Glutamic Acid (Glu) p-amino benzoic acid (PABA) 6-methyl pterin ...
Glutamic Acid (Glu) p-amino benzoic acid (PABA) 6-methyl pterin ...
Glutamic Acid (Glu) p-amino benzoic acid (PABA) 6-methyl pterin ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Investigation into the photochemical relationship between the<br />
<strong>pterin</strong> and <strong>PABA</strong> portions of Folic <strong>Acid</strong><br />
I. Research Interests in Folic <strong>Acid</strong><br />
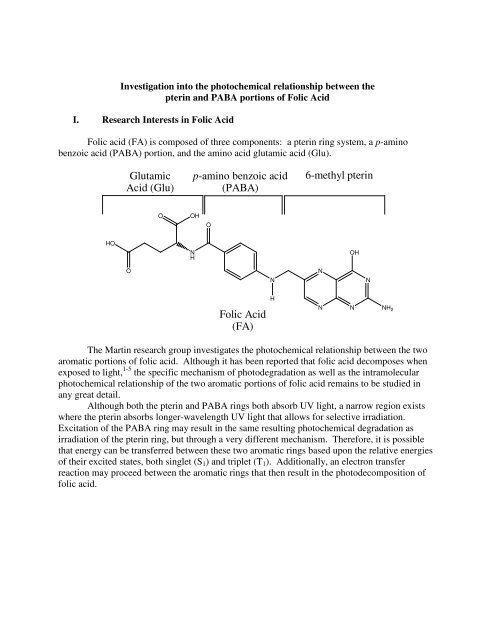
Folic <strong>acid</strong> (FA) is composed of three components: a <strong>pterin</strong> ring system, a p-<strong>amino</strong><br />
<strong>benzoic</strong> <strong>acid</strong> (<strong>PABA</strong>) portion, and the <strong>amino</strong> <strong>acid</strong> glutamic <strong>acid</strong> (<strong>Glu</strong>).<br />
HO<br />
<strong><strong>Glu</strong>tamic</strong><br />
<strong>Acid</strong> (<strong>Glu</strong>)<br />
O<br />
O OH<br />
N<br />
H<br />
p-<strong>amino</strong> <strong>benzoic</strong> <strong>acid</strong><br />
(<strong>PABA</strong>)<br />
O<br />
Folic <strong>Acid</strong><br />
(FA)<br />
N<br />
6-<strong>methyl</strong> <strong>pterin</strong><br />
The Martin research group investigates the photochemical relationship between the two<br />
aromatic portions of folic <strong>acid</strong>. Although it has been reported that folic <strong>acid</strong> decomposes when<br />
exposed to light, 1-5 the specific mechanism of photodegradation as well as the intramolecular<br />
photochemical relationship of the two aromatic portions of folic <strong>acid</strong> remains to be studied in<br />
any great detail.<br />
Although both the <strong>pterin</strong> and <strong>PABA</strong> rings both absorb UV light, a narrow region exists<br />
where the <strong>pterin</strong> absorbs longer-wavelength UV light that allows for selective irradiation.<br />
Excitation of the <strong>PABA</strong> ring may result in the same resulting photochemical degradation as<br />
irradiation of the <strong>pterin</strong> ring, but through a very different mechanism. Therefore, it is possible<br />
that energy can be transferred between these two aromatic rings based upon the relative energies<br />
of their excited states, both singlet (S1) and triplet (T1). Additionally, an electron transfer<br />
reaction may proceed between the aromatic rings that then result in the photodecomposition of<br />
folic <strong>acid</strong>.<br />
H<br />
N<br />
N<br />
OH<br />
N<br />
N<br />
NH 2
HO<br />
HO<br />
O<br />
O<br />
O OH<br />
N<br />
H<br />
O<br />
hν hν<br />
<strong>PABA</strong> S 1<br />
<strong>PABA</strong> T 1<br />
O OH<br />
N<br />
H<br />
O<br />
2<br />
N<br />
H<br />
energy transfer<br />
NH 2<br />
H 3C<br />
N<br />
N<br />
<strong>pterin</strong> S 1<br />
ISC ISC<br />
? ?<br />
energy transfer<br />
<strong>pterin</strong> T 1<br />
? ?<br />
Electron<br />
Transfer<br />
<strong>PABA</strong> <strong>pterin</strong><br />
?<br />
<strong>PABA</strong> 6-<strong>methyl</strong> <strong>pterin</strong><br />
N<br />
N<br />
OH<br />
N<br />
OH<br />
N<br />
N<br />
N<br />
NH 2<br />
NH 2
II. Research Tools and Approaches<br />
