes. Activated<br />
carbon can be used in lieu of an afterburner.
Collecting Dust<br />
As previously noted, fish meal does not contain<br />
a large amount of extremely fine particles, that<br />
is, those less than 10 microns. For this reason,<br />
cyclone separators are normally sufficient to<br />
prevent excessive emissions from the drier and<br />
subsequent pneumatic conveyors. If the meal<br />
from a particular plant were to contain appre-<br />
ciably more fine material than the sample shown<br />
in Table 218, more efficient dust collectors,<br />
such as small-diameter, multiple cyclones or<br />
baghouses, would have to be used.<br />
Controlling Edible-Fish Cookers<br />
Exhaust gases from both precooked and wet-fish<br />
process cookers consist essentially of water<br />
vapor. At tuna cookers, most of this vapor is<br />
condensed in the steamtraps on the cookers. If<br />
further control is desired, an afterburner,<br />
carbon adsorber, or low-temperature contact<br />
condenser is recommended.<br />
Reduction of Inedible Anlrnal Matter 815<br />
which are operated solely for the byproducts.<br />
In Figure 624, "deadstock" is shown awaiting<br />
dismemberment at a scavenger plant. Com-<br />
mon rendering cooker feedstocks are pictnred<br />
in Figure 625. In general, the materials pro-<br />
cessed in captive packing house systems are<br />
fresher than those handled at scavenger plants<br />
where feedstocks can be highly decayed. Typ-<br />
ical slaughterhouse yields of inedible offal,<br />
bone, and blood are listed in Table 220.<br />
The hot-exhaust boxes of wet-fish processes<br />
represent large odor sources that can be controlled<br />
with contact condensers, often at little<br />
expense to the operator. Most canneries are<br />
located near large bodies of water. Sea water<br />
or harbor water can be directed to contact condensers<br />
at little cost in these instances. Since<br />
exhaust box - eases are orincioallv water, there<br />
& ,<br />
is a marked reduction in volume across a condenser<br />
such as this, in addition to a decrease Figure 624. Dead stock awaiting skinning and dismemberment<br />
at a scavenger renderine plant (Califorin<br />
odor concentration.<br />
nia Rendering Co.,Ltd.. Los Angeles, Calif.).<br />
REDUCTION OF INEDIBLE<br />
ANIMAL MATTER<br />
Animal matter not suitable as food for humans<br />
or pets is converted into salable byproducts<br />
through various reduction processes. Animal<br />
matter reduction is the principal waste disposal<br />
outlet for slaughterhouses, butcher shops,<br />
poultry dressers, and other processors of<br />
flesh foods. In addition, it is used to dispose<br />
of whole animals such as cows, horses, sheep,<br />
poultry, dogs, and cats that have died through<br />
natural or accidental causes. If it were not<br />
for reduction facilities, these remains would<br />
have to be buried to prevent a serious health<br />
hazard. The principal products of reduction<br />
processes are proteinaceous meals, which find<br />
primary use as poultry and livestock feeds, and<br />
tallow.<br />
Much reduction equipment is operated in meat-<br />
packing plants to handle only the "captive" blood,<br />
meat, and bone scrap offal produced on the<br />
premises. Other reduction cookers and driers<br />
are located in scavenger rendering plants,<br />
Figure 625. Inedible animal matter in the receiving<br />
pit of a rendering system (California Rendering Co.,<br />
Ltd., Los Angeles, Calif.).
816 CHEMICAL PROCESSING EQUIPMENT<br />
Table 220. INEDIBLE, REDUCTION PROCESS<br />
RAW MATERIALS ORIGINATING<br />
FROM SLAUGHTERHOUSES<br />
(The Globe Co., Chicago, Ill. )<br />
Source, lb live wt<br />
Steers, I, 000<br />
Cows<br />
Calves, 200<br />
Sheeo . . 80<br />
Hogs, 200<br />
Inedible offal and bone,<br />
lblhead<br />
90 to 100<br />
110 to 125<br />
15 to 20<br />
(I to 10<br />
10 to 15<br />
Blood,<br />
Iblhead<br />
55<br />
55<br />
5<br />
4<br />
7<br />
The animal mstter reduction industry has been<br />
traditionally considered one of the "offensive<br />
trades. " The reputation is not undeserved.<br />
Raw materials and process exhaust gases are<br />
highly malodorous and capable of eliciting nuisance<br />
complaints in surrounding areas. In<br />
recognition of these facts, specific air pollution<br />
control regulations have been enacted requiring<br />
the control of odorous process vapors.<br />
BATCHWISE DRY RENDERING<br />
The most widely used reduction process is dry<br />
rendering, wherein materials containing tallow<br />
are heated indirectly, usually in a steam-jac-<br />
keted vessel. Heat breaks down the flesh and<br />
bone structure, allowing tallow to separate<br />
from solids and water. Ln the process, most<br />
of the moisture is evaporated. Emissions con-<br />
sist essentially of steam with small quantities<br />
of entrained tallow, solids, and gases.<br />
Dry rendering mav be performed batchwise or<br />
continuously and may be accomplished at pressures<br />
greater or less than atmospheric. A<br />
typical batch-type, steam-jacketed, dry rendering<br />
cooker is shown in Figure 626. These vessels<br />
are normally charged with 3,000 to 10,000<br />
pounds of animal matter per batch. The cookers<br />
are equipped with longitudinal agitators that are<br />
driven at 25 to 65 rpm. Each batch is cooked<br />
for 314 to 4 hours.<br />
Rendering is a specific heated reduction<br />
process wherein fat-containing materials are<br />
reduced to tallow and proteinaceous meal.<br />
Blood drying, feather cooking, and grease reclaiming<br />
are other reduction operations usually<br />
performed as companion processes in rendering<br />
plants.<br />
Pressures of 50 psig and greater are used to<br />
digest bones, hooves, hides, and hair. At the<br />
resulting temperature (about 300°F), these<br />
materials are reduced to a pulpy mass. In typical<br />
dry-pressure-rendering cycles, the cooker<br />
vent is initially closed to cause pressure and<br />
temperature to increase. Some materials are<br />
cooked as long - as 2 hours at elevated pressure<br />
Reduction processes are influenced largely by<br />
the makeup of feedstocks. As can be seen from<br />
Table 221, some materials, such as blood and<br />
feathers, are essentially grease free, while<br />
to obtain the necessary digestion. After pressures<br />
are reduced, the batch is cooked or dried<br />
to remove additional moisture and to complete<br />
tallow-solids separation.<br />
others contain more than 30 percent tallow. Some dry rendering operations are carried out<br />
Where no tallow is present, the reduction pro- under vacuum to remove moisture rapidly at<br />
cess becomes primarily evaporation with, possi- temperatures sufficiently low to inhibit degradahly,<br />
some thermal digestion, tion of products. Vacuum rendering processes<br />
Table 221. COMPOSITION OF TYPICAL INEDIBLE RAW MATERIALS<br />
CHARGED TO REDUCTION PROCESSES<br />
(The Globe Co., Chicago, Ill. )<br />
Source<br />
Packlng house offal and bone<br />
Steers<br />
Cows<br />
Calves<br />
Sheep<br />
Hogs<br />
Dead stock (whole anlmals)<br />
Cattle<br />
Cows<br />
Sheep<br />
Hogs<br />
Blood<br />
Feathers (from poultry houses)<br />
Butcher shop scrap<br />
Tallow or grease,<br />
wt %<br />
15 to 20<br />
10 to 20<br />
8 to 12<br />
25 to 35<br />
15 to 20<br />
12<br />
8 to 10<br />
22<br />
3 0<br />
.<br />
3 7<br />
Solids,<br />
wt %<br />
30 to 35<br />
20 to 30<br />
20 to 25<br />
20 to 25<br />
18 to 25<br />
25<br />
23<br />
2 5<br />
25 to 30<br />
12 to 13<br />
20 to 30<br />
25<br />
Moisture,<br />
wt %<br />
45 to 55<br />
50 to 70<br />
60 to 70<br />
45 to 55<br />
55 to 67<br />
6 3<br />
67 to 69<br />
5 3<br />
40 to 45<br />
87 to 88<br />
70 to 80<br />
3 8
are essentially all of the batch type. Thevacu-<br />
um is usually produced with a precondenser,<br />
steam ejector, and aftercondenser. Cooker<br />
pressures are close to atmospheric at the start,<br />
then diminish markedly as the moisture content<br />
of the charge decreases. Vacuum rendering<br />
produces high-quality tallow but has a disadvan-<br />
tage in that ternperatures are low and incomplete<br />
cooking of bones, hair, and so forth, may occur.<br />
CONTINUOUS DRY RENDERING<br />
Because of the increased demand for processing<br />
meat, bone, and offal, highly mechanized con-<br />
tinuous dry rendering processes are used today.<br />
The advantages readily become apparent when it<br />
is shown that one operator can process 1 - 114<br />
million pounds of material in 16 hours. It would<br />
Reduction of Inedible Animal Matter 817<br />
Figure 626. A horizontal, batch-type, dry-rendering<br />
cooker equipped with a charging elevator (Standard<br />
Steel Corp., Los Angeles, Calif.).<br />
otherwise take 40 batch cookers and an undeter-<br />
mined crew of men to produce the same volume<br />
of throughput. Some processes consist essen-<br />
tially of a series of grinders, steam-jacketed<br />
conveyor-cookers, and presses. The continuous<br />
system of Figure 627 uses recycle tallow and a<br />
vertical-tube vacuum cooker. Selected meat and<br />
bone scrap is ground and slurried withhottallow<br />
before being charged to the cooker. Slurry is<br />
circulated through the tubes, and vapors are<br />
vented to a contact condenser. Steam is con-<br />
densed ahead of the ejector, and a barometric<br />
leg is employed. Tallow and solids are continu-<br />
ously withdrawn from the bottom of the cooker.<br />
In another continuous system, material is con-<br />
veyedto ahasher or hogger before it is fed to the<br />
first cooker. Here the temperature is brought<br />
up to approximately 180" F. Then the material is<br />
fed to a high-speed hammer mill or blender be-<br />
fore it is conveyed to the next cooker. The mate-<br />
rial is brought up to a temperature of about 21 0" F<br />
in the second cooker and then continuously con-<br />
veyed to a stacked set of finishing cookers. The<br />
finishing cookers consist of long jacketed continu-<br />
ous-tube screw conveyors where the material is<br />
brought up to 275" F before it is discharged onto<br />
a screen or into a prepresser. The prepresser,<br />
a press with extra spacing, gently squeezes the<br />
crax before it is fed to a press, or many presses<br />
in parallel. The press-cake is ground in a high-<br />
speed hammer mill and emerges as a meat meal<br />
product. Pressed and free-running tallow is<br />
settled, centrifuged, bleached, dried, and fil-<br />
tered to a premium grade tallow.<br />
Another continuous process consists of a hasher<br />
and one large continuous-tube cooker without
818 CHEMICAL PROCESSING EQUIPMENT<br />
FLUlDlllNG TANK<br />
TRAMP METAL OISCHhRGF<br />
WATER SUPPLY<br />
STEAM SUPPLY<br />
BAROMETRIC CONDENSER STEhM SUPPLY<br />
FIUIOIIINO PUMP<br />
KlTER OISCHIIROE<br />
-AIR IN0 NUN CONOENSIBLE EJECTOR<br />
EAM CONOENSAI<br />
,. Y'8 . .<br />
FIT OISCIARGE 1<br />
STORAGE IANK<br />
Figure 62i. A continuous, vacuum rendering system employing tallow recycl ing (Carver-Greenf ield<br />
Process, The V.D. Anderson Co., Cleveland, Ohio).<br />
finishing cookers. The material is brought to<br />
250" to 300" F before being discharged to the<br />
prepresser. The feed rate to a unit without<br />
finishing cookers must necessarily be adjusted<br />
to allow more residence time in the single cooker.<br />
Another variation in the finishing cycle involves<br />
the use of a multiple-effect evaporator. The<br />
feed rate through the continuous-tube cooker may<br />
be approximately doubled and the material<br />
emerges half-cooked at approximately 210" to<br />
220" F. The steam driven off from the half-<br />
cooked material goes to an evaporator, where<br />
the remainder of the moisture is cooked off at a<br />
temperature of approximately 190" F and a<br />
vacuum of approximately 27 inches of water<br />
column.<br />
WET RENDERING<br />
One of the oldest reduction methods is the wet<br />
process, wherein animal matter is cooked in a<br />
closed vessel with live steam. There is little<br />
evolution of steam. Most of the contained mois-<br />
ture is removed as a liquid. Live steam is fed<br />
to a charge in a closed, vertical kettle until the<br />
internal pressure reaches approximately 60 psig<br />
(about 307°F). Heat causes a phase separation<br />
of water, tallow, and solids. After initial cook-<br />
ing, the pressure is released, and some steam<br />
is flashed from the system. The charge is then<br />
cooked at atmospheric pressure until tallow<br />
separation is complete. Water, tallow, and<br />
solids are separated by settling, pressing, and<br />
centrifuging.<br />
The water layer from a wet rendering process<br />
contains 6 to 7 percent solids. Soluble proteins<br />
can be recovered by evaporation, as in the<br />
processing of stick water at fish reduction<br />
plants.<br />
Wet rendering finds some use today in the han-<br />
dling of dead stock, namely whole animals that<br />
have died through accidents or natural causes.<br />
It has given way to dry rendering at most pack-<br />
ing houses and scavenger plants. Wet rendering<br />
is used to a limited degree in the production of<br />
edible fats and oils, as noted previously in this<br />
chapter.
REFINING RENDERED PRODUCTS<br />
At the completion of the cook cycle, tallow and<br />
sblids are run through a series of separation<br />
equipment as in the integrated plant of Figure<br />
628. Some systems are more complex than<br />
others, but the essential purpose is to produce<br />
dry, proteinaceous cracklings and clear, mois-<br />
ture-free tallow. In almost all cases, the cook-<br />
ers are discharged into perforated percolator<br />
pans that allow free-running tallow to drain from<br />
hot solids. The remaining solids are pressed to<br />
remove residual tallow. Dry cracklings are usu-<br />
ally ground to a meal before being marketed. In<br />
Figure 629, grease-laden cracklings are being<br />
dumped from a percolator pan after free tallow<br />
has been drained.<br />
Tallow from the percolators and presses is<br />
further treated to remove minor quantities of<br />
solids and water. Solids may he removed in<br />
desludging centrifuges, filters, or settling tanks.<br />
Traces of moisture are often removed from it<br />
by boiling or blowing air through heated tallow.<br />
Some operators remove moisture by settling in<br />
cone-bottom tanks, often with the aid of soda<br />
ash or sulfuric acid to provide better phase<br />
separation.<br />
Reduction of Inedible Animal Matter 819<br />
Belgian De Smet process, in which hexane is<br />
employed, has been adopted by some renderers<br />
in the United States and Canada. The entire<br />
process is enclosed in a vaportight building to<br />
minimize the explosion hazard. After extrac-<br />
tion, hexane is stripped from tallow and solids.<br />
The only measurable air contaminants, solvent<br />
vapors, are vented at one or more condensers.<br />
DRYING BLOOD<br />
Animal blood is evaporated and thermally di-<br />
gested to produce a dry meal used as a fertiliz-<br />
er, as a livestock feed supplement, and, to a<br />
limited degree, as a glue. Blood contains only<br />
10 to 15 percent solids and essentially no fat.<br />
At most packing houses, it is dried in horizontal,<br />
dry rendering cookers. In typical slaughtering<br />
operations, blood is continually drained from<br />
the kill floor to one or more cookers, throughout<br />
the day. Initially, while there is appreciable<br />
moisture in the blood, heat transfer through the<br />
jacket is reasonably rapid. As the moisture<br />
content decreases, however, heat transfer be-<br />
comes slower. During the final portion of the<br />
cycle, drying is extremely slow, and dusty meal<br />
can be entrained in exit gases.<br />
In some instances, solvents are used to extract In some instances, a tubular evaporator is used<br />
tallow from rendered solids. Solvent extraction to remove the initial portion of the water. When<br />
allows extremely fine control of products. he the moisture content decreases to about 65 per-<br />
Figure 628. An integrated dry rendering plant equipped with batch<br />
cookers, percolators, a crack1 ings press, and a tal low-settl ing tank.
