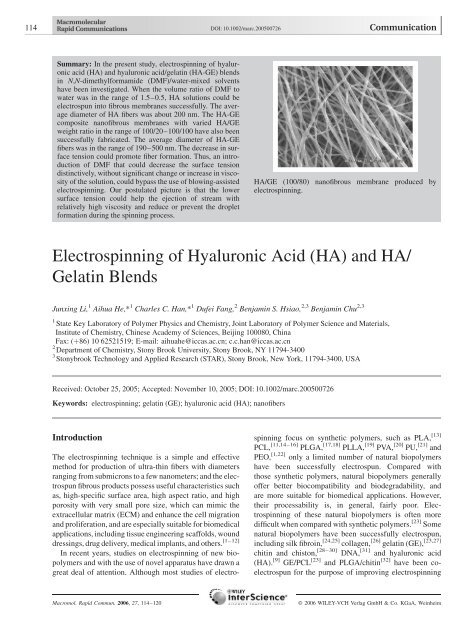
Electrospinning of Hyaluronic Acid (HA) and HA/Gelatin Blends
Electrospinning of Hyaluronic Acid (HA) and HA/Gelatin Blends
Electrospinning of Hyaluronic Acid (HA) and HA/Gelatin Blends
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
114 DOI: 10.1002/marc.200500726 Communication<br />
Summary: In the present study, electrospinning <strong>of</strong> hyaluronic<br />
acid (<strong>HA</strong>) <strong>and</strong> hyaluronic acid/gelatin (<strong>HA</strong>-GE) blends<br />
in N,N-dimethylformamide (DMF)/water-mixed solvents<br />
have been investigated. When the volume ratio <strong>of</strong> DMF to<br />
water was in the range <strong>of</strong> 1.5–0.5, <strong>HA</strong> solutions could be<br />
electrospun into fibrous membranes successfully. The average<br />
diameter <strong>of</strong> <strong>HA</strong> fibers was about 200 nm. The <strong>HA</strong>-GE<br />
composite nan<strong>of</strong>ibrous membranes with varied <strong>HA</strong>/GE<br />
weight ratio in the range <strong>of</strong> 100/20–100/100 have also been<br />
successfully fabricated. The average diameter <strong>of</strong> <strong>HA</strong>-GE<br />
fibers was in the range <strong>of</strong> 190–500 nm. The decrease in surface<br />
tension could promote fiber formation. Thus, an introduction<br />
<strong>of</strong> DMF that could decrease the surface tension<br />
distinctively, without significant change or increase in viscosity<br />
<strong>of</strong> the solution, could bypass the use <strong>of</strong> blowing-assisted<br />
electrospinning. Our postulated picture is that the lower<br />
surface tension could help the ejection <strong>of</strong> stream with<br />
relatively high viscosity <strong>and</strong> reduce or prevent the droplet<br />
formation during the spinning process.<br />
<strong>Electrospinning</strong> <strong>of</strong> <strong>Hyaluronic</strong> <strong>Acid</strong> (<strong>HA</strong>) <strong>and</strong> <strong>HA</strong>/<br />
<strong>Gelatin</strong> <strong>Blends</strong><br />
Junxing Li, 1 Aihua He,* 1 Charles C. Han,* 1 Dufei Fang, 2 Benjamin S. Hsiao, 2,3 Benjamin Chu 2,3<br />
1 State Key Laboratory <strong>of</strong> Polymer Physics <strong>and</strong> Chemistry, Joint Laboratory <strong>of</strong> Polymer Science <strong>and</strong> Materials,<br />
Institute <strong>of</strong> Chemistry, Chinese Academy <strong>of</strong> Sciences, Beijing 100080, China<br />
Fax: (þ86) 10 62521519; E-mail: aihuahe@iccas.ac.cn; c.c.han@iccas.ac.cn<br />
2 Department <strong>of</strong> Chemistry, Stony Brook University, Stony Brook, NY 11794-3400<br />
3 Stonybrook Technology <strong>and</strong> Applied Research (STAR), Stony Brook, New York, 11794-3400, USA<br />
Received: October 25, 2005; Accepted: November 10, 2005; DOI: 10.1002/marc.200500726<br />
Keywords: electrospinning; gelatin (GE); hyaluronic acid (<strong>HA</strong>); nan<strong>of</strong>ibers<br />
Introduction<br />
The electrospinning technique is a simple <strong>and</strong> effective<br />
method for production <strong>of</strong> ultra-thin fibers with diameters<br />
ranging from submicrons to a few nanometers; <strong>and</strong> the electrospun<br />
fibrous products possess useful characteristics such<br />
as, high-specific surface area, high aspect ratio, <strong>and</strong> high<br />
porosity with very small pore size, which can mimic the<br />
extracellular matrix (ECM) <strong>and</strong> enhance the cell migration<br />
<strong>and</strong> proliferation, <strong>and</strong> are especially suitable for biomedical<br />
applications, including tissue engineering scaffolds, wound<br />
dressings, drug delivery, medical implants, <strong>and</strong> others. [1–12]<br />
In recent years, studies on electrospinning <strong>of</strong> new biopolymers<br />
<strong>and</strong> with the use <strong>of</strong> novel apparatus have drawn a<br />
great deal <strong>of</strong> attention. Although most studies <strong>of</strong> electro-<br />
<strong>HA</strong>/GE (100/80) nan<strong>of</strong>ibrous membrane produced by<br />
electrospinning.<br />
spinning focus on synthetic polymers, such as PLA, [13]<br />
PCL, [11,14–16] PLGA, [17,18] PLLA, [19] PVA, [20] PU, [21] <strong>and</strong><br />
PEO, [1,22] only a limited number <strong>of</strong> natural biopolymers<br />
have been successfully electrospun. Compared with<br />