In our research group, we use a wide variety of tools for research. Theoretical<br />
calculations, using the Gaussian software package, allow us to investigate the relative energies of<br />
transient species as well as the distribution of unpaired electrons and bond-dissociation energies.<br />
We also synthesize small libraries of organic compounds for use as models for the larger<br />
biologically relevant targets. These libraries are then subjected at an array of irradiations,<br />
spectrophotometric analyses, and product study investigations. Finally, we investigate the effect<br />
of our target molecules on model phages, such as lambda phage, to investigate the possible antiviral<br />
action that these substances may possess.<br />
III. Background Information<br />
Folic <strong>Acid</strong>, also known as vitamin M, is an essential vitamin that is yellow-orange in<br />
color. It is found in liver, kidney, mushrooms, spinach, yeast, green leaves, and grasses. 6 Folic<br />
<strong>acid</strong> is reported to be present in photosensitive organs, various mammalian metabolic pathways,<br />
and possibly involved in photosynthesis. 7<br />
The electrochemical behavior of folic <strong>acid</strong> has been well studied. 8 However, reports on<br />
the photostability of folic <strong>acid</strong> are rare, 5 and those that exist often provide conflicting<br />
information: some reports state that the vitamin is photosensitive only under aerobic conditions, 5<br />
while most others report that folic <strong>acid</strong> is photosensitive under all conditions studied. Attempts<br />
to elucidate the intramolecular photochemical behavior of folic <strong>acid</strong> from the literature is<br />
difficult since studies of the aqueous stacking of the two aromatic portions of folic <strong>acid</strong> report<br />
both intramolecular stacked 9 and unstacked forms. 10 Therefore, the apparently contradictory<br />
nature of these reports makes it difficult to draw any clear conclusions, and more work is needed<br />
in this area of study.<br />
Literature indicates that photolysis of folic <strong>acid</strong> leads to cleavage between the <strong>pterin</strong><br />
benzylic position and the anilinic position of the <strong>PABA</strong> ring. 1,4,5,11 This may suggest that the<br />
reactive species responsible for the degradation of folic <strong>acid</strong> lies with the <strong>PABA</strong> ring and not the<br />
<strong>pterin</strong> core, although the <strong>pterin</strong> portion of folic <strong>acid</strong> is known to absorb UVA light and the <strong>PABA</strong><br />
portion does not. 12 It is known that <strong>pterin</strong>s will undergo electron transfer reactions with electron<br />
rich <strong>amino</strong> <strong>acid</strong>s to generate a charged radical pair. 13 The possibility of intramolecular electron<br />
transfer, or intramolecular energy transfer, has not been reported for folic <strong>acid</strong>. It is known that<br />
folic <strong>acid</strong> enhances the generation of reactive oxygen species generated by riboflavin in<br />
photolyzed solutions of cell culture media, although folic <strong>acid</strong> itself is not the source of the<br />