820 CHEMICAL PROCESSJNG EQUIPMENT<br />
Figure 629. Tallow-laden crack1 ings being dum~ed<br />
from a percolator after free tallow has been allowed<br />
to drain (California Render~ng Co.,<br />
California).<br />
Ltd., Los Angeles,<br />
cent, the material is transferred to a dry ren-<br />
dering cooker for final evaporation.<br />
Continuous dry rendering is also used to process<br />
blood into blood meal. The raw or green blood<br />
is screened and air blown, then fed to a continu-<br />
ous coagulator where the blood is "set" with live<br />
steam. It then goes to a separator, where serum<br />
is removed from the coagulated blood. The co-<br />
agulated blood is then fed into a recycling hot-air<br />
drying system called a Ring drier. The Ring<br />
drier system consists of a feed port and hammer<br />
mill where the coagulated blood is fed into the<br />
system. It is pulverized in an atmosphere of<br />
180" to 200" F and blown to the manifold where<br />
it meets the air from the furnace. The furnace<br />
gases of 600" to 800" F provide the make-up air<br />
to the system and enter at the control manifold.<br />
Control dampers are located in the manifold and<br />
regulate the recirculation rate of the material as<br />
well as the product take-off ratio. A cyclone col-<br />
lecting system also is ducted to the control mani-<br />
fold and driven by an exhaust fan at the outlet.<br />
Product is withdrawn through star valves located<br />
in the solids discharge leg of the cyclones.<br />
Some animal blood is spray dried to produce<br />
a plywood glue that commands a price con-<br />
siderably higher than that of fertilizer or live-<br />
stock feed. This is an air-drying process, and<br />
exhaust gases are markedly more voluminous<br />
than those of rendering equipment. Feedstocks<br />
are usually concentrated in an evaporator be-<br />
fore the spray drying.<br />
PROCESSING FEATHERS<br />
Poultry feathers are pressure cooked and sub-,<br />
sequently dried to produce a high-protein meal<br />
used principally as a poultry feed supplement.<br />
Feathers, like blood, contain practically no fat,<br />
and meal is the only product of the system.<br />
Feathers are pressure cooked at about 50 psig<br />
to hydrolyze the protein keratin, their principal<br />
constituent. Initial cooking is usually carried<br />
out in a dry rendering cooker. Final moisture<br />
removal may be accomplished in the cooker at<br />
ambient pressure or in separate air-drying<br />
equipment. Rotary steamtube air driers, such<br />
as that shown in Figure 630, are frequently<br />
used for this purpose. If separate driers are !<br />
employed, the material is transferred from<br />
cooker to drier at a moisture content of about<br />
50 percent.<br />
The Ring drier described under "Drying Blood''<br />
can also be used to dry feather meal. However,<br />
continuous dry rendering of poultry feathers still<br />
appears to be in the pilot stage. The pressure<br />
required to break down the feathers requires<br />
choking in the continuous-tube cookers. Another<br />
problem is the lack of free-running tallow for<br />
heat transfer. Economically, although a large<br />
continuous supply of feed feathers is available,<br />
continuous rendering does not show the tremendous<br />
advantage for feathers that it does for meat<br />
and bone for tallow and meat meal because of the<br />
relatively lower price for feather meal.<br />
ROTARY AIR DRIERS I<br />
Dlrect-fired rotary driers are seldom used in<br />
the reduction of inedible packing house waste<br />
or dead stock. As noted previously in this<br />
chapter, they find wide use in the reduction of<br />
fish scrap. Fired driers have been used to a<br />
limited degree to dry wet rendering tankage<br />
and some materials of low tallow content.<br />
Where air driers are required, steamtube<br />
units are generally more satisfactory from the<br />
standpoint of both ~roduct quality and odor emission.<br />
THE AIR POLLUTION PROBLEM<br />
Malodors are the principal air contaminants<br />
emitted irom inedible-rendering equipment and<br />
from other heated animal matter reduction pro-<br />
cesses, Reduction plant odors emanate from<br />
the handling and storage of raw materials and<br />
products as well as from heated reduction pro-<br />
cesses. Some feed materials are highly decayed,<br />
even before delivery to scavenger rendering<br />
plants, and the grinding, conveying, and storage<br />
of these materials cannot help but generate some<br />
malodors. Cooking and drying processes are,<br />
I
SIDE ELEVATION SHOW1 NG<br />
ARRANGEMENT OF TUBES<br />
Reduction of Inedible Animal Matter<br />
FRONT ELEVATION SHOWING STEAM FLOW<br />
CUTAWAY OF PIPES<br />
Figure 630. A rotary steamtube air drier of the type commonly<br />
used for the continuous drying of cooked feathers (The V.D.<br />
Anderson Co.. Cleveland, Ohio)<br />
nevertheless, considered the largest odor sources,<br />
and most odor control programs have been di-<br />
rected at them. Handling and storage odors can<br />
usually be kept to a tolerable minimum by fre-<br />
quently washing working surfaces and by pro-<br />
cessing uncooked feedstocks as rapidly as possible.<br />
McCord and Witheridge (19491, who discuss<br />
the "offensive trades" at length, attribute<br />
rendering plant malodors to a variety of com-<br />
pounds. Ronald (1935) identifies rendering<br />
odors as principally ammonia, ethylamines,<br />
and hydrogen sulfide, all decomposition products<br />
of proteins. Skatole, other amines, sulfides,<br />
and mercaptans are also usually present. Tallow<br />
and fats do not generate as great quantities of<br />
odorous materials. Aldehydes, organic acids,<br />
and other partial oxidation products are the<br />
principal odorous breakdown products of fats.<br />
Putrescine, NH2 (CH2)4 NH2, and cadaverine,<br />
NH2(CH2)5NH2, are two extremely malodorous<br />
diamines associated with decaying flesh and<br />
rendering plants. Several specific compounds<br />
have extremely low odor thresholds and are de-<br />
tectable in concentrations as small as 10 parts<br />
per billion (ppb). Odor threshold concentra-<br />
tions of compounds are listed in Table D2,<br />
Appendix D. Many suspected compounds have<br />
not been positively identified nor have their<br />
odor thresholds been determined.<br />
821
822 CHEMICAL PROCESSING EQUIPMENT<br />
Cookers As Prominent Odor Sources Odors From <strong>Air</strong> Driers<br />
When animal matter is subjected to heat, the<br />
cell structure breaks down liberating volatile<br />
gases and vapors. Further heating causes some<br />
chemical decomposition, and the resultant prod-<br />
ucts are often highly odorous. All these mal-<br />
odorous gases and vapors are entrained in ex-<br />
haust gases.<br />
Exhaust products from cooking processes con-<br />
sist essentially of steam. Entrained gases and<br />
vapors are, nevertheless, highly odorous and<br />
apt to elicit nuisance complaints in areas sur-<br />
rounding animal matter reduction plants. Odor<br />
concentrations measured in exhaust gases of<br />
typical reduction processes are listed in Table<br />
222. Evidently there is a wide variation in<br />
odor concentrations from similar equipment.<br />
For instance, dry-batch rendering processes<br />
range from 5,000 to 500,000 odor units per scf,<br />
depending principally upon the type and "ripe-<br />
ness" of feedstocks. Blood drying can be even<br />
more odorous, with concentrations as great as<br />
1 million odor units per scf if the blood is allowed<br />
to age for only 24 hours before processing. High-<br />
ly odorous steam emissions also are generated<br />
whenever fresh feed material is dropped into a<br />
hot cooker. Approximately 4 hours rest time<br />
would be required between loads to prevent fresh<br />
material from being distilled during the loading<br />
operation.<br />
Source<br />
-<br />
Renderine cooker.<br />
dry-batch typeb ' 1<br />
Blood cooker,<br />
dry-batch type b<br />
Feather drier,<br />
s teamtuheC<br />
Blood spray<br />
drierc'<br />
Grease-drying tank,<br />
air blowing<br />
156°F<br />
170°F<br />
2Z5'F<br />
As can be seen from Table 222, feather drier<br />
odor concentrations, though generally smaller,<br />
are more variable than those from rendering<br />
cookers. Their largest odor concentrations--<br />
25,000 odor units per scf--are associated with<br />
operations where feedstocks are putrefied or<br />
not completely cooked beforehand or where the<br />
meal is overheated in the drier. Under optimum<br />
conditions, odor concentrations from these driers<br />
should not exceed 2,000 odor units per scf. With<br />
blood spray driers, where extreme care is main-<br />
tained to ensure freshness of feedstocks, con-<br />
centrations can be less than 1,000 odor units per<br />
scf. In general, air drier odor concentrations<br />
are less than those of cookers for the following<br />
reasons: (1) In most instances feedskocks are<br />
cooked or partially evaporated before the air<br />
drying; (2) odorous gases are more dilute in<br />
drier exit gases; (3) feedstocks are often fresher.<br />
Odors and Dust From Rendered-Product Systems<br />
Some odors and dust are emitted from cooked<br />
animal matter as it is separated and refined.<br />
The heaviest points of odor emission are the<br />
percolators into which hot cooker contents are<br />
dumped. Steam and odors evolve from the hot<br />
material, particularly during times of cooker<br />
unloading. Cookers are normally dumped at or<br />
near 212°F.<br />
Table 222. ODOR CONCENTRATIONS AND EMISSION RATES FROM<br />
INEDIBLE REDUCTION PROCESSES<br />
Odor concentration,<br />
odor unit/scf<br />
Range ITypical average<br />
Typical moisture<br />
content of<br />
feeding stocks, 70<br />
5. 000 to I 50,000<br />
50<br />
500,000 1 I I<br />
10,000 to<br />
1 million<br />
600 to<br />
25, 000<br />
600 to<br />
1,000<br />
100,000<br />
2,000<br />
aAssumi~~g 5 percent moisture in solid products of system.<br />
b~oncondensable gases are neglected in determining emission rates.<br />
CExhaust gases are assumed to contain 25 percent moisture.<br />
d~lood handled in spray drier before any appreciable decomposition. occurs.<br />
800<br />
4, 500<br />
15,000<br />
60,000<br />
90<br />
5 0<br />
6 0<br />
< 5<br />
Exhaust products,<br />
scf/ton of feeda<br />
20.000<br />
38, 000<br />
77,000<br />
100,000<br />
100 scfm<br />
per tank<br />
Odor emission<br />
rate, odor unit/<br />
ton of feed
Table 223 shows odor threshold concentrations of<br />
emissions from cookers during the dumping oper-<br />
ation. The type of material being rendered has a<br />
great effect on the odor concentration. As indi-<br />
cated, meat and bone trimmings from restaurants,<br />
which contain large quantities of rancid grease.<br />
produce high odor concentrations which would<br />
require hooding and incineration in an afterburner.<br />
Entrails from turkeys and chickens would be bor-<br />
derline, as shown in the table. The type of mate-<br />
rial, quantity of tallow or grease in the batch,<br />
freshness of the material, and unloading tempera-<br />
tures all are factors which influence the odor con-<br />
centration. Each rendering operation should be<br />
sampled for odors before deciding whether or not<br />
air pollution control equipment is required for<br />
the dump operation.<br />
Odorous steam emissions and smoke generated<br />
by the presses usually require control. Centri-<br />
fuges and settling tanks where meal and tallow<br />
are heated to accomplish the desired separation<br />
also emit odorous steam.<br />
I The grinding of pressed solids, and subsequent<br />
meal conveying are the only points of dust emis-<br />
! sion from rendering systems. These particulates<br />
1 are reasonably coarse, and dust is usually not<br />
1 excessive.<br />
i Grease-Processing Odors<br />
I<br />
!<br />
I<br />
!<br />
, .<br />
Some odors are generated at processing tanks<br />
when moisture is removed from grease or tallow<br />
by boiling or by air blowing or both. If air is<br />
used for this purpose, exhaust volumes seldom<br />
Reduction of Inedible Animal Matter 823<br />
exceed 100 scfm, but odor concentrations are<br />
measurable. Odor concentration is a function<br />
of operating temperature. As shown in Table<br />
222, measured concentrations have been found<br />
to range from 4,500 odor units per scf at 150" F,<br />
to 60,000 odor units per scf at 225" F. Odor<br />
concentrations vary greatly with the type of<br />
grease processed and the air rate, as well as<br />
with temperature.<br />
Row-Materials Odors<br />
Some malodors emanate from the cutting and<br />
handling of raw materials. In most instances<br />
these emissions are not great. Odors usuaiiy<br />
originate at the point where raw material is<br />
first sliced, ground, or otherwise broken into<br />
smaller parts. Most feedstocks are ground in<br />
a hammer mill before the cooking. Large,<br />
whole animals (dead stock) must be skinned,<br />
eviscerated, and at least partially dismembered<br />
before being fed to rendering equipment. If the<br />
animal is badly decomposed, this skinning and<br />
cutting operation can evolve strong odors.<br />
HOODING AND VENTILATION REQUIREMENTS<br />
All heated animal matter reduction processes<br />
should be vented directly to control equipment.<br />
Hooding is used in some instances to collect<br />
malodors generated in the processing of raw<br />
materials and cooked products.<br />
The loading of material into a hot batch cooker<br />
generates highly odorous steam emissions. It is<br />
impractical to require a cooker to be cooled be-<br />
Table 223. ODOR CONCENTRATIONS OF EMISSIONS FROM INEDIBLE RENDERING COOKERS<br />
DURING THE DUMPING OPERATION<br />
Poultry feathers<br />
Type of material cooked<br />
Entrails from turkeys and chickens<br />
Meat and bone trimmings with large quantities<br />
of rancid restaurant grease<br />
Emissions during<br />
dump from cooker.<br />
odor unitslcf<br />
200<br />
2,000<br />
25,000<br />
40,000<br />
Fresh meat and bone trimmings from a beef 100<br />
slaughterhouse<br />
Mixture of dead cats and dogs, fish scrap,<br />
poultry offal, etc.<br />
Slaughterhouse viscera and bones<br />
a<br />
x = 7 minutes.<br />
b<br />
x = 18 minutes.<br />
1,000<br />
150<br />
200<br />
Emissions 5<br />
minutes after<br />
dumping,<br />
odor units/cf<br />
2 0<br />
500<br />
3,000<br />
200<br />
7 0<br />
1,500<br />
150<br />
Emissions x<br />
minutes after<br />
dumping,<br />
odor unitslcf<br />
-<br />
.<br />
-<br />
800a<br />
150b
824 CHEMICAL PROCESSING EQUIPMENT<br />
fore receiving the next charge. One method to<br />
prevent escape of air contaminants during the<br />
loading of a cooker is to provide a choked feed<br />
arrangement. A hopper may be constructed above<br />
the cooker that holds one full load. The material<br />
is dumped quickly and there is enough mass of<br />
material to prevent steaming. Another method<br />
is to blow the material into the cooker through a<br />
closed feed system.<br />
The methods described above, unfortunately, are<br />
not feasible for loading poultry feathers because<br />
of their density and texture. Grinding the feathers<br />
beforehand has not proven to be satisfactory. A<br />
successful method to control the loading of feathers,<br />
however, has been developed. The feather cooker<br />
is provided with a separate dome and vent at the<br />
drive end. Both exhaust systems may be valved<br />
at the dome. An indraft of 100 fpm through the<br />
charge door is usually adequate. The exhaust<br />
system used for cooking must be closed during<br />
the loading cycle and vice ver,sa.<br />
If highly decayed dead stock is being processed,<br />
the entire dead stock room should be ventilated<br />
at a rate of 40 or more air changes per hour for<br />
worker comfort. Areas should also be ventilated<br />
where raw materials are stored unrefrigerated<br />
for any appreciable time before processing.<br />
Hooding may be employed on raw-material<br />
grinders preceding cookers and percolator<br />
pans and expeller presses used to handle<br />
cooked products. Although the volume of<br />
steam and odors evolved at any of these points<br />
does not exceed 100 cfm, greater volumes are<br />
normally required to offset crossdrafts. In-<br />
draft velocities of 100 fpm under hoods are usu-<br />
ally satisfactory.<br />
Emission Rates From Cookers<br />
The ventilation rates of cookers can be esti-<br />
mated directly from the quantity of moisture<br />
removed and the time of removal. Maximum<br />
emission rates from dry cookers are approxi-<br />
mately twice the average moisture evaporation<br />
rates. In the determination of exhaust volumes,<br />
noncondensablegases can normally be neglected.<br />