those synthetic polymers, natural biopolymers generally<br />
<strong>of</strong>fer better biocompatibility <strong>and</strong> biodegradability, <strong>and</strong><br />
are more suitable for biomedical applications. However,<br />
their processability is, in general, fairly poor. <strong>Electrospinning</strong><br />
<strong>of</strong> these natural biopolymers is <strong>of</strong>ten more<br />
difficult when compared with synthetic polymers. [23] Some<br />
natural biopolymers have been successfully electrospun,<br />
including silk fibroin, [24,25] collagen, [26] gelatin (GE), [23,27]<br />
chitin <strong>and</strong> chiston, [28–30] DNA, [31] <strong>and</strong> hyaluronic acid<br />
(<strong>HA</strong>). [9] GE/PCL [23] <strong>and</strong> PLGA/chitin [32] have been coelectrospun<br />
for the purpose <strong>of</strong> improving electrospinning<br />
Macromol. Rapid Commun. 2006, 27, 114–120 ß 2006 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
<strong>Electrospinning</strong> <strong>of</strong> <strong>Hyaluronic</strong> <strong>Acid</strong> (<strong>HA</strong>) <strong>and</strong> <strong>HA</strong>/<strong>Gelatin</strong> <strong>Blends</strong> 115<br />
processability to fabricating into novel nan<strong>of</strong>ibrous<br />
composites.<br />
<strong>Hyaluronic</strong> acid, as a naturally occurring linear polysaccharide,<br />
consists <strong>of</strong> repeating disaccharide units (b-1,4-<br />
D-glucuronic acid <strong>and</strong> b-1,3-N-acetyl-D-glucosamine), is<br />
the main component <strong>of</strong> the ECM <strong>of</strong> connective tissues, <strong>and</strong><br />
has many important biological functions. [33–35] Due to its<br />
excellent properties <strong>of</strong> biocompatibility <strong>and</strong> biodegradability,<br />
<strong>HA</strong> <strong>and</strong> its derivatives have been used extensively in<br />
biomedical field, for example in wound dressings, tissue<br />
scaffolds, arthritis treatment, drug delivery, <strong>and</strong> components<br />
<strong>of</strong> implant materials. [35–38] However, medical studies<br />
on <strong>HA</strong> nan<strong>of</strong>ibrous membranes have hardly been reported<br />
in spite <strong>of</strong> its importance in medical applications.<br />
Generally, it was very difficult to carry out electrospinning<br />
<strong>of</strong> <strong>HA</strong> aqueous solution. The molecular weight <strong>of</strong><br />
native <strong>HA</strong> is typically several million. The unusually high<br />
viscosity <strong>and</strong> surface tension <strong>of</strong> <strong>HA</strong> were thought to be the<br />
key factors that hinder the electrospinning <strong>of</strong> <strong>HA</strong> solution.<br />
Additionally, the strong water retention ability <strong>of</strong> <strong>HA</strong> might<br />
lead to the fuse <strong>of</strong> nan<strong>of</strong>ibers electrospun on the collector<br />
due to the insufficient evaporation <strong>of</strong> solvents in electrospinning.<br />
The fabrication <strong>of</strong> <strong>HA</strong> into nan<strong>of</strong>ibrous nonwoven<br />
membranes from aqueous solution was successfully<br />
carried out only after the development <strong>of</strong> blowing-assisted<br />
electrospinning. [9]<br />
In this paper, the electrospinning process <strong>of</strong> <strong>HA</strong> <strong>and</strong><br />
hyaluronic acid/gelatin (<strong>HA</strong>-GE) blends in mixed solvents<br />
has been investigated. The main objectives are as follows:<br />
(a) Explore the electrospinning process <strong>of</strong> pure <strong>HA</strong><br />
solution in water <strong>and</strong> mixed solvents, find the key factor<br />
which hinders the electrospinning <strong>of</strong> <strong>HA</strong> aqueous solution,<br />
<strong>and</strong> provide the solution to this spinning difficulty.<br />
(b) Find a novel <strong>and</strong> simple way to electrospin <strong>HA</strong><br />
solutions with the common electrospinning setup.<br />
(c) Investigate the effects <strong>of</strong> GE on the electrospinning<br />
process <strong>of</strong> <strong>HA</strong> solutions. The characteristics <strong>of</strong> GE, such as<br />
relatively lower molecular weight <strong>and</strong> amphiphilic property,<br />
might favor the electrospinning <strong>of</strong> <strong>HA</strong> solution.<br />
(d) Obtain <strong>HA</strong>-GE nan<strong>of</strong>ibrous membranes with various<br />
