Debye Institute for Nanomaterials Science
Debye Institute for Nanomaterials Science
Debye Institute for Nanomaterials Science
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
42<br />
Inorganic Chemistry and Catalysis<br />
Development of a hydrothermal stable non-noble metal catalyst <strong>for</strong><br />
Aqueous Phase Re<strong>for</strong>ming<br />
Tomas van Haasterecht, T.vanHaasterecht@uu.nl, phone: 06 - 22736372<br />
Sponsor: Catchbio, since January 2009<br />
Supervisors: Prof. dr. ir. Krijn P. de Jong and dr. Johannes H. Bitter<br />
H 2 -Chemisorption, HPLC/RID, GC/TCD, AAS<br />
Aqueous phase re<strong>for</strong>ming (APR) of renewable carbohydrates is an attractive process <strong>for</strong> the<br />
sustainable and economical production of H . The generated H can be used <strong>for</strong> efficient power<br />
2 2<br />
generation in a PEM fuel cell. Using APR with microreactor technology and an integrated fuel<br />
cell, we aim <strong>for</strong> the development of a compact power producing unit <strong>for</strong> portable applications, this<br />
part of the project is in collaboration with the TUE.<br />
In the APR process biomass derived oxygenated hydrocarbons are converted into, preferably H2 and CO , around 500 K using heterogeneous metal catalysts.<br />
2<br />
So far the process has been most successful using expensive platinum based catalyst and glycols as<br />
feedstock. [1] This research focuses on the development of a stable, non noble metal catalyst <strong>for</strong> the<br />
efficient and selective production of H from various biomass feeds. The most promising catalyst<br />
2<br />
can be integrated in the microreactor system at the TUE to demonstrate the applicability.<br />
We have investigate the possibility of using Carbon Nanofiber (CNF) supported Co, Ni and Cu<br />
catalysts with a focus on their hydrothermal stability. These catalysts, and a commercial 5%Pt/Al O 2 3<br />
catalyst, were tested in a batch reactor <strong>for</strong> the APR of a 1% Ethylene Glycol (EG) solution. The<br />
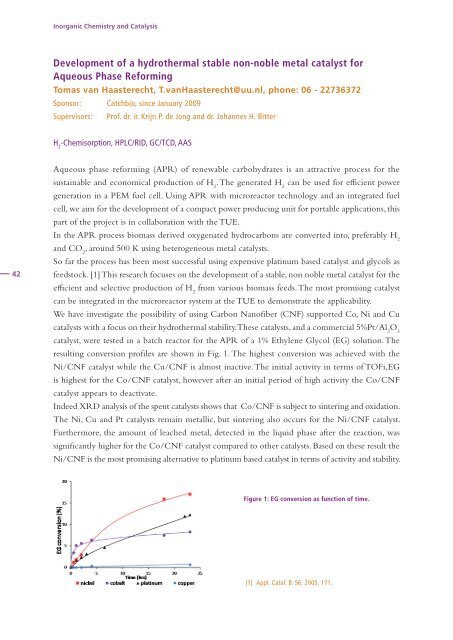
resulting conversion profiles are shown in Fig. 1. The highest conversion was achieved with the<br />
Ni/CNF catalyst while the Cu/CNF is almost inactive. The initial activity in terms of TOFi,EG<br />
is highest <strong>for</strong> the Co/CNF catalyst, however after an initial period of high activity the Co/CNF<br />
catalyst appears to deactivate.<br />
Indeed XRD analysis of the spent catalysts shows that Co/CNF is subject to sintering and oxidation.<br />
The Ni, Cu and Pt catalysts remain metallic, but sintering also occurs <strong>for</strong> the Ni/CNF catalyst.<br />
Furthermore, the amount of leached metal, detected in the liquid phase after the reaction, was<br />
significantly higher <strong>for</strong> the Co/CNF catalyst compared to other catalysts. Based on these result the<br />
Ni/CNF is the most promising alternative to platinum based catalyst in terms of activity and stability.<br />
Figure 1: EG conversion as function of time.<br />
[1] Appl. Catal. B. 56, 2005, 171.