Consider a batch cooker that removes 3, 000<br />
pounds of moisture from 6,000 pounds of animal<br />
matter in 3 hours, a relatively long cook cycle.<br />
The average rate of emission is 16.7 pounds<br />
per minute or 450 cfm steam at about 212°F.<br />
The instantaneous evaporation rate and cumula-<br />
tive moisture removal are plotted in Figure<br />
631. The maximum evolution rate apparently<br />
occurs near the initial portion of the cook at<br />
29 pounds per minute or 790 cfm at ZlZ'F. As<br />
moisture is removed from a batch cooker, the<br />
heat transfer rate decreases, the temperatures<br />
Figure 631. Steam emission pattern f ram a<br />
batch-type, dry render~ng cooker operated<br />
at ambient pressure.<br />
rise, and the evaporation rate falls off. The<br />
general shapes of the curves in Figure 631 are<br />
typical of batch-cooking cycles. Where cook<br />
times are appreciably shorter, evaporation<br />
rates are greater; nevertheless, the ratio of<br />
maximum to average evaporation rate is main-<br />
tained at approximately 2 to 1.<br />
The length of a cooking cycle, and the evapo-<br />
ration rate are dependent upon the temperature<br />
in the steam jacket, and the rotational speed<br />
of the agitator. The highest permissible agita-<br />
tor speeds (about 65 rpm) can result in cooking<br />
times of 45 minutes to 1 hour. Many operators,<br />
particularly at packing houses, use slower<br />
agitator speeds, and cycles are as long as 4<br />
hours.<br />
If vacuum cooking is employed, volume rates<br />
and temperatures decrease as the batch pro-<br />
gresses. With these systems, the vacuum-<br />
producing devices largely govern cooking times.<br />
The evaporation rate in a vacuum system is lim-<br />
ited by the rate at which steam can be removed,<br />
usually by condensation. If vapor cannot be<br />
condensed as fast as it is evaporated, the cycle<br />
is merely lengthened.<br />
Pressure cookers have a slightly dsferent emis-<br />
sion pattern, but maximum emission rates are<br />
again twice the average. During the initial<br />
portion of the cycle, there are no emissions<br />
while pressures are increasing to the desired<br />
maximum. The cooker is vented at elevated
pressure, usually about 50 psig. High-pres-<br />
sure vapors are relieved through small bypass<br />
lines so that the surge of steam is not more than<br />
the control system can handle. Most of the con-<br />
tained moisture is evaporated after pressures<br />
are reduced to ambient levels.<br />
Vapor emission rates from wet rendering cook-<br />
ers are considerably lower than those from dry<br />
cookers, comparable to initial volumes during<br />
pressure cooking. Only enough steam is flash<br />
evaporated to reduce the pressure to 1 atmos-<br />
phere. The large percentage of moisture in<br />
a wet rendering process is removed as water<br />
by physical separation rather than by evapora-<br />
tion.<br />
Emission rates from continuous, dry rendering<br />
processes are steady and can be calculated<br />
directly from the moisture content of feedstocks<br />
and products. To lower the moisture content<br />
from 36 to 6 percent in typical meat, bone, and<br />
offal scrap, 2,750 scfm or 110 pounds of steam<br />
per minute would be evaporated if the charge<br />
rate to the cooker were 20,000 pounds per hour.<br />
Since it is difficult to operate continuous systems<br />
gas-tight, it is necessary to produce an indraft<br />
at the feed and outlet ends of the system in order<br />
to ensure that cooker effluent does not escape<br />
into the atmosphere. Thus, another 490 scfm,<br />
or 15 percent by volume, must be accounted for<br />
in the emission rate from continuous tube systems.<br />
Emission rates from blood cookers are general- -<br />
ly lower than those from dry rendering cookers<br />
owing to the longer cook cycles employed. Blood<br />
is continually added to an operating cooker during<br />
a typical packinghouse workday. The emission<br />
rate fluctuates as a function of the moisture content<br />
in the cooker. A cook cycle may extend<br />
over 8 or 10 hours, and charging patterns can<br />
vary tremendously. Emission rates do not<br />
normally exceed 500 cfm, and at times, are considerably<br />
lower.<br />
Emission rates irom feather cookers follow the<br />
same pattern as those from other dry pressure<br />
cookers though rates are lower and cooking<br />
times usually longer. Inasmuch as feathers<br />
contain no appreciable tallow, heat transfer is<br />
relatively slow. At some plants, batches of<br />
feathers are cooked as long as 8 hours. Where<br />
separate driers are used, feathers are still<br />
cooked 2 to 4 hours, which reduces the moisture<br />
content to 50 percent before the charging to a<br />
drier.<br />
Emission Rates From Driers<br />
Most air-drying processes are operated on a<br />
continuous basis with no measurable fluctua-<br />
Reduction of Inedible Animal Matter 825<br />
tions in exhaust rates. Enough air and, in some<br />
instances, products of combustion are added to<br />
yield a moisture content of 10 to 30 percent by<br />
volume in the exit gas stream. To dry 2,000<br />
pounds of cooked feathers per hour from 50 to<br />
5 percent moisture requires a drier (steamtube)<br />
exhaust volume of 1,660 scfm at 20 percent<br />
moisture in the gases. Volumes from air driers<br />
are always much greater than those from cook-<br />
ing processes, and they contain far greater<br />
quantities of noncondensable gases .<br />
AIR POLLUTION CONTROL EQUIPMENT<br />
The principal devices used to control reduction<br />
plant odors are afterburners and condensers,<br />
installed separately and in combination. Ad-<br />
sorbers and scrubbers also find use. Dust is<br />
not a major problem at animal matter reduction<br />
plants, and simple cyclones are usually suffi-<br />
cient to prevent excessive emissions.<br />
Selection of odor control equipment is influenced<br />
greatly by the moisture content of the malodorous<br />
stream, or conversely, by the percentage of non-<br />
condensable gases. It is usually more costly to<br />
control noncondensable gases than moisture.<br />
Reduction plant exhaust streams fall into two<br />
general types: (1) Those consisting almost en-<br />
tirely (95 percent or greater) of water vapor,<br />
as from rendering cookers and blood cookers,<br />
and (2) air drier exhaust gases, which seldom<br />
contain more than 30 percent moisture by volume.<br />
Controlling High-Moisture Streams<br />
Condensing moisture from wet cooker gases is<br />
almost always economically attractive. Some<br />
malodors are usually condensed or dissolved in<br />
the condensate. In any case the volume is re-<br />
duced by a factor of 10 or more. The remaining<br />
noxious gases can be directed to a further control<br />
device such as an afterburner or carbon adsorber<br />
before being vented to the atmosphere.<br />
Selection of the condenser depends upon the par-<br />
ticular facilities of the operator. The principal<br />
types of condensers noted in Chapter 5 are adapt-<br />
able to reduction cooker exhaust streams. Con-<br />
tact condensers and air-cooled and water-cooled<br />
surface condensers have been successfully used<br />
for this purpose.<br />
Contact condensers are more efficient control<br />
devices than surface condensers are, though<br />
both types are highly effective when coupled with<br />
an afterburner or carbon adsorber. This is<br />
illustrated by data in Table 224. Odor concen-<br />
trations are seen to be considerably greater in<br />
gases from surface condensers than in those<br />
from contact condensers. With condensate at
826 CHEMICAL PROCESSING EQUIPMENT<br />
a<br />
Table 224. ODOR REMOVAL EFFICIENCIES OF CONDENSERS OR AFTERBURNERS,<br />
OR BOTH, VENTING A TYPICAL DRY RENDERING COOKER^<br />
(Calculated from Mills et al., 1963)<br />
Odors from cookers<br />
Concentration,<br />
odor unitslsci<br />
50,000<br />
80"F, a contact condenser reduces odor concen-<br />
trations by about 80 percent and odor emission<br />
rates by 99 percent. At the same condensate<br />
temperature, odor concentrations increase across<br />
a surface condenser. Either type of condenser,<br />
however, reduces the volume of cooker vapors<br />
by 95 percent or more. Thus, even a surface<br />
condenser lowers the odor emission rate by about<br />
50 percent.<br />
Contact condensers are relatively inexpensive<br />
to install hut require large quantities of one-<br />
pass cooling water. From 15 to 20 pounds of<br />
cooling water is necessary to condense and sub-<br />
cool adequately 1 pound of steam. Since cooling<br />
water and condensate are intimately mixed, the<br />
resultant liquid cannot be cooled in an atmospheric<br />
cooling tower without emission of malodors to the<br />
almosphere. The large condensate volume that<br />
must be disposed of can overload sewer facili-<br />
ties in reduction plant areas.<br />
Subcooling Condensate<br />
Emission rate,<br />
odor unitslmi"<br />
25,000, 000<br />
Condenser<br />
None<br />
Surface<br />
Surface<br />
Contact<br />
Contact<br />
Condensate<br />
temperature,<br />
OF<br />
Surface condensers, whether air cooled or water<br />
cooled, should be designed to provide subcooling<br />
of condensate to 140°F or lower. This may be<br />
accomplished iri several ways, as noted in Chapter<br />
5. The need for subcooling is negated when high<br />
vacuum is employed. With vacuum operation,<br />
volatile, malodorous gases are drawn off through<br />
the ejector or vacuum pump, and condensation<br />
temperatures are often less than 140°F. At a<br />
vacuum of 24 inches of mercury (2.9 psia), the<br />
condensation temperature of steam is 140°F.<br />
-<br />
.<br />
80<br />
140<br />
80<br />
140<br />
Afterburner<br />
temperature,<br />
OF<br />
Odor removal<br />
efficiency,<br />
70<br />
~ a on ahypothetical ~ ~ d cooker that emits 500 scfm ofvapor containing 5 percentnoncondensable gases.<br />
1,200<br />
None<br />
1,200<br />
None<br />
I. 200<br />
Odors from control system<br />
Concentration,<br />
odors<br />
unitslscf<br />
100 to 150<br />
(Mode 120)<br />
100.000 to<br />
10 million<br />
(Mode 500,000)<br />
50 to 100<br />
(Made 75)<br />
2,000 to<br />
20,000<br />
(Mode 10,000)<br />
20 to 50<br />
(Mode 25)<br />
Condenser Tube Materials<br />
99.40<br />
50<br />
99.93<br />
99<br />
99.99<br />
Reduction process vapors can be highly corro-<br />
sive to the metals commonly used in surface<br />
condenser tubes. Both acid and alkaline vapors<br />
can be present, sometimes alternately in the<br />
same equipment. Vapors from relatively fresh<br />
meat and bone scrap renderir 7 are mildly acidic,<br />
and some brasses are satisfactory. Brasses<br />
fail rapidly, however, under alkaline conditions.<br />
Mild steel tubes are adequate where the pH is<br />
greater than 7.0 but quickly corrode under acid<br />
conditions.<br />
Some operations, such as dead stock rendering,<br />
can produce alkaline and acid gases alternately<br />
during the cook cycle. Here neither brass nor<br />
mild steel is satisfactory. In these cases, stain-<br />
less steels have been successfully employed.<br />
With a relatively constant pH condition, less ex-<br />
pensive metals could be used.<br />
Where acid-base conditions are uncertain, a<br />
pH determination should be made. The vapors<br />
should be sampled over the complete process<br />
cycle with all representative feedstocks in the<br />
cookers.<br />
Interceptors in Cooker Vent Lines<br />
Modal emission<br />
rate, odor<br />
unitslmin<br />
90,000<br />
12,500,000<br />
6,000<br />
250,000<br />
2,000<br />
<strong>Air</strong> pollution control systems venting cookers<br />
should be equipped with interceptor traps to pre-<br />
vent fouling of condensers and other control de-<br />
vices. So-called wild blows are relatively com-<br />
mon in dry rendering operations. They result<br />
from momentary plugging of the cooker vent.<br />
Steam pressures increase until they are suffi-
cient to unblock the line. In the unblocking, a<br />
measurable quantity of animal matter is forced<br />
through the vent line at high velocity. If there<br />
is no interceptor, this material fouls condensers,<br />
hot wells, afterburners, and other connected con-<br />
trol devices. Although a wild blow is an opera-<br />
tional problem, it greatly affects the efficiency<br />
of odor control equipment.<br />
The systems shown in Figures 632 and 633 in-<br />
clude interceptors in the vent lines between the<br />
cookers and condensers. The installation de-<br />
picted in Figure 633 uses an air-cooled con-<br />
denser and afterburner. Most tanks are of suffi-<br />
cient size to hold approximately one-haIf of a full<br />
cooker charge. They are designed so that col-<br />
lected materials can be drained while the cooker<br />
and control system are in operation.<br />
Figure 632. A condenser-afterburner control<br />
system with an interceotor located between<br />
the rendering cooker and condenser.<br />
Vapor Incineration<br />
For animal matter reduction processes, as with<br />
most odor sources, flame incineration is the most<br />
positive control method. Afterburners have been<br />
used individually and in combination with other de-<br />
vices, principally condensers. Rule 64 of the<br />
Los Angeles County <strong>Air</strong> <strong>Pollution</strong> Control District<br />
(see Appendix A), which specifically governs heat-<br />
ed animal matter reduction processes, uses incin-<br />
eration at 1,200-F as an odor control standard.<br />
Any control method or device as effective as<br />
flame incineration at 1,200°F is acceptable under<br />
the regulation.<br />
Total incineration is used to control low-mois-<br />
tnre reduction process streams, as from driers,<br />
and various other streams of small volume. At<br />
reduction plants, steamtube driers are normal-<br />
ly the largest equipment controlled in this manner.<br />
Reduction of Inedible Animal Matter 827<br />
Gases from the driers are vented directly to after-<br />
burners, which are operated at temperatures of<br />
1,200" F or higher. Dustis usuallynotin sufficient<br />
concentration to impede incineration. If there is<br />
appreciable particulate matter in the gas stream,<br />
auxiliary dust collectors must be installed or the<br />
afterburner must be operated at 1.600" F or high-<br />
er. At 1,200" F, solids are only partially incin-<br />
erated.<br />
Flame incineration at 1.200" F reduces odor con-<br />
centrations from steamtube driers to 100 to 150<br />
odor units per scf where dust loading is not ex-<br />
cessive. Some variation can be expected when<br />
concentrations are greatly in excess of the nomi-<br />
nal 2,000 odor units per scf usually encountered<br />
in drier gases.<br />
Because of the large volumes exhausted from<br />
driers, afterburner fuel requirements are a<br />
major consideration. A drier emitting 3,000<br />
scfm requires about 4,800 scfh natural gas for<br />
1,200" F incineration. Several means of recov-<br />
ering waste heat from large afterburner streams<br />
have been used. The most common are the<br />
generation of steam and preheating of drier<br />
inlet gases.<br />
In the control of spray driers, dust collectors<br />
must often be employed ahead of the afterburner.<br />
High-efficiency centrifugal collectors, baghouses,<br />
or precipitators may he required as precleaners,<br />
depending upon the size and concentration of par-<br />
ticulates.<br />
Condensation-Incineration Systems<br />
As noted earlier, wet cooker vapors are seldom<br />
incinerated is a. While 100 percent incineration<br />
is feasible, operating costs are much greater<br />
than for condenser-afterburner combinations.<br />
Both types of control systems provide better than<br />
99 percent odor removal, but the combination system<br />
results in a much lower odor emission rate.<br />
The cooker control systems shown in Figures 632<br />
and 633 and in Chapter 5, illustrate typical com-<br />
binations of condensers and afterburners. Un-<br />
condensed gases are separated from condensate<br />
at either the condenser or hot well. Gases enter<br />
the afterburner near ambient temperature. Ei-<br />
ther contact or surface condensers serve to re-<br />
move essentially all particulates. The remaining<br />
"clean" uncondensed gases can be readily incin-<br />
erated at 1.200" F. In some instances there are<br />
minor concentrations of methane and other fuel<br />
gases in the stream. Uncondensed gases from<br />
surface condensers are richer in combustibles<br />
thanare those from contact condensers. As shown<br />
in Table 224, odor removal efficiencies greater than<br />
99.9 percent are possible with condenser-after-<br />
burner systems serving dry rendering cookers.