blending ratios. The <strong>HA</strong>-GE nan<strong>of</strong>ibrous membranes with<br />
different compositions <strong>and</strong> sized scale could have different<br />
clinical applications to drug delivery, wound healings, <strong>and</strong><br />
tissue engineering scaffolds.<br />
The present report shows that with the aid <strong>of</strong> N,Ndimethylformamide<br />
(DMF), <strong>HA</strong> can be electrospun successfully<br />
with common electrospinning setup at neutral pH,<br />
<strong>and</strong> <strong>HA</strong>-GE aqueous solutions were first co-electrospun to<br />
produce <strong>HA</strong>-GE composite fibrous non-woven membranes.<br />
Experimental Part<br />
Materials<br />
<strong>Hyaluronic</strong> acid (sodium salt, Mw ¼ 2 000 000) was purchased<br />
from Dali Co. (Nanning, China). Polymers <strong>of</strong> GE Type A<br />
(Approx. 220 Bloom, Mn ¼ 80 000), extracted from porcine<br />
skin by acidic process, were purchased from Sanhesheng<br />
<strong>Gelatin</strong> Co. (Wenzhou, China). DMF <strong>and</strong> ethanol were<br />
obtained from Beijing Chem. Co. (Beijing, China). All the<br />
solvents were used without further purification.<br />
Preparation <strong>of</strong> Spinning Solution<br />
<strong>HA</strong> Solutions in Water System<br />
Transparent aqueous solutions <strong>of</strong> <strong>HA</strong> were prepared by dissolving<br />
a specific amount <strong>of</strong> <strong>HA</strong> powder in the mixed solvents<br />
consisting <strong>of</strong> distilled water (w) <strong>and</strong> ethanol (e) (w/e ¼ 9/1,<br />
volume ratio). Pure <strong>HA</strong> solutions with concentrations <strong>of</strong> 1.3 w/<br />
v% (w in grams <strong>and</strong> v in milliliters) <strong>and</strong> 1.5 w/v% were<br />
obtained.<br />
1.5 w/v% <strong>HA</strong> Solutions in DMF/Water System<br />
Dissolve certain amounts <strong>of</strong> <strong>HA</strong> powder in DMF under gentle<br />
stirring for 10 min, <strong>and</strong> then add a specific amount <strong>of</strong> distilled<br />
water into the <strong>HA</strong> solution according to the volume ratio <strong>of</strong><br />
DMF to water (2, 1.5, 1 <strong>and</strong> 0.5), respectively, <strong>and</strong> continue to<br />
stir the solution for 20 min until the solution became<br />
transparent. The spinning solution <strong>of</strong> 1.5 w/v% <strong>HA</strong> in DMF/<br />
water system (2, 1.5, 1 <strong>and</strong> 0.5) was prepared.<br />
<strong>HA</strong>-GE <strong>Blends</strong> Solutions<br />
1.875 w/v% <strong>HA</strong> in DMF/water (volume ratio ¼ 1.5) solution<br />
was prepared using the same procedure as above. 1.5, 3, 4.5, 6,<br />
<strong>and</strong> 7.5 w/v% GE solutions with the solvent being pure water<br />
was prepared at 40 8C under gentle stirring for 20 min. Add the<br />
GE solution into the <strong>HA</strong> solution with specific volumes to<br />
obtain the <strong>HA</strong>-GE solutions (<strong>HA</strong>/GE ¼ 100/20, 100/40, 100/<br />
60, 100/80, 100/100, weight ratio), <strong>and</strong> in each solution the<br />
concentration <strong>of</strong> <strong>HA</strong> was fixed at 1.5 w/v%.<br />
<strong>Electrospinning</strong><br />
All electrospinning solutions, spinneret, <strong>and</strong> the environmental<br />
temperatures were controlled at 40 3 8C. The electrospinning<br />
solutions were placed into a 5 ml syringe with a capillary tip<br />
having an inner diameter <strong>of</strong> 0.3 mm. A syringe pump was used<br />
to feed the polymer solution <strong>and</strong> the feeding rate was fixed<br />
at 60 ml min 1 . A high-voltage power supply was employed<br />
to generate the electric field (0–50 kV). The applied voltage<br />
was fixed at 22 kV. The tip-to-collector distance was fixed at<br />
15 cm.<br />
<strong>Hyaluronic</strong> acid has strong water retention ability. The residual<br />
water could dissolve the electrospun fibers on the collector<br />
if the aluminum foil was used. In this study, the ethanol bath<br />
was used as a collector because ethanol is a poor solvent for<br />
both <strong>HA</strong> <strong>and</strong> GE. A piece <strong>of</strong> aluminum foil, connecting to the<br />
ground, was immersed in a vessel filled with ethanol. After<br />
electrospinning, the vessel was put into the vacuum oven at<br />