reactive oxygen species. 14 It has also been reported that folic <strong>acid</strong> is photochemically inactivated<br />
in the presence of riboflavin, and the reaction is inhibited by ascorbic <strong>acid</strong>, a known triplet state<br />
quencher. 15 Therefore it is very likely that the photo-decomposition of folic <strong>acid</strong> proceeds<br />
through an excited triplet state and folic <strong>acid</strong> may participate in photo-sensitization reactions.<br />
3
References<br />
(1) Lowry, O. H.; Bessey, O. A.; Crawford, E. J. "Photolytic and enzymatic transformations<br />
of pteroylglutamic <strong>acid</strong>," J. Biol. Chem. 1949, 182, 389-398.<br />
(2) Roe, D. A. "Photodegredation of carotenoids in human subjects," Fed. Proc. 1987, 46,<br />
1886-1889.<br />
(3) Suarez, G.; Cabrerizo, F. M.; Lorente, C.; Thomas, A. H.; Capparelli, A. L. "Study of the<br />
photolysis of 6-carboxy<strong>pterin</strong> in <strong>acid</strong> and alkaline aqueous solutions," J. Photochem.<br />
Photobiol. A 2000, 132, 53-57.<br />
(4) Thomas, A.; Einschlag, F. G.; Feliz, M. R.; Capparelli, A. L. "First steps in the<br />
photochemistry of folate in alkaline medium," J. Photochem. Photobio. A 1998, 116,<br />
187-190.<br />
(5) Thomas, A. H.; Suarez, G.; Cabrerizo, F. M.; Martino, R.; Capparelli, A. L. "Study of the<br />
photolysis of folic <strong>acid</strong> and 6-formyl<strong>pterin</strong> in <strong>acid</strong> aqueous solutions," J. Photochem.<br />
Photobiol. A 2000, 135, 147-154.<br />
(6) In The Merck Index; 12 ed.; Budavari, S., O'Neil, M. J., Smith, A., Heckelman, P. E.,<br />
Kinneary, J. F., Eds.; Merck Research laboratories: Whitehouse Station, NJ, 1996, p<br />
4253.<br />
(7) Chahidi, C.; Aubailly, M.; Momzikoff, A.; Bazin, M. "Photophysical and<br />
photosensitizing properies of 2-<strong>amino</strong>-4 pteridinone: a natural pigment," Photochem.<br />
Photobiol. 1981, 33, 641-649.<br />
(8) Spina Bifida and Hydrocephalus Association of Nova Scotia, "Folic <strong>Acid</strong> information,"<br />
http://reseau.chebucto.ns.ca/Health/SBANS/Folic_<strong>Acid</strong>.html<br />
(9) Thiery, C. "Etude spectroscopique de l'<strong>acid</strong>e folique," Eur. J. Biochem. 1973, 37, 100-<br />
108.<br />
(10) Lam, Y.-F.; Kotowycz, G. "Self Association of Folic <strong>Acid</strong> in Aqueous Solution by<br />
Proton Magnetic Resonance," Can. J. Chem. 1972, 50, 2357-2363.<br />
(11) Lowry, O. H.; Bessey, O. A.; Crawford, E. J. "Pterine Oxidase," J. Biol. Chem. 1949,<br />
180, 399-410.<br />
(12) Chahidi, C.; Morliere, P.; Aubailly, M.; Dubertret, L.; Santus, R. "Photosensitization by<br />
methotrexate photoproducts," Photochem. Photobiol. 1983, 38, 317-322.<br />
(13) Aubailly, M.; Santus, R. In Chemistry and Biology of Pteridines; Walter de Gruyter &<br />
Co.: Berlin, 1986, pp 99-102.<br />
4
(14) Grzelak, A.; Rychlik, B.; Bartosz, G. "Light-dependent generation of reactive oxygen<br />
species in cell culture media," Free Radical Biology and Medicine 2001, 30, 1418-1425.<br />
(15) Reusser, P. "Etude sur l'inactivation photochimique de l'<strong>acid</strong>e folique en presence de<br />
riboflavine et de son inhibition par l'<strong>acid</strong>e ascorbique," J. Internat. Vit<strong>amino</strong>l. 1970, 39,<br />
64-72.<br />
5