828 CHEMICAL PROCESSING EQUIPMENT<br />
Figure 633. A cooker control system including an interceptor,<br />
air-cooled condenser, and afterburner (California Protein Products,<br />
Los Angeles, Calif.).<br />
When the moisture content of the contaminated<br />
stream is from 15 to 40 percent, the use of<br />
condensers may or may not be advantageous.<br />
In these cases, a number of factors must be<br />
weighed including volumes, exit temperatures,<br />
fuel costs, water availability, and equipment<br />
costs among others.<br />
Carbon Adsorption of Odors<br />
Most of the malodorous gases emitted from reduction<br />
processes can be adsorbed on activated<br />
carbon to some degree. The capacities of<br />
activated carbons for hydrogen sulficle, uric<br />
acid, skatole, putrescine, and several other<br />
specific compounds found in reduction plant gases<br />
are considered "satisfactoryr' to "high. " For<br />
ammonia and low-molecular-weight amines,<br />
they have somewhat lower capacities. The latter<br />
compoul~ds tend to be desorbed as the carbon becomes<br />
saturated with high-molecular-weight<br />
compounds (Barnebey-Cheney Co., Bulletin<br />
T-642). For the mixture of inalodorous materials<br />
encountered at reduction plants, a highquality<br />
carbon would be expected to adsorb<br />
from 10 to 25 percent of its weight belore the<br />
breakthrough point is reached.<br />
Carbon adsorbers are as efficient as afterburn-<br />
ers but have limitations that often make them<br />
unattractive for cooker control. Their most<br />
useful application is the control of large volumes<br />
of relatively cool and dry gases. Adsorhers<br />
usually cannot be employed in reduction process<br />
streams without auxiliary dust collectors, con-<br />
densers, or coolers.<br />
Carbon adsorbers cannot be used to control<br />
emissions from wet cookers unless the adsorb-<br />
ers are preceded by condensers. Activated<br />
carbon does not adsorb satisfactorily at tem-<br />
peratures greater than 120°F. To cool cooker<br />
vapors, which are predominantly steam, to<br />
this temperature, most of the moisture must<br />
be recovered. At l2OsF, saturated air contains<br />
only 11.5 percent water vapor by volume. Con-<br />
denser-adsorber systems are reported to re-<br />
move odors as efficiently as condenser-after-<br />
burner systems. No comparative odor con-<br />
centration data are available.<br />
Drier exhaust streams can be controlled with<br />
adsorbers if inlet temperatures and dust con-<br />
centrations can be held suiIiciently low and<br />
small, respectively. Many driers are exhausted
at temperatures higher than 200°F and contain<br />
enough fine particulates to foul adsorbers. A<br />
scrubber-contact condenser is often a satis-<br />
factory means of removing particulates and low-<br />
ering temperatures hefore adsorption. If, how-<br />
ever, there are appreciable particulates of less<br />
than 10 microns diameter, more efficient dust<br />
control devices are necessary.<br />
Regeneration of activated carbon is a major con-<br />
sideration at animal matter reduction plants.<br />
Carbon life between regenerations can be as<br />
short as 24 hours, particularly where malodors<br />
are in heavy concentration, and the carbon has<br />
a low capacity for the compounds being adsorbed.<br />
Regeneration frequency is a function of many<br />
-<br />
factors, including malodor concentration. the<br />
quality and quantity of carbon, and the kind of<br />
compounds that must be adsorbed.<br />
Some means must be employed to contain or<br />
destroy the desorbed gases; otherwise, mal-<br />
odors are vented to the atmosphere in essentially<br />
the same form that they were collected. Inciu-<br />
eration at 1,ZOO"F or higher is the most common<br />
method of controlling these gases. For streams<br />
of low volume, afterburners used during regen-<br />
eration can be as large and as costly as those<br />
used to incinerate odors from the basic reduc-<br />
tion equipment. The need for incineration of<br />
desorbed gases usually offsets the advantages<br />
of carbon adsorption for streams of low volume.<br />
If the exhaust rate is sufficiently small, incin-<br />
erating vapors directly, as they are evolved<br />
from the reduction equipment or condenser, is<br />
considerably simpler.<br />
Odor Scrubbers<br />
ELECTROPLATING 829<br />
Odor Masking and Counteraction<br />
Masking agents and odor counteractants have<br />
been used with some success to offset in-plant<br />
odors. These materials are added to cooker<br />
feedstocks and sprayed in processing and storage<br />
areas. They are repdrted to provide a degree<br />
of nuisance elimination and worker comfort,<br />
particularly in high-odor areas such as dead<br />
stock skinning rooms. Masking agents and<br />
counteractants, however, are not recommended<br />
for the control of odors from heated animal<br />
matter reduction equipment.<br />
ELECTROPLATING<br />
Electroplating is a process used to deposit,<br />
or plate, a coating of metal upon the surface<br />
of another metal by electrochemical reactions.<br />
In variations of this process, nonmetallic sur-<br />
faces have been plated with metals, and a non-<br />
metal such as rubber has been used as a plating<br />
material. Industrial and commercial applica-<br />
tions of electroplating are numerous, ranging<br />
from manufactured parts for automobiles, tools,<br />
other hardware, and furniture to toys. Brass,<br />
bronze, chromium (chrome), copper, cadmium,<br />
iron, lead, nickel, tin, zinc, and the precious<br />
metals are most commonly electroplated.<br />
Platings are applied to decorate, to reduce<br />
corrosion, to improve wearing qualities and<br />
other mechanical properties, or to serve as<br />
a base for subsequent plating with another metal.<br />
The purpose and type of plating determine the details<br />
of the process employed and, indirectly,<br />
the air pollution potential, which is a function of<br />
the type and rate of "gassing, " or release of gas<br />
bubbles from plating solutions with entrainment<br />
of droplets of solution as a mist. The degree<br />
of severity of air pollution from these processes<br />
may vary from being an insignificant problem<br />
to a nuisance.<br />
Conventional scrubbers are seldom used to<br />
control reduction process odors. Of course,<br />
contact condensers provide some scrubbing<br />
of cooker gases; nevertheless, these devices<br />
are principally condensers, and tail waters<br />
An electroplating system consists of two eleccannot<br />
be recirculated. It is conceivable that<br />
trodes--an anode and a cathode--immersed in<br />
alkaline or acid scrubbers would he effective<br />
for drier gases if all the odorous compounds<br />
an electrolyte and connected to an external<br />
source of direct-current electricity. The base<br />
reacted in the same manner. Unfortunately,<br />
material upon which the plating is to be deposited<br />
the malodorous mixtures encountered in typical<br />
makes up the cathode. In most electroplating<br />
reduction processes are not homogenous from<br />
systems, a bar of the metal to be deposited is<br />
the acid-base standpoint.<br />
used as the anode. The electrolyte is a solution<br />
containing: (1) Ions of the metal to be deposited<br />
Strong oxidizing solutions, such as chlorine and (2) additional dissolved materials to aid in<br />
dioxide, are reported to destroy many of the electrical conductivity and produce desirable<br />
odorous organic materials (Woodward and characteristics in the deposited plating.<br />
Fenrich, 1952). With any type of recirculating<br />
chemical scrubber, the contaminated stream When an electric current is passed through the<br />
would first have to be cooled to ambient tem- electrolyte, ions from the electrolyte are reperature,<br />
by condensation if necessary. duced, or deposited, at the cathode, and an
830 CHEMICAL PROCESS11 NG EQUIPMENT<br />
equivalent amount of either the same or a dif-<br />
ferent element is oxidized or dissolved at the<br />
anode. In some systems, for example, chrome<br />
plating, the deposited metal does not dissolve<br />
at the anode, and hence, insoluble anodes are<br />
used, the source of the deposited metal being<br />
ions formed from salts of that metal previously<br />
dissolved in the electrolyte.<br />
The character of the deposited metal is affected<br />
by many factors, including the pH of the electro-<br />
lyte, the metallic ion concentration, the sim-<br />
plicity or complexity of the metallic ion (includ-<br />
ing its primary and secondary ionization prod-<br />
ucts), the anodic and cathodic current densities,<br />
the temperature of the electrolyte, and the<br />
presence of modifying or "addition agents." By<br />
varying these factors, the deposit can be varied<br />
from a rough, granular, loosely adherent plat-<br />
ing to a strong, adherent, mirror-finish plating.<br />
If the electromotive force used is greater than<br />
that needed to deposit the metal, hydrogen is<br />
also formed at the cathode, and oxygen forms<br />
at the anode. When insoluble anodes are used,<br />
oxygen or a halogen (if halide salts are used in<br />
the electrolyte) is formed at the anode. Both of<br />
these situations produce gas sing.<br />
A potential air pollution problem can also occur<br />
in the preparation of articles for plating. These<br />
procedures, primarily cleaning processes, are<br />
as important as the plating operation itself for<br />
the production of high-quality finishes of im-<br />
pervious, adherent metal coatings. The clean-<br />
ing of metals before electroplating generally<br />
requires a multistage procedure as follows:<br />
1. Precleaning by vapor degreasing or by soak-<br />
ing in a solvent, an emulsifiable solvent, or<br />
an emulsion (used for heavily soiled items);<br />
2. intermediate cleaning with an alkaline bath<br />
soak treatment;<br />
3.<br />
electrocleaning with an alkaline anodic or<br />
cathodic bath treatment, or both (the chem-<br />
ical and mechanical [gassing] action created<br />
by passing a current through the bath between<br />
the immersed article and an electrode pro-<br />
duces the cleaning);<br />
4. pickling with an acid bath soak treatment,<br />
~ith or without electricity.<br />
The selection of an appropriate cleaning method<br />
in any given case depends upon three important<br />
factors: The type and quantity of the soil, com-<br />
position and surface texture of the base metal,<br />
and the degree of cleanliness required. In gen-<br />
eral, oil, grease, and loose dirt are removed<br />
first; then scale is removed, and, just before<br />
the plating, the pickling process is employed.<br />
The articles to be plated are thoroughly rinsed<br />
after each treatment to keep them from contami-<br />
nating succeeding baths. A cold rinse is usually<br />
used after the pickling to keep the articles from<br />
drying before their immersion in the plating bath.<br />
Electrocleaning and electropickling are generally<br />
faster than similar soak procedures; however,<br />
the electroprocesses always produce more gas-<br />
sing (hydrogen at the cathode and oxygen at the<br />
anode) than the nonelectroprocesses. The gas-<br />
sing from cleaning solutions tends to create<br />
mists that may, but usually do not, cause signi-<br />
ficant air pollution problems.<br />
THE AIR POLLUTION PROBLEM<br />
The electrolytic processes do not operate with<br />
100 percent efficiency, and some of the current<br />
decomposes water in the bath, evolving hydrogen<br />
and oxygen gases. In fact, the chief advantage<br />
of electrocleaning is the mechanical action pro-<br />
duced by the vigorous evolution of hydrogen at<br />
the cathode, which tends to lift off films of oil,<br />
grease, paint, and dirt. The rate of gassing<br />
varies widely with the individual process. If<br />
the gassing rate is high, entrained mists of<br />
acids, alkaline materials, or other bath con-<br />
stituents are discharged to the atmosphere.<br />
Most of the electrolytic plating and cleaning pro-<br />
cesses are of little interest from a standpoint of<br />
air pollution because the emissions are inoffen-<br />
sive and of negligible volume, owing to low gas-<br />
sing rates. Generally, air pollution control<br />
equipment is not required for any of these pro-<br />
cesses except the chromium-plating process.<br />
In this process, large volumes of hydrogen and<br />
oxygen gases are evolved. The bubbles rise<br />
and break the surface with considerable energy,<br />
entraining chromic acid mist, which is dis-<br />
charged to the atmosphere. Chromic acid mist<br />
is very toxic and corrosive and its discharge<br />
to the atmosphere should be prevented.<br />
Chromic acid emissions have caused numerous<br />
nuisance complaints and frequently cause property<br />
damage. Particularly vulnerable are automobiles<br />
parked downwind of chrome-plating installations.<br />
Tne acidmist spots car finishes severely.<br />
The amounts of acid involved are relatively<br />
small but are sufficient to cause damage. In a<br />
typical decorative chromium-plating installation<br />
with an exhaust system but without mist control<br />
equipment, a stack test disclosed that 0.45 pound<br />
of chromic acid per hour was being discharged<br />
from a 1,300-gallon tank.<br />
Chromium-plating processes can be divided into<br />
two general classes, one of which offers a con-
siderably greater air pollution problem than the<br />
other. "Hard chrome" plating, which causes<br />
the more severe problem, produces a thick,<br />
hard, smooth, corrosion-resistant coating.<br />
This plating process requires a current density<br />
of about 250 amperes per square foot, which<br />
results in a high rate of gassing and a heavy<br />
evolution of acid mist. The less severe problem<br />
is presented by the process called "decorative<br />
chrome" plating, which requires a current<br />
density of only about 100 amperes per square<br />
foot and results in a definitely lower gassing<br />
rate.<br />
HOODING AND VENTILATING REQUIREMENTS<br />
Local exhaust systems are installed on many<br />
electroplating tanks to reduce the concentra-<br />
tions of steam, gases, and mists to what are<br />
commonly accepted as safe amounts for person-<br />
nel in the plating room. In the past, these ex-<br />
haust systems were often omitted altogether,<br />
and the resulting working conditions were often<br />
unhealthful.<br />
In 1951, the American Standards Association<br />
introduced Code Z9. 1 for Ventilation and Oper-<br />
ation of Open Surface Tanks. This code is an<br />
organized engineering approach designed to re-<br />
place the rule-of-thumb methods applied in the<br />
past. The use of this code in designing plating<br />
tank exhaust systems is recommended by public<br />
health officials and industrial hygienists.<br />
Mast exhaust systems use slot hoods to capture<br />
the mists discharged from the plating solutions.<br />
These hoods have been found satisfactory when<br />
properly designed. To obtain adequate distribu-<br />
tion of ventilation along the entire length of the<br />
slot hood, the slot velocity should be high, 2,000<br />
fpm or more, and the plenum velocity should<br />
be one-half of the slot velocity or less. With<br />
hoods over 10 feet in length, either multiple<br />
takeoffs or splitter vanes are needed. Enough<br />
takeoffs or splitter vanes should be used to re-<br />
duce the length of the slot to sections not more<br />
than 10 feet long.<br />
Ventilation rates for tanks, as previously dis-<br />
cussed in Chapter 3, are for tanks located in<br />
areas having no crossdrafts. In drafty areas,<br />
ventilation rates must be increased and baffles<br />
should be used to shield the tank.<br />
AIR POLLUTION CONTROL EQUIPMENT<br />
Scrubbers<br />
The device most commonly used to control air<br />
contaminants in hard-chrome-plating tank ex-<br />
haust gases is a wet collector. This type of<br />
Electr oplating<br />
831<br />
equipment is also suitable for controlling mists<br />
from any other type of plating or cleaning tank<br />
that may cause a problem. Figure 634 shows<br />
a ventilation system with a spray-type scrubber<br />
used to control mists from two 18-foot chrome-<br />
plating tanks. Many other types of commercial<br />
wet collectors are available, constructed of<br />
various corrosion-resistant materials. Water<br />
circulation rates are usually 10 to 12 gpm per<br />
1, 000 cfm. If the water is recirculated, the<br />
makeup rate is about 2.5 to 4 gph per 1,000 cfm.<br />
The scrubber water, of course, becomes con-<br />
taminated with the acid discharged from the plat-<br />
ing tank; therefore, efficient mist eliminators<br />
must be used in the scrubber to prevent a con-<br />
taminated water mist from discharging to the<br />
atmosphere.<br />
The scrubber water is commonly used for plat-<br />
ing tank makeup. This procedure not only re-<br />
moves the acid from the scruhber but also re-<br />
duces the amount of makeup acid needed for the<br />
plating solution. In some scrubbers, a very<br />
small quantity of fresh water is used to collect<br />
the acid mist; the resulting solution is continu-<br />
ously drained from the scrubber either into the<br />
plating tank or into a holding tank, from which<br />
it can be taken for plating solution makeup.<br />
The mists collected by the air pollution control<br />
system are corrosive to iron or steel; therefore,<br />
hood, ducts, and scrubbers of these materials<br />
must be lined with, or replaced hy, corrosion-<br />
resistant materials. Steel ducts and scrubbers<br />
lined with materials such as polyvinyl chloride<br />
have been found to resist adequately the corro-<br />