50 8C for 30 min to dry <strong>of</strong>f ethanol <strong>and</strong> other solvents. Then the<br />
aluminum foil can be taken out. Through this method, <strong>HA</strong> <strong>and</strong><br />
<strong>HA</strong>-GE films can be peeled <strong>of</strong>f easily.<br />
Macromol. Rapid Commun. 2006, 27, 114–120 www.mrc-journal.de ß 2006 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
116 J. Li, A. He, C. C. Han, D. Fang, B. S. Hsiao, B. Chu<br />
Characterization<br />
The morphologies <strong>of</strong> the electrospun fibers were observed<br />
by means <strong>of</strong> scanning electron microscopy (SEM, HITACHI<br />
S-4300) at an accelerating voltage <strong>of</strong> 5 or 15 kV. The<br />
conductivities <strong>and</strong> the surface tensions <strong>of</strong> spinning solutions<br />
were measured at 40 8C by conductivity meter (DDS-307A,<br />
Shanghai Rex Instruments) <strong>and</strong> surface tension meter (DCAT<br />
21, Dataphysics) with the Wilhelmy plate method, respectively.<br />
The shear viscosities <strong>of</strong> the spinning solutions were<br />
measured by using Advanced Rheometric Expansion System<br />
(Ares, TA Instruments) at 40 8C. The 17 mm 16 mm couette<br />
geometry was used for the measurements. The shear rate was<br />
controlled from 1 to 1 000 s 1 .<br />
Results <strong>and</strong> Discussion<br />
<strong>Electrospinning</strong> <strong>of</strong> Pure <strong>HA</strong><br />
<strong>Hyaluronic</strong> acid could be dissolved in water easily, but<br />
could not be dissolved in most organic solvents. In the present<br />
study, water-ethanol mixed solvent was used to dissolve<br />
<strong>HA</strong> in order to increase the evaporation rate <strong>and</strong> to<br />
decrease the surface tension <strong>of</strong> the <strong>HA</strong> solution. The applied<br />
voltage was fixed at 22 kV, <strong>and</strong> the distance between the<br />
spinneret tip <strong>and</strong> the collector was set at 25 cm. The electrospinning<br />
<strong>of</strong> <strong>HA</strong> solution at different <strong>HA</strong> concentrations<br />
was investigated. The electrospinning process was rather<br />
discontinuous as the SEM results being shown in Figure 1(a)<br />
<strong>and</strong> (c). It can be seen from SEM images that many large<br />
irregular beads with few fibers were produced on the collector.<br />
However, when the distance between the electrode <strong>and</strong><br />
the collector was decreased to 15 cm, the membrane morphology<br />
became more uniform when compared with that<br />
electrospun at the 25 cm distance, as shown in Figure 1(b)<br />
<strong>and</strong> (d). The bead-string structure was formed at both 1.3<br />
<strong>and</strong> 1.5 w/v% <strong>HA</strong> aqueous solution <strong>and</strong> the beads were<br />
more like spindles in Figure 1(b) <strong>and</strong> (d). Therefore, an<br />
increase in the electric field strength could benefit the fiber<br />
formation. However, the electrospinning process under<br />
these conditions was not satisfactory, as large bead formation<br />
occurred even when the voltage was increased to 40 kV.<br />
It meant that it was impossible to improve the electrospinning<br />
process <strong>of</strong> <strong>HA</strong> aqueous solution by adjusting the<br />
distance <strong>and</strong> applied voltage.<br />
The above results are well consistent with that in ref. [9]<br />
Although with the assistance <strong>of</strong> air-blowing <strong>HA</strong> aqueous<br />
solution could be electrospun fluently, what we want to<br />
know is that which is the key factor hindering the electrospinning<br />
<strong>of</strong> <strong>HA</strong> solution with common electrospinning<br />
setup.<br />
In our study, the physical properties <strong>of</strong> <strong>HA</strong> solution have<br />
been studied in detail. A solvent mixture <strong>of</strong> DMF-water was<br />
used. DMF was chosen because it is a polar solvent with<br />
poor solubility for <strong>HA</strong>. <strong>HA</strong>/DMF-water solutions at a<br />
volume ratio <strong>of</strong> DMF to water in the range <strong>of</strong> 0.5–2:1 were<br />
used for electrospinning. The morphologies <strong>of</strong> obtained<br />
membranes are shown in Figure 2. At a volume ratio <strong>of</strong><br />
Figure 1. Effect <strong>of</strong> the distance <strong>of</strong> tip to collector (d) on the morphology <strong>of</strong> electrospun<br />