sive action of the mists. In recent years, hoods,<br />
ducts, and scrubbers made entirely of polyester<br />
resins reinforced with glass fibers have been<br />
used in air pollution control systems handling<br />
acid or alkaline solutions. These systems have<br />
been found to be very resistant to the corrosive<br />
effects of plating solutions.<br />
The scrubber removes chromic acid mist with<br />
high efficiency. A commonly used field method<br />
of determining chromic acid mist evolution<br />
consists of holding a sheet of white paper over<br />
the surface of the tank or scrubber discharge.<br />
Any mist contacting the paper immediately<br />
stains it. A piece of paper held in the discharge<br />
of a well-designed scruhber shows no signs of<br />
staining.<br />
Mist Inhibitors<br />
The mist emissions from a decorative-chromeplating<br />
tank and from other tanks with lesser<br />
mist problems can be substantially eliminated<br />
by adding a suitable surface-active agent to the<br />
plating solutions. The action of the surface-
832 CHEMICAL PROCESSING EQUIPMENT<br />
Flgure 634. Two control systems wlth scrubbers venting four chrome-<br />
plating tanks. Each scrubber vents two tanks (Industrial Systems, Inc.,<br />
South Gate, Calif.).<br />
active agent reduces the surface tension, which,<br />
in turn, reduces the size of the hydrogen bubbles.<br />
Their rates of rise, and the energy of their evolu-<br />
tion are greatly reduced, and the amount of mist<br />
is also greatly reduced. Several of these mist<br />
inhibitors are commercially available.<br />
If the proper concentration of mist inhibitor is<br />
maintained, a sheet of paper placed 1 inch above<br />
the bath surface shows no spotting.<br />
INSECTICIDE MANUFACTURE<br />
The innumerable substances used commercially<br />
as insecticides can be conveniently classified<br />
according to method of action, namely: (1) Stomach<br />
poisons, which act in the d~gestive system;<br />
(2) contact poisons, which act by dlrect external<br />
contact with the insect at some stage of its life<br />
cycle; and (3) fumigants, which attack the<br />
respiratory system.<br />
A few of the commonly used insecticides, clas-<br />
sified according to method of action, are shown<br />
in Table 225. The classification is somewhat<br />
arbitrary in that many poisons, such as nicotine,<br />
possess the characteristics of two or three<br />
classes.<br />
Table 225. SOME COMMON INSECTICIDES<br />
CLASSLFIED ACCORDING TO<br />
METHOD OF ACTION<br />
stomach po~sons I contact polsons I Fumigants<br />
Cryolite<br />
Nicotine<br />
Hydrocyanic acid<br />
Naphthalene<br />
Human threshold limit values of various insecticides<br />
are shown in Table 226. They represent<br />
conditions under which it is believed that nearly<br />
all workers may be repeatedly exposed day after<br />
day, without adverse effect. The amount by which<br />
these figures may be exceeded for short periods<br />
without injury to health depends upon factors such<br />
as (1) the nature of the contaminant, (2) whether<br />
large concentrations over short periods produce<br />
acute poisoning, (3) whether the effects are<br />
cumulative, (4) the frequency with which large<br />
concentrations occur, and (5) the duration of<br />
these periods.<br />
METHODS OF PRODUCTION<br />
Production of the toxic substances used in in-<br />
secticides involves the same operations employed<br />
for general chemical processing. Similarly,
chemical-processing equipment, that is, reac-<br />
tion kettles, filters, heat exchangers, and so<br />
forth, are the same as discussed in other sec-<br />
tions of this chapter. Emphasis is given, there-<br />
fore, to the equipment and techniques encoun-<br />
tered in the compounding and blending of com-<br />
mercial insecticides to achieve specific chemi-<br />
cal and physical properties.<br />
Insecticide Manufacture<br />
Table 226. THRESHOLD LLMIT VALUES OF VARIOUS INSECTICIDES<br />
Substance<br />
Aldrin (1, 2, 3, 4, 10, 10-hexachloro-<br />
1,4,4a, 5.8, 8a-hexahydro-1, 4, 5,sdimethanonaphthalene)<br />
Arsenic<br />
Calcium arsenate<br />
Chlordane (1, 2,4,5,6, 7, 8,E-octachloro-3a, 4, 7,<br />
7a-tetrahydro-4,7 -methanoindane)<br />
Chlorinated camphene, 60%<br />
2, 4-D (2,4-dichlorophenoxyacetic acid)<br />
DDT (2,Z-bis(p-chlorophenyl)<br />
-1, 1, l -trichloroethane)<br />
Dieldrin (1, 2, 3, 4, 10, 10-hexachloro-6, 7,<br />
epoxy-1, 4,4a, 5,6,7,8, Ba-octahydro-<br />
1, 4, 5, 8-dimethano-naphthalene)<br />
Dinitro-o-cresol<br />
EPN (O-ethyl O-p-nitrophenyl thionoben~ene~hosphonate)<br />
Ferbam (ferric dimethyl dithiocarbamate)<br />
Lead arsenate<br />
Lindane (hexachlorocyclohexane gamma isomer)<br />
Malathion (0, O-dimethyl dithiophosphate of<br />
diethyl mercaptosuccinate)<br />
Methoxychlor (2, 2-di-p-methoxyphenyl-1, 1,ltrichloroethane)<br />
Nicotine<br />
Parathion (0, O-diethyl-O-p-nitrophenyl<br />
thio~hos~hate)<br />
Pentachlorophenol<br />
Phosphorus pentasulfide<br />
Picric acid<br />
Pyrethrum<br />
Rotenone<br />
TEDP (tetraethyl dithionopyrophosphate)<br />
TEPP (tetraethyl pyrophosphate)<br />
Thiram (tetramethyl thiuram disulfide)<br />
Warfarin (3-(a-acetonylben~~l) 4-<br />
hydroxycoumarin)<br />
Most commercial insecticides are used as either<br />
dusts or sprays. Insecticides employed as dusts<br />
are in the solid state in the 0.5- to 10-micron<br />
size range. Insecticides employed as sprays<br />
may be manufactured and sold as either solids<br />
or liquids. The solids are designed to go into<br />
solution in an appropriate solvent or to form a<br />
colloidal suspension; liquids may be either solu-<br />
tions or water base emulsions. No matter what<br />
Threshold limit value,<br />
mg/meter3<br />
0. 25<br />
0.5<br />
1<br />
0.5<br />
0. 5<br />
10<br />
1<br />
0. 25<br />
0.2<br />
0.5<br />
15<br />
0. 15<br />
0.5<br />
15<br />
15<br />
0.5<br />
0.1<br />
0.5<br />
1<br />
0. 1<br />
5<br />
5<br />
0.2<br />
0. 05<br />
5<br />
0.1<br />
physical state or form is involved, insecticides<br />
are usually a blend of several ingredients in<br />
order to achieve desirable characteristics. A<br />
convenient means of classifying equipment and<br />
their related processing techniques is to differ-<br />
entiate them by the state of the end product.<br />
Equipment used to process insecticides where<br />
the end product is a solid is designated solid-<br />
insecticide-processing equipment. Equipment<br />
used to process insecticides where the end<br />
product is a liquid is designated as liquid-in-<br />
secticide-processing equipment.<br />
Solid-Insecticide Production Methods<br />
Solid mixtures of insecticides may be com-<br />
pounded by either (1) adding the toxicant in<br />
833
R34 CHEMICAL PROCESSING EQUIPMENT<br />
liquid state to a dust mixture or (2) adding a<br />
solid toxicant to the dust mixture.<br />
Figure 635 illustrates equipment used if the<br />
toxicant in liquid state is sprayed into a dust<br />
mixture during the blending process. After<br />
leaving the rotary sifter, the solid raw mate-<br />
rials are carried by elevator to the upper<br />
mixer where the liquid toxicant is introduced<br />
by means of spray nozzles. This particular<br />
unit has discharge gates at each end of the up-<br />
per mixer, which permit the wetted mixture<br />
to be introduced either directly into the second<br />
mixer or into the high-speed fine-grinding pul-<br />
verizer and then into the second mixer. From<br />
the sec0n.d mixer, a discharge gate with a built-<br />
in feeder screw conveys themixture toa second<br />
elevator ior transfer to the holding bin where the<br />
finished batch is available for packaging. Al-<br />
though as much as 50 percent by weight of liq-<br />
uid toxicant may be added to the blend, the di-<br />
luent clays are porous and absorb the liquid<br />
to such a degree that the ingredients of the mix<br />
are essentially solids and act as such. In in-<br />
secticide processing, the type of mixer general-<br />
ly employed to blend liquids with dusts is the<br />
ribbon blender.<br />
Figure 636 is an illustration of a ribbon blender<br />
screw. This screw consists of two or more<br />
ribbon flights of different diameters and opposite<br />
hand, mounted one within the other on the same<br />
shaft by rigid supporting lugs. Ingredients of<br />
the mix are moved forward by one flight and<br />
backward by the other, which thereby induces<br />
positive and thorough mixing with a gradual<br />
propulsion of the mixed material to the discharge.<br />
An example of an insecticide compound produced<br />
by this method is toxaphene dust. A commonly<br />
used formulation is:<br />
Toxaphene (chlorinated<br />
camphene) 40<br />
Kerosine 4. 5<br />
Finely divided porous clay 55. 5<br />
The toxaphene is melted and mixed with the<br />
kerosine, then sprayed into the clay and thor-<br />
oughly blended.<br />
When the toxicant is in the solid state, the in-<br />
gredients of the blend are intimately ground, usu-<br />
ally in stages, and blended by mechanical mixing<br />
operations. The equipment employed consists of<br />
standard grinding and size reduction machines<br />
Figure 635. Sol id-insecticide-processing unit (Poulsen Company, Los Angeles, Calif.)
Insecticide Manufacture<br />
Figure 636. R~hhon hlender screw (L~nk-Belt Company, Los Angeles, Cal I f.).<br />
such as ball mills, hammer mills, air mills,<br />
disc mills, roller mills, and others. A spe-<br />
cific example oi a grinding and blending facility<br />
for solid insecticide is shown in Figures 637 and<br />
638. This installation is used for compounding<br />
DDT dust. The grinding and blending operations<br />
are done in two stages. First, the material is<br />
processed in the premix grinding unit and then<br />
transferred to the final grinding and blending unit.<br />
Figure 637. Premix grinding unit.<br />
Figure 637 illustrates the equipment comprising<br />
the premix grinding unit. This unit is used for<br />
the initial grading and blending of DDT and silica<br />
mixtures. DDT flakes, 75 percent of which have a<br />
particle size of 1-centimeter diameter, are emp-<br />
tied from sacks into a hopper. A conveyor takes<br />
this DDT to a crusher irom which it is conveyed<br />
to a pulverizer. Finely ground silica (0. 2- to<br />
2-micron size) is introduced to the pulverizer.<br />
Silica is added because DDT becomes waxy at<br />
temperatures approaching its melting point and<br />
has a tendency to cake and resist grinding.<br />
Silica acts as a stabilizing agent. The coarsely<br />
ground silica-DDT mixture is then discharged<br />
into a ribbon blender for thorough mixing be-<br />
fore being conveyed to a barrel-filling unit,<br />
which packs the mixture for aging before its<br />
further grinding.<br />
835<br />
The final grinding unit shorn in Figure 638 takes<br />
the coarsely ground DDT-silica mixture and sub-<br />
jects it to fine grinding and blending. The aged<br />
DDT-silica mixture is fed into a ribbon blender<br />
where additional silica and wetting agents are<br />
added to the mix. The mix is then screw con-<br />
veyed to a high-speed grinding mill that uses<br />
rotating blades to shear the insecticidal mix-<br />
ture. A pneumatic conveying system carries<br />
the material to a cyclone separator from which<br />
it drops into another blender. After this mix-<br />
ing operation, the blend is finely ground by high-<br />
pressure air in an airmill. The blend is air<br />
conveyed to a reverse-jet baghouse that dis-<br />
charges into another blender. Additional air<br />
grinding is then repeated before the barrel<br />
filling and packing.<br />
1iquid.lnsecticide Production Methods<br />
Liquid insecticides may be produced as either<br />
solutions, emulsions, or suspensions. The<br />
most common means oi production consists of<br />
introducing a solid toxicant into a liquid carrier,<br />
which results in either a solution, emulsion,<br />
or suspension.<br />
Figure 639 shows equipment employed in a liq-<br />
uid-emulsion insecticide plant that makes the<br />
emulsion by introducing a solid toxicant into a<br />
liquid carrier in the presence of an emulsifying<br />
agent. A typical iormulation is:<br />
Ib<br />
-<br />
DDT (technical) 2 00<br />
Emulsiiying agent No. 1 12<br />
Emulsifying agent No. 2 12<br />
Organic solvent 569 (79. 5 gal)
836 CHEMICAL PROCESSWG EQUIPMENT<br />
The operation consists of adding the DDT to the<br />
mixing tank, the DDT being held on a horizontal<br />
wire screen located at the vertical midpoint of<br />
the tank. Organic solvent and emulsifying agents<br />
are then pumped into the mixing tank at the approximate<br />
level of the dry DDT. The mixture is<br />
continually agitated, both during and after the<br />
-<br />
addition of the liquids, until the desired emulsified<br />
state is achieved. The finished product is<br />
then pumped to the drum-filling station for packaging.<br />
THE AIR POLLUTION PROBLEM<br />
As can be seen from the installations just de-<br />
scribed, air pollutants generated by the insecti-<br />
cide industry are of two types--dusts and or-<br />
ganic solvent vapors.<br />
To collect insecticide dusts, high-efficiency<br />
collectors are mandatory, since in many in-<br />
stances, the dust is extremely toxic and cannot<br />
be allowed to escape into the atmosphere, even<br />
in small amounts. The moderate fineness, 0.5<br />
to 10 microns, of the dust necessitates using<br />
collectors that are effective in these particle<br />
Figure 638. Final grinding and blending unit.<br />
size ranges. For the most part, the dusts en-<br />
countered are noncorrosive.<br />
Organic solvent vapors emitted from liquid-in-<br />
secticide production processes ordinarily orig-<br />
inate from relatively nonvolatile solvents. These<br />
vapors are of such concentration, nature, and<br />
quantity as to be inoffensive from a viewpoint of<br />
air pollution.<br />
HOODING AND VENTILATION REQUIREMENTS<br />
Because of the toxicity of the dusts used in the<br />
manufacture of insecticides, it is important<br />
that all sources of dust be enclosed or tightly<br />
hooded to prevent exposure of this dust to per-<br />
sonnel in the working area. Wherever possible,<br />
the sources should be completely enclosed and<br />
ventilated to an air pollution control device.<br />
Some of the sources emitting dust are bag pack-<br />
ers, barrel fillers, hoppers, crushers, con-<br />
veyors, blenders, mixing tanks, and grinding<br />
mills. Of these, the crushing and grinding<br />
operations are the largest sources of emission.<br />
In most cases, these are not conducive to corn-<br />
plete enclosure, and hoods must be employed.<br />
i<br />
I<br />
!<br />
I<br />
I
Insecticide Manufacture 837<br />
The contaminants emitted are extremely<br />
fine dust and, since no elevated temperatures<br />
a.re encountered and the materials handled are<br />
n'dt particularly corrosive to cloth, can be<br />
easily collected by simple cloth bag filters.<br />
If extremely large throughputs are encountered,<br />
a conventional baghouse may be required. Since<br />
no extreme conditions of operation are general-<br />
ly involved, the most widely used filter material<br />
is a cotton sateen cloth.<br />
In larger installations, such as those illustrated<br />
in Figures 637 and 638 for compounding DDT<br />
dust, several baghouses are usually used. In<br />
the premix grinding unit shown in Figure 637,<br />
the air pollution control equipment consists of<br />
an exhaust system discharging into a baghouse,<br />
which is equipped with a pullthrough exhaust<br />
fan. The exhaust ducting connects to both the<br />
DDT and the silica hoppers, the DDT crusher,<br />
the blender, and the barrel-filling unit. Dust<br />
collected in the baghouse is conveyed to the<br />
barrel-filling unit for packaging.<br />
Figure 639. Liquid-insecticide-formulating unit.<br />
The final grinding unit, shown in Figure 638,<br />
uses air pollution control equipment consisting<br />
of a baghouse that serves the receiving hopper,<br />
the blenders, and the cyclone air discharge of<br />
the high-speed grinding mill. Dust collected<br />
in this baghouse is recycled to the feed blender.<br />
The final blenders and the barrel filler and<br />
Indraft velocities through openings in hoods<br />
around crushers and mills should be 400 fpm<br />
or higher. Velocities through hood openings<br />
for the other operations, where dust is released<br />
with low velocities, should be 200 to<br />
300 fpm.<br />
packer are vented to one of the reverse-jet<br />
baghouses serving the fluid energy mill. As<br />
in the case of mixing liquid toxicant with dust,<br />
the material collected is not corrosive to<br />
cotton cloth, and no elevated temperatures are<br />
encountered. In this installation, cotton sateen<br />
bags of 1.12 to 1.24 pounds weight per yard<br />
with an average pore size of 0.004 inch are<br />
AIR POLLUTION CONTROL EQUIPMENT employed as the filtering medium.<br />
Baghouses employing cotton sateen bags are the<br />
most common means of controlling emissions<br />
from the insecticide-manufacturing industry.<br />
In some applications, water scrubbers, of both<br />
the spray chamber and the packed-tower types,<br />
are used to control dust emissions. Inertial<br />
separators such as cyclones and mechanical<br />
centrifugal separators are not used because<br />
collection efficiencies are not high enough to<br />
prevent the smaller size toxic particles from<br />
being emitted into the atmosphere.<br />
In the solid-insecticide-processing unit previ-<br />
ously discussed and illustrated in Figure 635,<br />
air pollution control is achieved by dust pickup<br />
hoods located at the inlet rotary sifter and at<br />
the automatic bag packer. The dust picked up<br />
at these points is filtered by the use of cloth<br />
bags. Most units of this type are entirely<br />
enclosed, air contaminants being discharged<br />
only at the inlet to the unit and at the outlet.<br />
In liquid-insecticide manufacturing, air pollu-<br />
tion control problems usually entail collection<br />
of dusts in a wet airstream. Baghouses can-<br />
not, therefore, be used, and some type of<br />
scrubber must be employed. For the liquid-<br />
emulsion insecticide plant shown in Figure 639,<br />
the air pollution control equipment for the solid<br />
and liquid aerosols consists of a packed tower<br />
that vents the mixing tank. The tower is packed<br />
with l-inch Intalox saddles, the packing being<br />
4- 112 feet high, which equals a volume of 14<br />
cubic feet. The water rate through the tower<br />
is approximately 20 gpm. The tower is used<br />
to control dust emissions from the mixing tank,<br />
which occur when dry material is charged to the<br />
tank, and also occur during the first stages of<br />
agitation. Solvent vapors are not effectively<br />
prevented by the tower from entering the atmo-<br />
sphere since the solvent is insoluble in water.<br />
Solvent emissions originate from the storage<br />
tank and drum-filling unit. In the installation
838 CHEMICAL PROCESSING EQUIPMENT<br />
described, no provision is made to prevent the<br />
solvint from escaping to the atmosphere since<br />
total solvent emissions are calculated to be<br />
only 5.4 per day.<br />
HAZARDOUS RADIOACTIVE MATERIAL<br />
Although the responsibility for overseeing the<br />
control of radioactive materials is predomi-<br />
nantly that of the Federal government, more<br />
and more responsibility is expected to be placed<br />
at state and local levels. For this reason,<br />
those concerned with air pollution must become<br />
acquainted with the problems associated with<br />
this new field, particularly those problems<br />
arising as more and smaller industries make<br />
use of radioactive materials.<br />
HAZARDS IN THE HANDLING OF RADIOISOTOPES<br />
THE AIR POLLUTION PROBLEM<br />
Radioactive materials used in industry are a<br />
definite hazard today and will become an in-<br />
creasing rather than a diminishing hazard in<br />
the future. In industry, the maximum per-<br />
missible dose of direct, whole-body radiation<br />
of persons from all radioactive materials,<br />
airborne or nonairborne, is 5,000 millirem<br />
per year. There is greater likelihood that this<br />
limit will be reduced than that it will be in-<br />
creased. <strong>Air</strong>borne radiological hazards can<br />
result from routine or accidental venting of<br />
radioactive mists, dusts, metallurgical fumes,<br />
and gases and from spillages of liquids or<br />
solids. Presently existing governmental regu-<br />
lation of the rate of venting airborne, radio-<br />
active materials consists primarily of spe-<br />
cific limitations based upon individual chemi-<br />
cal compounds or upon concentrations of ra-<br />
dioactivity from single vents. No concepts<br />
have been promulgated concerning methods<br />
of controlling total radioactive air pollution<br />
from all sources in an entire area. Whether<br />
it will be either desirable or necessary to<br />
find a solution or solutions to these problems<br />
is an unanswered question.<br />
The characteristics of radioactive, gaseous<br />
or airborne, particulate wastes vary widely<br />
depending upon the nature of the operation from<br />
which they originate. In gaseous form they may<br />
range . from rare gases, such as argon (A~')<br />
from air-cooled reactors, to highly corrosive<br />
gases, such as hydrogen fluoride from chemical<br />
and metallurgical processes. Particulate<br />
The hazards encountered in handling of radioisotopes<br />
may be classified in order of importance<br />
as follows: (1) Deposition of radioisotope<br />
in the body, (2) exposure of the whole<br />
body to gama radiation, (3) exposure of the<br />
body to beta radiation, and (4) exposure of the<br />
hands or other limited parts to beta or gamma<br />
radiation. Deposition of a radioisotope in the<br />
.body occurs by ingestion, inhalation, or absorption<br />
through either the intact or injured<br />
body surface. Inhalation of a radioactive gas,<br />
vapor, spray, or dust may occur. Spray or<br />
matter or aerosols may be organic or inorganic<br />
and range in size from less than 0. 05 micron<br />
to 20 microns. The smaller particles originate<br />
from metallurgical fumes caused by oxidation<br />
or vaporization. The larger particles may be<br />
acid mist droplets, which are low in specific<br />
gravity and remain suspended in air or gas<br />
streams for longer periods (Liberman, 1957).<br />
dust is particularly hazardous because of the<br />
large fraction of contamination retained by the<br />
Characteristics of Solid, Radioactive Waste<br />
lungs (National Bureau of Standards, 1949).<br />
Solid, radioactive wastes are of two general<br />
Types of radiation are listed in Table 227. The<br />
ranges of activity may be defined as: (1) Tracer<br />
level, less than 1 x curie; (2) low level,<br />
1 x 10-6 to 1 x 10-3 curie; (3) medium level,<br />
1 x 10-3 curie to 1 curie; and (4) high level,<br />
1 curie and over. The handling of tracer quantities<br />
of radioisotopes usually presents no external<br />
hazard. Ordinary laboratory manipulaclasses<br />
--combustible and noncombustible.<br />
Typical combustible solid wastes are paper,<br />
clothes, filters, and wood. Noncombustible,<br />
solid wastes may include nonrecoverable scrap,<br />
evaporator bottoms, contaminated process equipment,<br />
floor sweepings, and broken glassware.<br />
If inadequate provisions are made for proper<br />
handling and disposal of these wastes, a distinct<br />
nuisance, and, under certain circumstances,<br />
tions are performed with special precautions even a hazard, could result.<br />
to prevent absorption of radioactive material<br />
by the body.<br />
Characteristics of Liquid, Radioactive Waste<br />
Liquid, radioactive wastes are evolved in all<br />
nuclear energy operations-from laboratory<br />
research to full-scale production. Liquid<br />
wastes with relatively small concentrations<br />
of radioactivity originate in laboratory oper-<br />
ations where relatively small quantities of<br />
radioactive materials are involved. Other<br />
sources are the processing of uranium ore and<br />
feed material; the normal operation of essen-<br />
tially all reactors, particularly water-cooled<br />
types; and the routine chemical processing of<br />
reactor fuels. High-activity liquid wastes
Type of<br />
radiation<br />
Alpha (a)<br />
. . Beta (8)<br />
~~ . .<br />
. . .<br />
. . . .. . . . .<br />
... .. . ... .<br />
Gamma (y)<br />
Neutron (r~)<br />
Physical nature<br />
Heavy particle,<br />
helium nucleus,<br />
double positive<br />
charge<br />
Light-particle<br />
electron, single<br />
negative charge<br />
Ray, similar to<br />
X-ray<br />
Moderately heavy<br />
particle, neutral<br />
charge<br />
are produced by the chemical processing of<br />
reactor fuels.<br />
Problems in Control of <strong>Air</strong>borne, Radioactive Waste<br />
Hazardous Radioactive Material 839<br />
Table 227. TYPES OF RADLATION<br />
Distance of<br />
travel in air<br />
Removal of radioactive suspended particles,<br />
vapors, and gases from "hot" (radioactive)<br />
exhaust systems before discharge to the atmosphere<br />
is a se~ious problem confronting<br />
all nuclear energy and radiochemistry installations.<br />
Removal is necessary in order to prevent<br />
dangerous contamination of the immediate<br />
and neighboring areas. <strong>Air</strong> pollution brought<br />
about through discharge of radioactive stack<br />
gas wastes from ventilation systems is only<br />
partially avoided by filter devices, no matter<br />
how efficient they may be, if the discharge<br />
contains radioactive gases. In systems using<br />
filter media such as paper, cloth, glass fiber,<br />
and so forth, activity eventually builds up in<br />
the filter media through dust loading; the same<br />
situation applies to electrical precipitators.<br />
I<br />
Few inches maximum<br />
Few yards maximum<br />
Very long<br />
Very long<br />
Another problem in the control of airborne,<br />
radioactive waste is the low dust loading of<br />
exhaust streams. The dust concentration of<br />
ambient air is usually about 1 grain per 1,000<br />
cubic feet. At installations handling radioactive<br />
material, owing to precleaning of the<br />
entering air, aerosols may have concentrations<br />
as small as 10-<br />
2<br />
to 10-3 grain per 1,000<br />
cubic feet. In contrast, loadings of some industrial<br />
gases may reach several hundred<br />
grains per cubic foot, though values of 20<br />
grains or less per cubic foot are more common.<br />
An outstanding feature to consider with air-<br />
cleaning requirements for many nuclear oper-<br />
Effective shielding<br />
Skin or thin layer<br />
of any solid mate-<br />
rial<br />
One-half inch of<br />
any solid material<br />
Lead, other heavy<br />
metals, concrete,<br />
tlghtly packed so11<br />
Water, paraffin<br />
Usual means of<br />
detection ~~-...<br />
Proportional counter,<br />
ion chamber, scin.<br />
tillation counter<br />
Geiger counter, film<br />
badge, dosimeter<br />
Geiger counter, ion<br />
chamber, film badge,<br />
dosimeter<br />
Proportional counter<br />
contalnlng borlc compound,<br />
lon chamber<br />
with cadmium shield<br />
ations is the extremely small permissible con-<br />
centrations of various radioisotopes in the at-<br />
mosphere (see Table 228). Often, removal ef-<br />
ficiencies of about 99. 9 percent or greater for<br />
particles less than 1 micron in diameter are<br />
necessary. This high removal efficiency lim-<br />
its the selection of control equipment for ra-<br />
dioactive applications.<br />
HOODING AND VENTILATION REQUIREMENTS<br />
Hooding<br />
Hooding for radiochemical processes must pre-<br />
vent radioactive contaminants, such as dust<br />
and fmes, from escaping into thk work area<br />
and must deliver them to suitable control de-<br />
vices. Radioactive sources require proper<br />
shielding to prevent the escape qf radiation<br />
and are not considered in this section. The<br />
materials used for construction for hoods depend<br />
upon the type and quantities of radioactivity and<br />
the nature of the process. Stainless steel,<br />
masonite, transite, or sheet steel, surfaced<br />
with a washable or strippable paint, can be<br />
used (Ward, 1952). Where it is necessary in<br />
a process to handle material that may cause<br />
dusts or fumes to form, a completely enclosed<br />
hood should be used, equipped with a glove box<br />
or dry box. Any tools used for manipulation<br />
should not be removed from the hood.<br />
Ventilation<br />
The recommended airflow for toxic material<br />
across the face of a hood is 150 fpm (Manufac-<br />
turing Chemists' Association, 1954). Turbu-<br />
lence of air entering a hood can be reduced by
840 CHEMICAL PROCESSING EQUIPMENT<br />
Table 228. PROPERTIES OF RADIOISOTOPES (Benedict and Pigford, 1957)<br />
the addition of picture frame airfoils to the edges.<br />
Hoods should not be located where drafts will<br />
affect their operation. When more than one hood<br />
is located in a room, fan motors should be oper-<br />
ated by a single switch. The fan should freely<br />
discharge to the atmosphere and be connected<br />
to the outlet side of any control device, the<br />
motors being located outside the air ducts to<br />
prevent their contamination. Hood and ducts<br />
should be equipped with manometers to indi-<br />
cate that they are operating under a negative<br />
pressure.<br />
AIR POLLUTION CONTROL EQUIPMENT<br />
Reduction of Radioactive, Particulate Matter at Source<br />
Reduction at the source has been defined as the<br />
design of processes so as to minimize the initial<br />
release of particulate matter at its source. The<br />
principle is not new; it is applied, for example,<br />
in the ceramics industry where dry powders<br />
are wetted and mixed as a slurry to minimize<br />
the production of dust. But its application to
adioactive aerosols is particularly worthwhile<br />
since it (1) provides a cleaner effluent, (2) re-<br />
duces radiation hazards involved in the mainte-<br />
nance of air-cleaning equipment or those re-<br />
sulting from the buildup of dust activity, (3) per-<br />
mits the use of simpler and less expensive air-<br />
cleaning equipment, and (4) becomes a part of<br />
the process once reduction has been established.<br />
In general, preventing the formation of highly<br />
toxic aerosols is preferable to cleaning by<br />
secondary equipment.<br />
The design or redesign of processes for reduc-<br />
tion at the source should be based upon a study<br />
of the quantity and physical characteristics of<br />
the contaminant, and the manner in which it is<br />
released. Examples of this concept are instal-<br />
lation of glass fiber filters on the lnlet of ven-<br />
tilating or cooling alr to minimize the irradia-<br />
tion of ambient dust particles, and treatment<br />
of ducts to minimlze corrosion and flaking<br />
(Friedlander et al., 1952).<br />
Design of Suitable <strong>Air</strong>-Cleaning Equipment<br />
The most satisfactory control of particulate<br />
contamination with air-cleaning equipment re-<br />
sults from using combinations of the various<br />
collectors. These installations should be de-<br />
signed to terminate with the most efficient<br />
separator possible, the nature of the gases<br />
being considered. To reduce maintenance,<br />
less efficient cleaners capable of holding or<br />
disposing of most of the weight load should be<br />
placed before the final stage. It is good prac-<br />
tice to arrange the equipment in order of in-<br />
creasing efficiency. A typical example of such<br />
an arrangement is a wet collector such as a<br />
centrifugal scrubber to cool the gases and re-<br />
move most of the larger particles, an efficient<br />
dry filter such as a glass fiber filter to remove<br />
most of the remaining particulate matter, and<br />
a highly efficient paper filter to perform the<br />
final cleaning. If the gases are moist, as in<br />
this example, the paper filter should be pre-<br />
ceded by a preheater to dry the gases (Fried-<br />
lander, 1952).<br />
An air-cleaning installation for highly toxic<br />
aerosols should fulfill the following require-<br />
ments (Friedlander et al., 1952):<br />
1. "It should discharge innocuous air.<br />
2. "The equipment should require only occa-<br />
sional replacement and should be designed<br />
for easy maintenance. Frequent replace-<br />
ment or cleaning entails excessive exposure<br />
to radiation and the danger of redispersing<br />
the collected material.<br />
Hazardous Ra rdioactive Material 84 1<br />
3. "The particulate matter should be separated<br />
in a form allowing easy disposal. The use<br />
of wet collectors, for example, poses the<br />
additional problem of disposing of volumes<br />
of contaminated liquid. Wet collection does,<br />
however, reduce considerably the danger of<br />
redispersion.<br />
4. "Initial and maintenance costs, as well as<br />
operating costs, should be as low as possi-<br />
ble while fulfilling the preceding three con-<br />
ditions. In this respect, pressure drop is<br />
generally an important consideration. "<br />
Reverse-jet haghouse<br />
One type of commercially available dust collector<br />
that meets the requirements of filtering airborne,<br />
radioactive particles from ventilation exhaust<br />
streams is a bag filter employing what is called<br />
reverse-jet cleaning. This type of baghouse<br />
(described in Chapter 4) has an efficiency as<br />
high as the conventional cloth bag or cloth screen<br />
collector and is particularly adapted to an in-<br />
stallation where the grain loading of the effluent<br />
is low. The bag material is a hard wool felt of<br />
the pressed type, about 1116-inch thick, or a<br />
cloth woven of glass fibers. The gas flow is<br />
likely to he around 10 to 40 cfm per square foot<br />
of bag area when the pressure drop is maintained<br />
at usual values such as 2 to 7 inches water col-<br />
umn (Anderson, 1958).<br />
The conventional cloth bag or cloth screen col-<br />
lectors, which are cleaned periodically by auto-<br />
matic shaking devices, may allow a puff of dust<br />
to escape after the shaking operation. The prob-<br />
lem of maintenance in this instance presents a<br />
contamination and radiation hazard. For this<br />
reason, the reverse-jet baghouse is generally<br />
preferred.<br />
Wet collectors<br />
Another method of treating contaminated ex-<br />
haust air before discharge to the atmosphere<br />
involves the use of wet collectors of various<br />
types. These collectors are relatively effec-<br />
tive on gases. Investigation covering changing<br />
of water supply or recirculating has shown the<br />
latter procedure useful for considerable periods<br />
of time without apparent adverse effect. Evapo-<br />
ration is compensated for by fresh supply. In-<br />
soluble radioactive salts, soluble salts, and<br />
other radioactive particles that may form a<br />
solution, suspension, or sludge in the reser-<br />
voir result in fairly high radioactivity of the<br />
scrubbing media. Precautions must be taken<br />
during maintenance to avoid carryover of the<br />
scrubbing media since the radioactive con-<br />
tamination of entrained liquid would be trans-
842 CHEMICAL PROCESSING EQUIPMENT<br />
ferred to the preheater or filter, resulting in 2. "With uneven airflow, the air velocity<br />
high radiation levels at those points. through some of the collector cells may<br />