fibers <strong>of</strong> pure <strong>HA</strong> solutions in water system ( 5k). Distilled water <strong>and</strong> ethanol mixture was<br />
used as solvent (volume ratio <strong>of</strong> water to ethanol was 9/1), the voltage was fixed at 22 kV.<br />
(a) <strong>HA</strong> 1.3 w/v%, d ¼ 25 cm; (b) <strong>HA</strong> 1.3 w/v%, d ¼ 15 cm; (c) <strong>HA</strong> 1.5 w/v%, d ¼ 25 cm; (d)<br />
<strong>HA</strong> 1.5 w/v%, d ¼ 15 cm.<br />
Macromol. Rapid Commun. 2006, 27, 114–120 www.mrc-journal.de ß 2006 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
<strong>Electrospinning</strong> <strong>of</strong> <strong>Hyaluronic</strong> <strong>Acid</strong> (<strong>HA</strong>) <strong>and</strong> <strong>HA</strong>/<strong>Gelatin</strong> <strong>Blends</strong> 117<br />
Figure 2. SEM images <strong>of</strong> <strong>HA</strong> (1.5 w/v%) fibers at different volume ratio <strong>of</strong> DMF to water<br />
( 5k). The voltage was fixed at 22 kV<strong>and</strong> the distance <strong>of</strong> tip to collector was fixed at 15 cm.<br />
(a) DMF/water ¼ 0.5, (b) DMF/water ¼ 1, (c) DMF/water ¼ 1.5, <strong>and</strong> (d) DMF/water ¼ 2.<br />
DMF to water was 0.5, the fibers were rather thin, but large<br />
polymer beads occurred on the collector, as shown in<br />
Figure 2(a). The average fiber diameter was about 120 nm.<br />
When the volume ratio <strong>of</strong> DMF to water was 1, the electrospinning<br />
process could proceed very smoothly <strong>and</strong> the<br />
average fiber diameter was in the order <strong>of</strong> 200 nm. When the<br />
volume ratio <strong>of</strong> DMF to water was 1.5, the solution still<br />
remained transparent (implying no <strong>HA</strong> precipitation). SEM<br />
images showed that uniform fibers without beads could be<br />
produced with an average fiber diameter <strong>of</strong> about 250 nm. It<br />
was found that <strong>HA</strong> could not be completely dissolved in the<br />
mixed solvent when the volume ratio <strong>of</strong> DMF to water<br />
was 2. Therefore, the proper volume ratio <strong>of</strong> DMF to water<br />
was 0.5–1.5:1 for <strong>HA</strong> with a molecular weight in the two<br />
million range.<br />
Table 1. Physical data <strong>of</strong> <strong>HA</strong> <strong>and</strong> <strong>HA</strong>/GE spinning solutions.<br />
It was found that both the surface tensions <strong>and</strong> conductivities<br />
<strong>of</strong> <strong>HA</strong> solutions decreased with increasing in DMF<br />
content, as shown in Table 1 (samples 2, 3, 4, 5, <strong>and</strong> 6). It can<br />
be concluded that the decrease in surface tension could<br />
benefit the electrospinning process <strong>and</strong> make the beads<br />
disappear. The average diameter <strong>of</strong> <strong>HA</strong> fibers increased<br />
when DMF was introduced.<br />
<strong>Electrospinning</strong> <strong>of</strong> <strong>HA</strong>/<strong>Gelatin</strong> <strong>Blends</strong><br />
<strong>Gelatin</strong> is a natural biopolymer derived from collagen by<br />
controlled hydrolysis <strong>and</strong> has been widely used in biomedical<br />
fields. [39–43] Studies on blends <strong>of</strong> <strong>HA</strong> <strong>and</strong> GE have<br />
indicated that GE can endow <strong>HA</strong> with protein properties to<br />
improve the cell attachment <strong>and</strong> the blends <strong>of</strong> <strong>HA</strong>-GE have<br />
Sample <strong>HA</strong>/GE weight <strong>HA</strong> w/v% DMF/water in <strong>HA</strong> Surface tension Conductivity Average diameter<br />
number ratios<br />
solution v/v<br />
mN m 1<br />
mS cm 1<br />
nm<br />
1 100/0 1.3 0 52.99 2 870 –<br />
2 100/0 1.5 0 52.33 3 170 –<br />
3 100/0 1.5 0.5 50.95 1 612 120<br />
4 100/0 1.5 1 47.85 1 178 200<br />
5 100/0 1.5 1.5 45.91 970 250<br />
6 100/0 1.5 2 44.66 773 –<br />
7 100/20 1.5 1.5 47.46 1 250 190<br />
8 100/40 1.5 1.5 46.12 1 214 260<br />
9 100/60 1.5 1.5 44.97 1 210 320<br />
10 100/80 1.5 1.5 46.55 1 116 350<br />
11 100/100 1.5 1.5 39.83 1 067 500<br />
Macromol. Rapid Commun. 2006, 27, 114–120 www.mrc-journal.de ß 2006 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
118 J. Li, A. He, C. C. Han, D. Fang, B. S. Hsiao, B. Chu<br />
the potential use in tissue repair. [44] GE is also an ampholyte<br />
polymer with interesting protein characteristics <strong>and</strong> generally<br />