Some important disadvantages of wet collectors<br />
make them less attractive than other types . . of<br />
collectors. Wet collectors present the difficult<br />
problem of separating the radioactive, solid<br />
material from the water in which it is suspended.<br />
Maintenance and corrosion are serious problems.<br />
Considerable quantities of water are required,<br />
and, if the radioactive solids are not separated<br />
from the water, this in turn leads to a final<br />
storage and disposal problem.<br />
Electrical precipitators<br />
Radioactive, airborne particles, when given an<br />
electrical charge, can be collected on grounded<br />
surfaces. The fact that the particles are radioactive<br />
has very little to do with their behavior<br />
in an electrical precipitator. Experiments conducted<br />
with precipitators using the alpha emitter<br />
polonium and the beta emitter sulfur 35 indicate<br />
that neither material behaves in a way different<br />
from nonradioactive material.<br />
Water-flushed-type, single-stage, industrial<br />
precipitators, and air-conditioning-type, two-<br />
stage precipitators are used for separating ra-<br />
dioactive dusts and fumes from gases at atomic<br />
energy plants and laboratories. A small elec-<br />
trical precipitator of the water-flushed type with<br />
a design capacity of 200 cfm was installed to<br />
test efficiency of collecting and removing par-<br />
ticulate radioactivity from the offgas system<br />
of an isotope recovery operation. This precip-<br />
itator consists of 23 vertical collecting pipes<br />
with an ionizing wire centered in each pipe. The<br />
inside surfaces of the pipes serve as collecting<br />
walls. For wet operation, the collecting walls<br />
are water flushed by means of spray nozzles in-<br />
stalled at the top of each pipe. This water is re-<br />
cycled continuously at a rate of 35 gpm over the<br />
collecting walls while high voltage is applied to<br />
the electrodes. This unit reportedly collects<br />
more than 99. 99 percent of the particulate radio-<br />
activity in the offgas at 50 to 55 kilovolts when<br />
the concentration of radioactivity as solids is<br />
greater than 5.0 x microcuries per cubic<br />
centimeter of offgas (Anderson, 1958).<br />
Based upon tests made at the Oak Ridge Nation-<br />
al Laboratory, Anderson (1958) makes the follow-<br />
ing evaluation of precipitators used in radio-<br />
active applications:<br />
1. "Electrical precipitators are not intended<br />
to collect the ultra fine particles which<br />
may be discharged from radiochemistry<br />
installations.<br />
be sufficientlv above velocitv limits to blow<br />
off collected wastes which would then be<br />
discharged to the atmosphere.<br />
3. "Efficient operation depends a great deal<br />
on the regularity with which the unit is<br />
cleaned. At best the electrical precipitator<br />
is only approximately 90 percent<br />
efficient. This may he demonstrated by<br />
the fact that dense clouds of tobacco smoke<br />
fed into the precipitator will escape from<br />
it in concentrations great enough so that the<br />
escaping smoke can be seen. The blue<br />
color of tobacco smoke is evidence that<br />
most of its particles have a diameter less<br />
than the wavelength of light, which is<br />
roughly 0.5 micron.<br />
4. "For absolute efficiency an after-filter of<br />
the Cambridge or MSA Ultra-<strong>Air</strong>e type is<br />
necessary to catch the dirt should the pre-<br />
cipitator short circuit.<br />
5. "Difficulty may be experienced if the dust-<br />
load builds up faster than it can be removed,<br />
eventually becoming so heavy that arcing<br />
occurs between the dirt bridges resulting<br />
in a fire hazard.<br />
6.<br />
"Devices such as the single-stage indus-<br />
trial precipitator and the air-conditioning<br />
type two-stage precipitator accomplish<br />
only one phase of the problem. The final<br />
disposal of radioactive wastes collected<br />
and accumulated during operation and main-<br />
tenance still remains. "<br />
Glass fiber filters<br />
Glass fiber or glass fiber paper is often used<br />
as a filter medium and is effective in the oper-<br />
ation of :adiochemistry hoods, canopies, and<br />
gloved b'oxes. One of the most efficient light-<br />
weight, inorganic filters developed to date is<br />
made with a continuous, pleated sheet of micro-<br />
glass fiber paper. The pleats of the glass paper<br />
are separated by a corrugated material (paper,<br />
glass paper, aluminum foil, plastic, or as -<br />
bestos paper) for easy passage of air to the deep<br />
pleats of the filter paper. The assembly of the<br />
filter paper and corrugated separators is sealed<br />
in a frame of wood, cadmium plated steel, stain-<br />
less steel, or aluminum. This construction per-<br />
mits a large area of filter paper to be presented<br />
to the airstream of a correspondingly low re-<br />
sistance (Flanders Filters, Inc. , Riverhead,<br />
N. Y. ).
Glass fiber, from which filters are made, with-<br />
stands temperatures up to 1, OOO°F. It is non-<br />
combustible and has extremely low thermal<br />
conductivity and low heat capacity. The fibers<br />
are noncellular, are like minute rods of glass,<br />
and do not absorb moisture; however, water<br />
can enter the interstices. The material is<br />
relatively nonsettling, noncorrosive, and durable.<br />
It is resistant to acid fumes and vapors, except<br />
hydrogen fluoride.<br />
The installation and replacement costs of glass<br />
fiber filters are low. Final disposal of used<br />
filters may be accomplished by incinerating at<br />
over 1,000"F with provisions for decontaminating<br />
the stack gases. This melts the glass fibers,<br />
reducing the physical mass to the size of a glass<br />
bead. Thus, glass fiber filters provide, in part,<br />
a very good answer to the problem of control<br />
and final disposal of radioactive contaminants.<br />
Paper filters<br />
A highly efficient paper filter medium can be<br />
used with adequate effectiveness on incoming<br />
ventilating air and as a final cleaner in many<br />
instances. This type filter is composed of as-<br />
bestos cellulose paper. A more recently de-<br />
veloped filter has a glass fiber web. It is de-<br />
signed and manufactured in corrugated form<br />
to increase the available filter area and load-<br />
ing capacity and to reduce initial resistance.<br />
The filter units are tested at rated capacity<br />
with standard U.S. Army Chemical Corps test<br />
equipment for resistance and initial penetra-<br />
tion and are unconditionally guaranteed to be<br />
at least 99.95 percent effective against 0. 3-<br />
micron-diameter dioctyl phthalate particles.<br />
This filter performs as well as, or better<br />
than, the earlier paper types and under tem-<br />
peratures up to l,OOOeF.<br />
<strong>Air</strong>borne, radioactive wastes are only part of<br />
the control and disposal problem of nuclear<br />
energy and radiochemistry installations. Solid<br />
and liquid, radioactive wastes are subject to<br />
the same limitations on disposal to the environ-<br />
ment.<br />
The methods of disposing of the final waste from<br />
the collection systems present additional prob-<br />
lems, as follows (Anderson, 1958):<br />
1. "Incineration results in stack gas and par-<br />
ticle discharge which is a cycle of the en-<br />
tire problem repeated over again.<br />
2. "Direct burial results in redispersal and<br />
ground contamination with associated prob-<br />
lems related to the ground water table.<br />
Hazardous Radio active Material 843<br />
3. "High dust or particle loading capacity re-<br />
sults in high radioactivity of the collecting<br />
media.<br />
4. "Vapors, acid fumes and unfilterable gases<br />
may cause rapid deterioration and disinte-<br />
gration of filter media resulting in a main-<br />
tenance and health hazard problem.<br />
5. "Mechanical replacement costs are high<br />
because of the remote handling involved.<br />
6. "An auxiliary unit for emergency or main-<br />
tenance shutdown must be available to pre-<br />
vent the possibility of reverse flow of the<br />
air stream out of "hot" equipment into<br />
controlled rooms and areas. "<br />
Disposol and Control of Solid, Radioactive Waste<br />
The most common method of disposal of solid,<br />
radioactive wastes is Land burial at isolated<br />
and controlled areas. The earth cover over<br />
these burial pits is usually about 12 feet, and<br />
the surface is monitored regularly. A method<br />
used for disposal of low-level, radioactive,<br />
solid wastes consists of putting the wastes in<br />
concrete and dumping it at sea. Incineration<br />
of combustible, solid wastes is practiced, with<br />
provisions for decontaminating the flue gases<br />
(Shamos and Roth, 1950).<br />
Disposal and Control of Liquid, Radioactive Worte<br />
Low-level, radioactive, liquid wastes, under<br />
proper environmental conditions, are suscepti-<br />
ble to either direct disposal to nature or dis-<br />
posal after minimum treatment. Treatment<br />
processes used include coprecipitation, ion ex-<br />
change, biological systems similar to sewage<br />
treatment methods, and others. Only to the<br />
extent that it is absolutely safe, maximum use<br />
is made of the dilution factors that may be avail-<br />
able in the environment and that can be assessed<br />
quantitatively.<br />
High-activity, liquid wastes associated with the<br />
chemical processing of reactor fuels constitute<br />
the bulk of the engineering problem of disposal<br />
of radioactive wastes. Highly radioactive, liq-<br />
uid wastes are currently stored in specially de-<br />
signed tanks. Since the effective life of the fis-<br />
sion products constituting the wastes may be<br />
measured in terms of hundreds of years, tank<br />
storage is not a permanent solution to the dis-<br />
posal problem. Evaporation before storage is<br />
generally practiced to reduce storage volume<br />
and cost. The degree to which evaporation is<br />
carried out is limited in some instances by the
844 CHEMICAL PROCESSIl \iG EQUIPMENT<br />
percentage of solids present in the waste or by<br />
considerations of corrosion.<br />
There are several ~ractical approaches to<br />
ultimate, safe disposal of high-activity, liquid<br />
wastes. The actual fission products in radioactive<br />
waste material may be fixed in an inert,<br />
solid carrier so that the possibility of migration<br />
of the radioactivity into the environment<br />
is eliminated or reduced to acceptable and safe<br />
limits. The carrier containing the radioactive<br />
material could then be permanently stored or<br />
buried in selected locations. Fixation on clay,<br />
incorporation in feldspars, conversion to oxide,<br />
elutriation of the oxide, and fixation of the<br />
elutriant are examples of systems under development.<br />
Because of the particular radiotoxicity and long<br />
half-life of strontium-90 and cesium-137, the<br />
removal and separate fixation and handling of<br />
these two isotopes would substantially reduce<br />
the effective life and activity of the waste and<br />
facilitate its final disposal. With cesium and<br />
strontium removed, the possibilities of safe<br />
disposal into the environment under controlled<br />
conditions are greatly increased.<br />
It may be practical to dispose of the wastes<br />
underground in some cases without any treat-<br />
ment, into formations such as (1) spaces pre-<br />
pared by dissolution in salt beds or salt domes,<br />
(2) deep basins containing connate brines and<br />
with no hydraulic or hydrologic connection to<br />
potable waters or other potentially valuable<br />
natural resources, and (3) special excavations<br />
in selected shale formations (Liberman, 1957).<br />
OIL AND SOLVENT RE-REFINING<br />
Many millions of gallons of oils and solvents<br />
are used annually for lubricating vehicle en-<br />
gines and other machinery, transmitting pres-<br />
sure hydraulically, cleaning manufactured arti-<br />
cles and textiles, and dissolving or extracting<br />
soluble materials. In the course of their usage,<br />
these oils and solvents accumulate impurities,<br />
decompose, and lose effectiveness. The im-<br />
purities include dirt, scale, water, acids, de-<br />
composition products, and other foreign mate-<br />
rials. Reclaiming some of these oils and sol-<br />
vents for reuse by removal of the impurities<br />
can he effected in many instances by re-refining<br />
processes.<br />
Most re-refiners must practice stringent econ-<br />
omies to survive, and for this reason, second-<br />
hand, cannibalized, or makeshift equipment is<br />
often employed. Many re-refiners also neglect<br />
maintenance, repairs, and general housekeeping<br />
in order to keep operating costs low. As a result,<br />
air pollution control is minimal or lacking unless<br />
made mandatpry by legislation.<br />
RE-REFINING PROCESS FOR OILS<br />
Lubricating oils collected from service stations<br />
are the main source of supply. A typical scheme<br />
for re-refining lubricating oil is shown in Figure<br />
640. Re-refining is normally a batch process.<br />
Treating clay, for example, Fuller's earth, is<br />
added to the contaminated oil at ambient tem-<br />
perature to aid in the removal of carbon mate-<br />
rials. The mixture is next circulated through a<br />
fired heater, usually a pipe or tube still, to a flash<br />
tower for removal of diluent hydrocarbons and<br />
water. The oil being reclaimed and the products<br />
desired determine the final temperature (300"<br />
to 600°F). Live steam, introduced at the base<br />
of the flash tower, is used to assist in this phase<br />
of the operation. Besides distilling off the light<br />
fractions contained in the oil, the steam pre-<br />
vents excessive cracking of the oil at the higher<br />
temperatures.<br />
A barometric condenser maintains a vacuum on<br />
the tower. The overhead vapors containing<br />
steam, low-boiling organic materials, and en-<br />
trained hydrocarbons are aspirated through the<br />
condenser to a separator tank. The condensate,<br />
consisting of light gas, oil, and water, is col-<br />
lected and separated in the separator tank. Non-<br />
condensible gases are usually incinerated in<br />
fireboxes of adjacent combustion equipment. The<br />
light oil condensate is decanted from the water<br />
and is suitable as liquid fuel. The contaminated<br />
water is piped to a skimming pond where it is<br />
cooled and either reused or disposed of by drain-<br />
ing to a sewer. The oil..-clay mixture is with-<br />
drawn from the tower and filtered. The oil is<br />
blended with additives and is canned or drummed.<br />
The clay is usually hauled to a dump.<br />
In some re-refineries, the process is preceded<br />
by a dehydration operation. Water is removed<br />
from the oil by using sodium silicate, sodium<br />
hydroxide, and heat. Dehydrated oil is decanted<br />
from the mixture and charged to the still. Sul-<br />
furic acid treabent is also employed at some<br />
re-refineries before the refining process. The<br />
acid-treated oil is settled, decanted from the<br />
acid sludge, and neutralized with caustic. Be-<br />
fore the clay is added, sulfuric acid treabent<br />
or air blowing may also be used to improve<br />
color of the re-refined oil.<br />
RE-REFINING PROCESS FOR ORGANIC SOLVENTS<br />
The typical organic solvent re-refining process<br />
is similar to that described for oil re-refining.<br />
The prime difference between the processes is<br />
that the volatilities of the organic solvents re-
efined are much greater than those of lubrica-<br />
ting oils. Mineral spirits, benzene, toluene,<br />
xylene, ketones, esters, alcohols, trichloro-<br />
ethylene, and tetrachloroethylene from paint,<br />
lacquer, degreasers, and dry cleaners are ex-<br />
amples of solvents reclaimed by re -refining.<br />
Figure 641 illustrates a typical solvent recov-<br />
ery system. The mixture to he processed is<br />
introduced into a settling tank to permit the<br />
solids to settle out. The supernatant liquid is<br />
then preheated and charged to a pot still topped<br />
by a fractionating section, which may be under<br />
RECOVERABLE<br />
+<br />
Oil and Solvent Re-Refining 845<br />
Figure 640. Composite flow sheet for re-refining process.<br />
CDNDENSER<br />
t lTER OUT<br />
FRACTlONIITlNG<br />
EsEcrloM ~ " SOGlUM cClRBONliTE ' E<br />
QRCHtIITER<br />
Sllll<br />
OEHIORATING<br />
CGNOENSIITI lATER PRODUCT<br />
TO SBtR<br />
Figure 641. Typical solvent re-refining installation.<br />
vacuum. Vapors from the still are condensed<br />
in a water-cooled surface condenser. Reflux-<br />
ing may or may not be done, depending upon the<br />
product, the degree of purity desired, and the<br />
contaminants present. The condensate is ac-<br />
cumulated in a holding tank, where a salt such<br />
as sodium carbonate is added to "break" the<br />
water from the solvent. After the water settles<br />
out, it is removed, and the solvent is drurnmed . .<br />
off as product.<br />
, .<br />
r.<br />
THE AIR POLLUTION PROBLEM i<br />
<strong>Air</strong> <strong>Pollution</strong> From Oil Re-refining ~<br />
The two primary air pollution problems connected<br />
with oil re-refining are odors and hydrocarbon<br />
vapors.<br />
Chief odor sources are the contaminated water<br />
and the noncondensible gases from the separator<br />
tank and dehydration tank. Obnoxious odors !<br />
emanate from the skimming pond. Odors also<br />
occur from the barometric condenser leg. If<br />
the process water is aerated in a cooling tower<br />
or spray pond, a serious odor problem occurs.<br />
Other odors can originate from the dehydration<br />
operation and from sulfuric acid sludges and<br />
clay filter cakes. !<br />
i<br />
I<br />
I<br />
j
846 CHEMICAL PROCESSING EQUIPMENT<br />
In addition to air pollution from odors, oil re- sions from the bottom drawoff of the still are<br />
refining processes can emit some hydrocarbons slight since most of the volatiles have been<br />
into the atmosphere. These originate from the flashed off. Emissions from the settling tank<br />
noncondensible gases and the layers of light, and the product receivers are normally too<br />
volatile hydrocarbons on the surface of the sep- small to create any problems, but they can be<br />
arator tank and the skimming pond. controlled by being vented also to a boiler firebox.<br />
<strong>Air</strong> <strong>Pollution</strong> From Solvent Re-refining<br />
As in oil re-refining, - the chief air pollution<br />
problems are odors but these are less severe<br />
than those occurring from re-refining of lubricating<br />
oil. Sources of emissions are the settling<br />
tanks during filling and sludge drawoff, the drawoff<br />
of bottoms from the still, the product receivers,<br />
and the water jet reservoir (if vacuum is<br />
produced by a barometric water jet). By creating<br />
a vacuum, the water jet entraps the solvent vapors<br />
from the still.<br />
AIR POLLUTION CONTROL EQUIPMENT<br />
Oil Re-refining<br />
The most acceptable method of controlling emis-<br />
sions from re-refining is incineration. Usually<br />
the firebox of a boiler or heater provides ade-<br />
quate incineration. The separator tank must be<br />
covered and vented to a firebox. The vent line<br />
should be equipped with a knockout drum and a<br />
flashback arrester. Additional safety protection<br />
can be achieved by introducing live steam into<br />
the vent line upstream from the firebox. Other<br />
vessels, for example, dehydrating tanks and<br />
mixing tanks, may be tied into this system.<br />
Emissions from the barometric, or contact,<br />
condenser can be controlled by maintaining a<br />
closed recycle water system or by modifying<br />
the operation by substituting a shell-and-tube-<br />
type condenser.<br />
Recycle water, highly odorous from contact<br />
with the oil and heated by contact with the hot<br />
vapors, must be allowed to cool before reuse.<br />
It can he controlled by cooling in a covered<br />
settling tank that is properly vented to an<br />
operating boiler or heater firebox. Con-<br />
taminated recycle water must not be cooled<br />
by aerating in a spray pond or cooling tower.<br />
Solvent Re-refining<br />
Usually, in the solvent re-refining industry, air<br />
pollution control is lacking without enforcement,<br />
and solvent vapors are allowed to escape into<br />
the atmosphere. If, however, control is re-<br />
quired, it can easily be accomplished by venting<br />
the barometric water jet vacuum system to a<br />
boiler firebox, provided appropriate flashback<br />
prevention measures have been taken. Emis-<br />
CHEMICAL MILLING<br />
The chemical milling process was developed<br />
by the aircraft industry as a solution to the<br />
problem of making lightweight parts of intri-<br />
cate shapes for missiles. These parts could<br />
not be formed if mechanically milled first, and<br />
no machines were available that could mill them<br />
after they were formed. Chemical milling is<br />
based upon the theory that an appropriate etch<br />
solution dissolves equal quantities of metal per<br />
given time from either flat or curved surfaces.<br />
The process was quickly adopted by the aircraft<br />
industry, and etchants were developed for<br />
chemically milling many metals used in aircraft<br />
and missiles, including aluminum, titanium,<br />
stainless steel, and magnesium.<br />
DESCRIPTION OF THE PROCESS<br />
Before an article can be chemically milled, the<br />
surface of the metal must be clean. The usual<br />
metal surface preparation includes (1) degreas-<br />
ing, (2) alkaline cleaning, (3) pickling, and<br />
(4) surface passivation. The cleaning is needed<br />
to provide a clean surface in order to ensure uni-<br />
form dissolving of the metal when it is submerged<br />
in the milling solution. The passivation is needed<br />
to protect the surface from oxidation in air and<br />
provide a surface that will accept and hold a<br />
masking agent or material.<br />
Maskings are either tapes with pressure-sensi-<br />
tive adhesives or paint-like substances that are<br />
applied by brushing, dipping, spraying, or flow-<br />
coating. Figure 642 shows a sheet of stainless<br />
steel being flow-coated with a rubber base mask-<br />
ing material. These paint-like maskings must<br />
be cured, usually in a bake oven. After curing,<br />
the masking is removed or stripped from those<br />
areas to be milled. Figure 643 shows one meth-<br />
od of scribing the masking by use of a template.<br />
Milling is accomplished by submerging the pre-<br />
pared article in an appropriate etching solution.<br />
The depth of the cut is controlled by the length<br />
of time the article is held in the etching solu-<br />
tion. To stop the milling action, the article is<br />
removed from the etchant and the adhering solu-<br />
tion is rinsed off with water. During the milling<br />
step, some metals are discolored by their etch-<br />
ing solutions. The smutty discoloration is re-
Chemical Milling 847<br />
This process is patented and licensed by<br />
Fieilre . .-- fi47. An 18- hv 6-foot stainless steel Turco Products Co., Wilmington, Calif.<br />
~ - ... - .. ., . ~~ ... . -.. ~ .-. .<br />
sheet being masked b; flow-coating with a rub- Figure 644. A flow diagram showing the typical<br />
ber-based masking (U.S. Chemical Milling Gorp., steps necessary to the chem-milling process<br />
Manhatten Beach, Calif.). (Scheer, 1956).<br />
Figure 643. The masking on a titanium part is being<br />
scribed bv use of a template. After scribing, the<br />
masking will be stripped from those areas shown by<br />
the holes in the templates. The stripped areas will<br />
then be milled. The black part in tile foreground and<br />
those in the background have not yet been scribed<br />
(U.S. Chemical Mi l ling Corporation, Manhatten Beach,<br />
Calif.).<br />
moved in a brightening solution such as cold,<br />
dilute nitric acid. A flow diagram of the pro-<br />
cess is shorn in Figure 644.<br />
percent high-boiling alcohols, and is then<br />
stripped off by hand. Figure 645 shows the in-<br />
spection of a part. The metal thickness is mea-<br />
sured before the masking is removed. Figure<br />
646 shows the masking being removed from a<br />
section of a wing skin. The entire side shown<br />
was masked, and some areas of the other side<br />
were etched. In Figure 647, the masking is<br />
being stripped from a milled part.<br />
Figure 645. Inspection of milled parts. The in-<br />
After the milling, the paint-like masking is strument measures the metal thickness before the<br />
softened in a solution consisting, for example, masking is removed (U.S. Chemical Mi l l ing Corpora-<br />
of 80 percent chlorinated hydrocarbons and 20 tion, Manhatten Beach. Calif.),
848 CHEMICAL PROCESSING EQUIPMENT<br />
ETCHANT SOLUTIONS<br />
Figure 646. Stripping masking from a.section of a wing skin of a<br />
B-58. The entire side shown was masked. Some areas of the other<br />
side were mil led (U.S. Chemical Milling Corporation, Manhatten Beach,<br />
Calif.).<br />
Etchants range from sodium hydroxide solution<br />
for aluminum to aqua regia for stainless steel.<br />
For milling a specific metal, the concentration<br />
of the chemical in the solution may vary widely<br />
between different operators: however, each<br />
operator controls the concentration of his solu-<br />
tion to within very close limits. The concen-<br />
Figure 647. Masking being stripped, milled parts with masking still<br />
in lace, and mil led parts with masking removed (U.S. Chemical Mi l-<br />
ling Corporation, Manhatten Beach, Calif.).<br />
tration of the solution affects the milling rat'e;<br />
therefore, it must be closely controlled to ob-<br />
tain the desired rate. For milling aluminum,<br />
the solutions in use contain from 7 to 30 per-<br />
cent sodium hydroxide. For lnilling magnesi-<br />
um, dilute sulfuric acid solutions are adequate.<br />
Stainless steels require strong solutions, USU-<br />
ally aqua regia fortified with sulfuric acid. In<br />
most of the milling solutions, surface-active
agents are used to ensure smooth, even cuts.<br />
The surface-active agents also reduce the ten-<br />
dency toward mist formation by reducing the<br />
surface tension of the solution. The solutions,<br />
during milling operations, are generally main-<br />
tained at constant temperatures ranging from<br />
105" to 190" F.<br />
THE AIR POLLUTION PROBLEM<br />
The air contaminants emitted in the prepara-<br />
tion of metals by chemical milling consist of<br />
mists, vapors, gases, and organic solvents.<br />
Mists<br />
A mist of the etching solution used in a milling<br />
process is discharged from the milling tank<br />
owing to entrainment of droplets of the solution<br />
hy the gas hubbles formed by the chemical<br />
action of the etchant on the metal. The amount<br />
of mist generated depends upon factors such as<br />
the nature of the chemical reaction, the solu-<br />
tion temperature, and the surface tension of the<br />
solution. Since the solutions from which the<br />
mists are formed are very corrosive, the mists,<br />
too, are very corrosive and are capable of caus-<br />
ing annoyance, or a nuisance, or a health hazard<br />
to persons, or damage to property.<br />
Some of the acid solutions used, such as hydro-<br />
chloric and nitric, have high vapor pressures<br />
at the temperatures used for the milling pro-<br />
cess; therefore, appreciable amounts of acid<br />
vapors are discharged. Unlike the discharge<br />
of mists, which occurs only during the milling,<br />
the vapors are discharged continuously from the<br />
hot solution. Under certain atmospheric condi-<br />
tions, the vapors condense, forming acid mists<br />
in the atmosphere.<br />
Gases<br />
Since hydrogen is formed in chemical milling,<br />
proper ventilation must be provided to prevent<br />
the accumulation of dangerous concentrations of<br />
this gas.<br />
Solvents<br />
Organic solvent vapors may be emitted from the<br />
vapor degreaser, the maskant area, and the<br />
curing station in the cleaning and masking pro-<br />
cesses. This type of air contaminant, and the<br />
method of controlling it are described elsewhere<br />
in this manual. Alkaline cleaning, pickling, and<br />
passivating tanks from the other phases of the<br />
cleaning processes have been found to be minor<br />
sources of air pollution.<br />
:a1 Milling 849<br />
HOODING AND VENTILATION REQUIREMENTS<br />
The air contaminants released from chemical<br />
milling tanks can be captured by local exhaust<br />
systems. Since open tanks are used to provide<br />
unobstructed working area, most exhaust sys-<br />
tems employ slotted hoods to capture the mists<br />
and vapors. In designing slot hoods for chem-<br />
ical milling equipment, it is particularly im-<br />
portant to provide for the elimination of exces-<br />
sive cross-drafts as well as for adequate dis-<br />
tribution of ventilation along the entire length<br />
of the hoods. The minimum ventilation rates<br />
previously mentioned in Chapter 3 are for tanks<br />
located in an area having no cross-drafts. If<br />
the tank is to be located outside or in a very<br />
drafty building, either the ventilation rate will<br />
have to be greatly increased or baffles must be<br />
used to shield the tank from winds or drafts.<br />
In some instances, both baffles and increased<br />
ventilation are needed.<br />
Adequate distribution of ventilation along the<br />
entire length of a slot can be attained by pro-<br />
viding a high slot velocity and a relatively low<br />
plenum velocity. The slot velocity should be at<br />
least 2, 000 fpm, and the plenum velocity should<br />
be not more than half of the slot velocity. With<br />
hoods more than 10 feet in length, either multi-<br />
ple takeoffs or splitter vanes are needed. Enough<br />
takeoffs or splitters should be used to reduce<br />
the length of the slot to sections not more than<br />
10 feet long.<br />
Under excessively drafty conditions, a hood en-<br />
closing the tank can be used to advantage. The<br />
hood should cover the entire tank and have suf-<br />
ficient height to accommodate the largest metal<br />
sections that can be handled in the tank. Vari-<br />
ous methods have been used to get work into and<br />
out of the tank. In one installation, the hood has<br />
doors on one end, and a monorail, suspended<br />
below the hood roof, that runs out through the<br />
doors. The work is carried on the monorail<br />
into the hood and above the solution. After the<br />
work is lowered into the solution, the doors are<br />
closed, when necessary, to ensure complete<br />
capture of the air contaminants created. In<br />
another installation, the hood is left open on one<br />
end, and a slot hood placed across the opening.<br />
The top of the hood is slotted to provide for the<br />
movement of the crane cable. This slot is<br />
nominally closed with rubber strips, which are<br />
pushed aside by the cable during movement of<br />
the crane.<br />
AIR POLLUTION CONTROL EQUIPMEN1<br />
Many types of wet collectors that can control<br />
the emissions from chemical milling tanks are<br />
commercially available. The one most common-
850 CHEhiIICAL PROCESS<br />
ly used is the spray and baffle type, owing prob-<br />
ably to its low cost. and ease of coating with cor-<br />
rosion-inhibiting materials. Moreover, the oper-<br />
ation and maintenance of this type are simple<br />
and inexpensive compared with those of other<br />
types of scrubbers.<br />
Figure 648 shows an exhaust and mist control<br />
system employing two scrubbers, one for each<br />
side oi a 24-foot-long by 6-foot-wide tank used<br />
for chemically milling stainless steel and ti-<br />
tanium. The etching solution is a mixture of<br />
hydrochloric, nitric, and sulfuric acids and is<br />
heated to 150°F. Acid vapors discharged from<br />
the solution are captured by slot hoods, one on<br />
each side of the tank. The ducts from each<br />
hood exit downward from the center. Each hood<br />
has four splitter vanes, which divide it into four<br />
sections. The overall hood length is 24 feet,<br />
the end-sections and those adjacent being 4 feet<br />
long each, and the center section being 8 feet<br />
long. Distribution of ventilation is excellent.<br />
Each hood is supplied with 18, 000 cfm ventila-<br />
Figure 648. A tank used for the chemical milling of<br />
stainless steel, and Dart of its air oollution<br />
control system. The hoods, ductwork, and scrubbers<br />
shown are made ent~rely of polyester resin reinforced<br />
wlth flberglas. The fans and discharge ducts,<br />
not shown, are steel-coated wlth polyester resln.<br />
(U S Chem~cal MI ll lnE Coraoratlon, Manhatten Beach,<br />
tion, and the slot is sized to give an intake ve-<br />
locity of 2, 000 fpm. The plenum velocity is<br />
less than 1, 000 fpm. It is estimated that this<br />
system provides sufficient ventilation to capture<br />
at least 95 percent of the vapors emerging from<br />
the process.<br />
The scrubbers are of the spray and baffle type,<br />
as shown in Figure 649. They are cylindrical,<br />
two baffles forming three concentric chambers.<br />
Gases enter at the top and flow down through<br />
the center cylindrical section. Water from a<br />
bank of sprays scrubs the gases as they enter<br />
this section. The bottom of the scrubber is<br />
filled with water to a depth of 1 foot. The gases<br />
and scrubbing water flow downward through the<br />
center section and impinge on the water. The<br />
gases turn 180 degrees and flow upward through the<br />
second chamber. Most of the scrubbing water<br />
remains in the sump. The depth of water in the<br />
sump is maintained at a uniform level with a<br />
float valve and an overflow line. The scrubber<br />
is equipped with a pump to circulate the sump<br />
water to the sprays. In this installation, how-<br />
ever, only fresh water is used, the sump being<br />
kept full and overflowing all the time.<br />
The gases flowupward throughthe second sectionand<br />
over the secondbaffle. Theyturn 180 degrees to enter<br />
the third section. In the third section, the gas-<br />
es flow down and around to the outlet port. Most<br />
of the entrained moisture entering the second<br />
section is removed either by impingement on<br />
the walls of that section or by centrifugal im-<br />
pingementduring the 180-degree change of direction<br />
into the third section. The last of the entrained<br />
water is deposited on the walls of the third sec-<br />
tion. The gases then flow from the scrubber to<br />
the fan, from which they are discharged to the<br />
atmosphere through ducts.<br />
The hoods, the scrubber, and the ductwork con-<br />
necting the hoods to the scrubbers and the scrub-<br />
bers to the fans are made entirely of polyester<br />
resin reinforced with glass fibers. The fans and<br />
discharge ducts are made of steel coated with<br />
polyester resin.<br />
The existing system provides satisfactory con-<br />
trol of the vapors. It captures an estimated 95<br />
percent of the vapors at the tank, and the gases<br />
discharged have only a slight acid odor.<br />
Corrosion Problems<br />
Whenever moisture is present in an exhaust<br />
system, the iron or steel surfaces should be<br />
coated to prevent corrosion. However, since<br />
zinc is soluble in both acid and alkaline solu-<br />
tions, galvanized iron cannot be used when<br />
chemical milling tanks are vented. A coating
Model<br />
Motora<br />
ho<br />
.".<br />
Chemical Milling 851<br />
DRA l N<br />
Pump Drain<br />
rac.htb A B C D E F 6 H I K L<br />
'~otor is 440/220 volts, 3 phase. 60 cycle. Exhaust fan and motor furnished upon request<br />
b D ~ ~ not s include recirculating motor and pump.<br />
Figure 649. A scrubber used to control the acid vapors discharged<br />
from a tank used to mill stainless steel (Lin-0-Coat scrubber,<br />
manufactured by Diversified Plastics. Inc., Paramount, Calif.).<br />
such as polyvinylchloride (PVC), which is not<br />
attacked by either dilute acids or dilute alkalies,<br />
should be used. It has been found, however,<br />
that the PVC linings in ducts and scrubbers can-<br />
not withstand the strongly oxidizing acids used<br />
for stainless steel and titanium milling. These<br />
highly corrosive acids have been successfully<br />
handled in exhaust systems made of polyester<br />
resins reinforced with fiberglas. Hoods, ducts,<br />
and scrubbers are available made entirely of<br />
polyester-fiberglas material. Figure 648 shows<br />
i<br />
Spray<br />
nozzles<br />
Drain<br />
size. it,.<br />
Mi".-range-max.<br />
lorn cfm i fom I r fm<br />
an air pollution control system venting a 24-foot-<br />
long tank for stainless steel chemical milling.<br />
The hoods, ductwork up to the blowers, and the<br />
scrubbers are made entirely of polyester-fiber-<br />
glas material. The steel blowers and discharge<br />
ducts are coated with polyester resin. Some<br />
blower manufacturers are now advertising blow-<br />
ers with scrolls made entirely of polyester-<br />
fiberglas and with steel wheels coated with the<br />
same material.<br />
!<br />
1