has lower molecular weight when compared with that<br />
<strong>of</strong> <strong>HA</strong>. The purpose <strong>of</strong> the GE used in this study was to<br />
improve the electrospinning processability <strong>and</strong> to endow<br />
the fibrous membranes with protein characteristic. Therefore,<br />
<strong>HA</strong>-GE/DMF-water solutions were prepared in this<br />
study to investigate the electrospinning process <strong>of</strong> this two<br />
natural biopolymer blend. The concentration <strong>of</strong> <strong>HA</strong> was<br />
fixed at 1.5 w/v% <strong>and</strong> the volume ratio <strong>of</strong> DMF to water was<br />
1.5. The electrospinning <strong>of</strong> <strong>HA</strong>-GE solutions could be<br />
carried out successfully with the electrospinning processes<br />
becoming smoother on increasing the GE content. SEM<br />
images at both low <strong>and</strong> high magnifications indicated that<br />
uniform <strong>HA</strong>-GE nan<strong>of</strong>ibrous membranes could be fabricated<br />
(Figure 3). <strong>HA</strong>-GE fibrous membranes at different<br />
Figure 3. SEM images <strong>of</strong> <strong>HA</strong>-GE composite fibrous membranes. <strong>HA</strong> ¼ 1.5 w/v%, DMF/<br />
water ¼ 1.5, volume ratio. Weight ratio <strong>of</strong> (a) <strong>HA</strong>/GE ¼ 100/20, (b) <strong>HA</strong>/GE ¼ 100/40,<br />
(c) <strong>HA</strong>/GE ¼ 100/60, (d) <strong>and</strong> (f) <strong>HA</strong>/GE ¼ 100/80, (e), (g), <strong>and</strong> (h) <strong>HA</strong>/GE ¼ 100/100. In<br />
each solution, the concentration <strong>of</strong> <strong>HA</strong> was fixed at 1.5 w/v%. Magnification: panel (a–e,<br />
10k); panel (f, 200); panel (g, 100), <strong>and</strong> panel (h, 50).<br />
Macromol. Rapid Commun. 2006, 27, 114–120 www.mrc-journal.de ß 2006 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
<strong>Electrospinning</strong> <strong>of</strong> <strong>Hyaluronic</strong> <strong>Acid</strong> (<strong>HA</strong>) <strong>and</strong> <strong>HA</strong>/<strong>Gelatin</strong> <strong>Blends</strong> 119<br />
<strong>HA</strong>/GE compositions <strong>and</strong> different average diameters<br />
ranging from 190 to 500 nm could be produced by electrospinning.<br />
In the present procedure, the overall polymer<br />
concentration increased with increasing GE content, <strong>and</strong><br />
the higher polymer concentration led to an increase in the<br />
average diameter <strong>of</strong> <strong>HA</strong>-GE nan<strong>of</strong>ibers as well as the decrease<br />
in conductivity <strong>of</strong> <strong>HA</strong> solution, as shown in Table 1. The<br />
results <strong>of</strong> surface tension data (Table 1) also showed that<br />
the addition <strong>of</strong> GE, acting as a kind <strong>of</strong> surfactant, decreased<br />
the surface tension <strong>of</strong> the solution (samples 5, 7, 8, 9, 10, <strong>and</strong><br />
11 in Table 1) <strong>and</strong> rendered better electrospinning<br />
processability.<br />
Rheological measurements showed that the viscosity<br />
changed very little after DMF was added into the <strong>HA</strong><br />
solution, as shown in Figure 4(a). Both <strong>HA</strong> <strong>and</strong> <strong>HA</strong>-GE<br />
solutions in DMF-water had similarly high viscosities,<br />
which meant that electrospinning <strong>of</strong> <strong>HA</strong> solution with<br />
unusually high viscosity could be proceeded successfully.<br />
The normal force <strong>of</strong> spinning solutions, as shown in<br />
Figure 4(b), could reflect the viscoelastic part <strong>of</strong> the solutions,<br />
which was a necessary factor in the spinning process<br />
a)<br />
Normal force (N) Viscosity (Pa*s)<br />
100<br />
10<br />
1<br />
0.1<br />
1 10 100 1000<br />
Shear rate (s -1 )<br />
b)<br />
0.1<br />
0.01<br />
1E-3<br />
1E-4<br />
<strong>HA</strong>1.3w/v%<br />
<strong>HA</strong>1.5w/v%<br />
<strong>HA</strong> in DMF/water=1.5<br />
<strong>HA</strong>/GE=100/100<br />
<strong>HA</strong>1.3w/v%<br />
<strong>HA</strong>1.5w/v%<br />
<strong>HA</strong> in DMF/water=1.5<br />
<strong>HA</strong>/GE=100/100<br />
10 100 1000<br />
Shear rate (s -1 )<br />
Figure 4. Viscosity (a) <strong>and</strong> normal force (b) <strong>of</strong> pure <strong>HA</strong> <strong>and</strong> <strong>HA</strong>-<br />
GE solutions.<br />
including dry spinning <strong>and</strong> electrospinning. The viscoelasticity<br />
<strong>of</strong> <strong>HA</strong> (<strong>and</strong> <strong>HA</strong>-GE) in DMF/water was higher than<br />
that <strong>of</strong> <strong>HA</strong> in the water-ethanol solvent mixture.<br />
Therefore, the decrease in surface tension could play an<br />
important role in the electrospinning <strong>of</strong> <strong>HA</strong> <strong>and</strong> the formation<br />
<strong>of</strong> smooth fibers. In contrast, for the electrospinning <strong>of</strong><br />
<strong>HA</strong> in water-ethanol solution at a 15 cm distance, the<br />
relatively high surface tension led to the bead-on-string<br />
structure, as shown in Figure 1.<br />
The <strong>HA</strong>-GE fibrous membranes obtained from electrospinning<br />
looked like gauzes with ultra-thin fibers at low<br />
magnifications. This kind <strong>of</strong> nanostructures could have<br />
many potential clinical applications, especially in the field<br />
<strong>of</strong> artificial skin. Also, <strong>HA</strong>-GE fibrous membranes at<br />
different <strong>HA</strong>/GE compositions obtained by electrospinning<br />
are expected to have different cell proliferation properties<br />
<strong>and</strong> biodegradability, <strong>and</strong> have potential applications in<br />
wound dressings, such as hemostatic gauzes, tissue engineering<br />
scaffolds, <strong>and</strong> drug delivery.<br />
Conclusion<br />
In this study, pure <strong>HA</strong> solution could be successfully<br />
electrospun in DMF/water system without the assistance <strong>of</strong><br />
air blowing. The electrospinning processability <strong>of</strong> <strong>HA</strong>-GE<br />
solutions was also investigated. It was found that the<br />
processability <strong>of</strong> <strong>HA</strong> had been improved greatly by using a<br />
DMF-water solvent mixture or/<strong>and</strong> by adding GE into the<br />
<strong>HA</strong> solution. Nan<strong>of</strong>ibrous membranes with different<br />
average fiber diameters <strong>and</strong> different <strong>HA</strong>/GE compositions<br />
could be obtained. The average diameters <strong>of</strong> <strong>HA</strong> or <strong>HA</strong>based<br />
nan<strong>of</strong>ibrous membranes became larger <strong>and</strong> the<br />
conductivity <strong>of</strong> <strong>HA</strong> or <strong>HA</strong>-based solution decreased with<br />
increasing DMF content in the solvent mixture or increasing<br />
GE content. Measurements on viscosity indicated that<br />
the viscosity <strong>of</strong> <strong>HA</strong> solution in DMF-water mixed solvent<br />
did not change much when compared with that in waterethanol.<br />
<strong>HA</strong> solution with high viscosity could be electrospun.<br />
The decrease in surface tension contributed to the<br />
fiber formation <strong>of</strong> <strong>HA</strong> <strong>and</strong> <strong>HA</strong>/GE by electrospinning.<br />
Therefore, the study provided not only a novel <strong>and</strong> simpler<br />
way to electrospin the natural polyanion <strong>HA</strong> solution but<br />
also the fundamental physical insight <strong>and</strong> solution to this<br />
spinning difficulty. The <strong>HA</strong>-GE nan<strong>of</strong>ibrous membranes<br />
at different <strong>HA</strong>/GE compositions are expected to be<br />
useful in the biomedical field as novel scaffolds for many<br />
applications.<br />
Acknowledgements: This work was financially supported by<br />
the National Nature Science Foundation <strong>of</strong> China (Funds No.<br />
50503023 <strong>and</strong> 50373048), the National ‘‘973’’ Project (G2003<br />
CB615605), the MSC Creative Project <strong>of</strong> CAS (CMS-CX200503),<br />
the National Institutes <strong>of</strong> Health SBIR grant (GM63283-02) in the<br />
U.S, administered by the Stonybrook Technology <strong>and</strong> Applied<br />
Research, Inc.<br />
Macromol. Rapid Commun. 2006, 27, 114–120 www.mrc-journal.de ß 2006 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
120 J. Li, A. He, C. C. Han, D. Fang, B. S. Hsiao, B. Chu<br />
[1] H. Fong, I. Chun, D. H. Reneker, Polymer 1999, 40, 4585.<br />
[2] D. Li, Y. Xia, Adv. Mater. 2004, 16, 1151.<br />
[3] Z.-M. Huang, Y.-Z. Zhang, M. Kotaki, S. Ramakrishna,<br />
Compos. Sci. Technol. 2003, 63, 2223.<br />
[4] Y. M. Shin, M. M. Hohman, M. P. Brenner, G. C. Rutledge,<br />
Polymer 2001, 42, 9955.<br />
[5] W. He, Z. W. Ma, T. Yong, W. E. Teo, S. Ramakrishna,<br />
Biomaterials 2005, 26, 7606.<br />
[6] S. A. Riboldi, M. Sampaolesi, P. Neuenschw<strong>and</strong>er, G. Cossu,<br />
S. Mantero, Biomaterials 2005, 26, 4606.<br />
[7] M. G. McKee, C. L. Elkins, T. E. Long, Polymer 2004, 45,<br />
8705.<br />
[8] W.-J. Li, C. T. Laurencin, E. J. Caterson, R. S. Tuan, F. K. Ko,<br />
J. Biomed. Mater. Res. 2002, 60, 613.<br />
[9] I. C. Um, D. Fang, B. S. Hsiao, A. Okamoto, B. Chu,<br />
Biomacromolecules 2004, 5, 1428.<br />
[10] X. Zong, S. Ran, K.-S. Kim, D. Fang, B. S. Hsiao, B. Chu,<br />
Biomacromolecules 2003, 4, 416.<br />
[11] W.-J. Li, R. Tuli, X. Huang, P. Laquerriere, R. S. Tuan,<br />
Biomaterials 2005, 26, 5158.<br />
[12] M. Schindler, I. Ahmed, J. Kamal, A. N.-E.- Kamal, T. H.<br />
Grafe, H. Y. Chung, S. Meiners, Biomaterials 2005, 26, 5624.<br />
[13] Y. You, B.-M. Min, S. J. Lee, T. S. Lee, W. H. Park, J. Appl.<br />
Polym. Sci. 2005, 95, 193.<br />
[14] D. H. Reneker, W. Kataphinana, A. Theronb, E. Zussmanb,<br />
A. L. Yarin, Polymer 2002, 43, 6785.<br />
[15] H. Yoshimoto, Y. M. Shin, H. Terai, J. P. Vacanti,<br />
Biomaterials 2003, 24, 2077.<br />
[16] M.-S. Khil, S. R. Bhattarai, H.-Y. Kim, S.-Z. Kim, K.-H. Lee,<br />
J. Biomed. Mater. Res. 2005, 72B, 117.<br />
[17] H. Jiang, D. Fang, B. S. Hsiao, B. Chu, W. Chen,<br />
Biomacromolecules 2004, 5, 326.<br />
[18] M. Li, M. J. Mondrinos, M. R. G<strong>and</strong>hi, F. K. Ko, A. S. Weiss,<br />
P. I. Lelkes, Biomaterials 2005, 26, 5999.<br />
[19] X. Zong, H. Bien, C.-Y. Chung, L. Yin, D. Fang, B. S. Hsiao,<br />
B. Chu, E. Entcheva, Biomaterials 2005, 26, 5330.<br />
[20] L. Yao, T. W. Haas, A. G. Elie, D. G. Simpson, G. L. Bowlin,<br />
G. E. Wnek, Chem. Mater. 2003, 15, 1860.<br />
[21] M.-S. Khil, D.-I. Cha, H.-Y. Kim, I.-S. Kim, N. Bhattarai,<br />
J. Biomed. Mater. Res. 2003, 67B, 675.<br />
[22] R. Jaeger, H. Schonherr, G. J. Vancso, Macromolecules<br />
1996, 29, 7634.<br />
[23] Y. Zhang, H. Ouyang, C. T. Lim, S. Ramakrishna, Z.-M.<br />
Huang, J. Biomed. Mater. Res. 2005, 72B, 156.<br />
[24] W. H. Park, L. Jeong, D. I. Yoo, S. Hudson, Polymer 2004, 45,<br />
7151.<br />
[25] B.-M. Min, G. Lee, S. H. Kim, Y. S. Nam, T. S. Lee, W. H.<br />
Park, Biomaterials 2004, 25, 1289.<br />
[26] J. A. Matthews, G. E. Wnek, D. G. Simpson, G. L. Bowlin,<br />
Biomacromolecules 2002, 3, 232.<br />
[27] Z.-M. Huang, Y. Z. Zhang, S. Ramakrishna, C. T. Lim,<br />
Polymer 2004, 45, 5361.<br />
[28] K. Ohkawa, D. Cha, H. Kim, A. Nishida, H. Yamamoto,<br />
Macromol. Rapid Commun. 2004, 25, 1600.<br />
[29] B.-M. Min, S. W. Lee, J. N. Lim, Y. You, T. S. Lee, P. H.<br />
Kang, W. H. Park, Polymer 2004, 45, 7137.<br />
[30] X. Geng, O.-H. Kwon, H. Jang, Biomaterials 2005, 26,<br />
5427.<br />
[31] X. Fang, D. H. Reneker, J. Macromol. Sci., Part B: Phys.<br />
1997, 36, 169.<br />
[32] B.-M. Min, Y. You, J.-M. Kim, S. J. Lee, W. H. Park,<br />
Carbohydr. Polym. 2004, 57, 285.<br />
[33] D. Campoccia, P. Doherty, M. Radice, P. Brun, G. Abatagelo,<br />
D. F. Williams, Biomaterials 1998, 19, 2101.<br />
[34] P. Bulpitt, D. Aeschlimann, J. Biomed. Mater. Res. 1999, 47,<br />
152.<br />
[35] X. Z. Shu, Y. Liu, F. S. Palumbo, Y. Luo, G. D. Prestwich,<br />
Biomaterials 2004, 25, 1339.<br />
[36] Y. H. Yun, D. J. Goetz, P. Yellen, W. Chen, Biomaterials<br />
2004, 25, 147.<br />
[37] S.-N. Park, H. J. Lee, K. H. Lee, H. Suh, Biomaterials 2003,<br />
24, 1631.<br />
[38] S. Hokputsa, K. Jumel, C. Alex<strong>and</strong>er, S. E. Harding,<br />
Carbohydr. Polym. 2003, 52, 111.<br />
[39] Y. S. Choi, S. R. Hong, Y. M. Lee, K. W. Song, M. H. Park,<br />
Y. S. Nam, J. Biomed. Mater. Res., Part B: Appl. Biomater.<br />
1999, 48, 631.<br />
[40] Y. Liu, X. Z. Shu, S. D. Gray, G. D. Prestwich, J. Biomed.<br />
Mater. Res. 2004, 68A, 142.<br />
[41] J. Mao, L. Zhao, K. Yao, Q. Shang, G. Yang, Y. Cao,<br />
J. Biomed. Mater. Res. 2003, 64A, 301.<br />
[42] S. B. Lee, H. W. Jeon, Y. W. Lee, Y. M. Lee, K. W. Song,<br />
M. H. Park, Y. S. Nam, H. C. Ahn, Biomaterials 2003, 24,<br />
2503.<br />
[43] S. Matsuda, H. Iwata, N. Se, Y. Ikada, J. Biomed. Mater. Res.<br />
1999, 45, 20.<br />
[44] X. Z. Shu, Y. Liu, F. Paulo, G. D. Prestwich, Biomaterials<br />
2003, 24, 3825.<br />
Macromol. Rapid Commun. 2006, 27, 114–120 www.mrc-journal.de ß 2006 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim