Tetracycline-inducible protein expression in pancreatic cancer cells
Tetracycline-inducible protein expression in pancreatic cancer cells
Tetracycline-inducible protein expression in pancreatic cancer cells
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
A<br />
Migration (relative units)<br />
Tonack S et al . Pancreatic <strong>cancer</strong> Tet-on cell clones; effects of CapG over<strong>expression</strong><br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
0.0<br />
Control<br />
Dox<br />
P = 0.564<br />
B 2.4<br />
Control<br />
Motility <strong>in</strong>dex<br />
2.0<br />
1.6<br />
1.2<br />
0.8<br />
0.4<br />
0.0<br />
P = 0.032<br />
Panc-1 were all capable of stably harbour<strong>in</strong>g the pN1βact<strong>in</strong>rtTA2S-M2-IRES-EGFP<br />
vector. Moreover, <strong><strong>in</strong>ducible</strong> gene<br />
over<strong>expression</strong> was observed for GST-P, CYP2E1, and<br />
S100A6 <strong>in</strong> Suit-2 and for CapG <strong>in</strong> all three <strong>in</strong>vestigated cell<br />
l<strong>in</strong>es. This <strong>in</strong>dicates that the system functions for a variety<br />
of different <strong>prote<strong>in</strong></strong>s. Equally, the method was efficient with<br />
80% of pN1βact<strong>in</strong>-rtTA2S-M2-IRES-EGFP transfected<br />
Suit-2 clones and 60% of MiaPaca-2 clones show<strong>in</strong>g greater<br />
than five-fold luciferase <strong>in</strong>duction. Only Panc-1 <strong>cells</strong> showed<br />
relatively low efficiency, with only 14.4% of the selected cell<br />
clones show<strong>in</strong>g a greater than five-fold luciferase <strong>in</strong>crease.<br />
A clear advantage of the Tet-on system is the time and<br />
dose-dependent activation of <strong>expression</strong> of the gene of<br />
<strong>in</strong>terest. Investigation of stable CapG overexpress<strong>in</strong>g Suit-2<br />
cell clones showed that CapG <strong>prote<strong>in</strong></strong> <strong>expression</strong> was dox<strong><strong>in</strong>ducible</strong><br />
<strong>in</strong> a time dependent manner. The level of CapG<br />
<strong>in</strong>creased from 12 h after <strong>in</strong>duction, and was ma<strong>in</strong>ta<strong>in</strong>ed up<br />
to 72 h after <strong>in</strong>duction. A similar time-dependent <strong>in</strong>duction<br />
of gene <strong>expression</strong> <strong>in</strong> the liver carc<strong>in</strong>oma <strong>cells</strong>, HepG2 has<br />
been described previously [14] . However, we did not observe<br />
a l<strong>in</strong>ear correlation between dox concentration and <strong>prote<strong>in</strong></strong><br />
<strong>expression</strong>, as was reported <strong>in</strong> the HepG2 <strong>cells</strong> [14] . Instead,<br />
we detected little <strong>in</strong>crease <strong>in</strong> CapG <strong>prote<strong>in</strong></strong> concentration<br />
between 0-10 ng/mL dox, and over<strong>expression</strong> of CapG<br />
WJG|www.wjgnet.com<br />
P = 0.0171<br />
TET29 C35 C43<br />
Dox<br />
P = 0.4311<br />
P = 0.0006<br />
P = 0.031<br />
TET29 C35 C43<br />
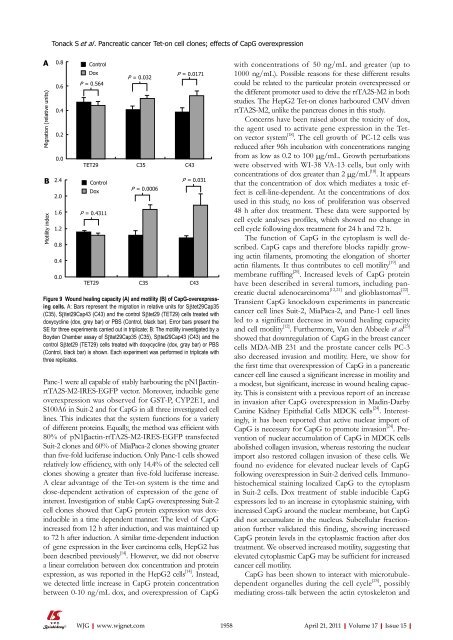
Figure 9 Wound heal<strong>in</strong>g capacity (A) and motility (B) of CapG-overexpress<strong>in</strong>g<br />
<strong>cells</strong>. A: Bars represent the migration <strong>in</strong> relative units for Sβtet29Cap35<br />
(C35), Sβtet29Cap43 (C43) and the control Sβtet29 (TET29) <strong>cells</strong> treated with<br />
doxycycl<strong>in</strong>e (dox, grey bar) or PBS (Control, black bar). Error bars present the<br />
SE for three experiments carried out <strong>in</strong> triplicate; B: The motility <strong>in</strong>vestigated by a<br />
Boyden Chamber assay of Sβtet29Cap35 (C35), Sβtet29Cap43 (C43) and the<br />
control Sβtet29 (TET29) <strong>cells</strong> treated with doxycycl<strong>in</strong>e (dox, gray bar) or PBS<br />
(Control, black bar) is shown. Each experiment was performed <strong>in</strong> triplicate with<br />
three replicates.<br />
with concentrations of 50 ng/mL and greater (up to<br />
1000 ng/mL). Possible reasons for these different results<br />
could be related to the particular <strong>prote<strong>in</strong></strong> overexpressed or<br />
the different promoter used to drive the rtTA2S-M2 <strong>in</strong> both<br />
studies. The HepG2 Tet-on clones harboured CMV driven<br />
rtTA2S-M2, unlike the pancreas clones <strong>in</strong> this study.<br />
Concerns have been raised about the toxicity of dox,<br />
the agent used to activate gene <strong>expression</strong> <strong>in</strong> the Teton<br />
vector system [18] . The cell growth of PC-12 <strong>cells</strong> was<br />
reduced after 96h <strong>in</strong>cubation with concentrations rang<strong>in</strong>g<br />
from as low as 0.2 to 100 μg/mL. Growth perturbations<br />
were observed with WI-38 VA-13 <strong>cells</strong>, but only with<br />
concentrations of dox greater than 2 μg/mL [18] . It appears<br />
that the concentration of dox which mediates a toxic effect<br />
is cell-l<strong>in</strong>e-dependent. At the concentrations of dox<br />
used <strong>in</strong> this study, no loss of proliferation was observed<br />
48 h after dox treatment. These data were supported by<br />
cell cycle analyses profiles, which showed no change <strong>in</strong><br />
cell cycle follow<strong>in</strong>g dox treatment for 24 h and 72 h.<br />
The function of CapG <strong>in</strong> the cytoplasm is well described.<br />
CapG caps and therefore blocks rapidly grow<strong>in</strong>g<br />
act<strong>in</strong> filaments, promot<strong>in</strong>g the elongation of shorter<br />
act<strong>in</strong> filaments. It thus contributes to cell motility [19] and<br />
membrane ruffl<strong>in</strong>g [20] . Increased levels of CapG <strong>prote<strong>in</strong></strong><br />
have been described <strong>in</strong> several tumors, <strong>in</strong>clud<strong>in</strong>g <strong>pancreatic</strong><br />
ductal adenocarc<strong>in</strong>oma [12,21] and glioblastomas [22] .<br />
Transient CapG knockdown experiments <strong>in</strong> <strong>pancreatic</strong><br />
<strong>cancer</strong> cell l<strong>in</strong>es Suit-2, MiaPaca-2, and Panc-1 cell l<strong>in</strong>es<br />
led to a significant decrease <strong>in</strong> wound heal<strong>in</strong>g capacity<br />
and cell motility [12] . Furthermore, Van den Abbeele et al [23]<br />
showed that downregulation of CapG <strong>in</strong> the breast <strong>cancer</strong><br />
<strong>cells</strong> MDA-MB 231 and the prostate <strong>cancer</strong> <strong>cells</strong> PC-3<br />
also decreased <strong>in</strong>vasion and motility. Here, we show for<br />
the first time that over<strong>expression</strong> of CapG <strong>in</strong> a <strong>pancreatic</strong><br />
<strong>cancer</strong> cell l<strong>in</strong>e caused a significant <strong>in</strong>crease <strong>in</strong> motility and<br />
a modest, but significant, <strong>in</strong>crease <strong>in</strong> wound heal<strong>in</strong>g capacity.<br />
This is consistent with a previous report of an <strong>in</strong>crease<br />
<strong>in</strong> <strong>in</strong>vasion after CapG over<strong>expression</strong> <strong>in</strong> Mad<strong>in</strong>-Darby<br />
Can<strong>in</strong>e Kidney Epithelial Cells MDCK <strong>cells</strong> [24] . Interest<strong>in</strong>gly,<br />
it has been reported that active nuclear import of<br />
CapG is necessary for CapG to promote <strong>in</strong>vasion [24] . Prevention<br />
of nuclear accumulation of CapG <strong>in</strong> MDCK <strong>cells</strong><br />
abolished collagen <strong>in</strong>vasion, whereas restor<strong>in</strong>g the nuclear<br />
import also restored collagen <strong>in</strong>vasion of these <strong>cells</strong>. We<br />
found no evidence for elevated nuclear levels of CapG<br />
follow<strong>in</strong>g over<strong>expression</strong> <strong>in</strong> Suit-2 derived <strong>cells</strong>. Immunohistochemical<br />
sta<strong>in</strong><strong>in</strong>g localized CapG to the cytoplasm<br />
<strong>in</strong> Suit-2 <strong>cells</strong>. Dox treatment of stable <strong><strong>in</strong>ducible</strong> CapG<br />
expressors led to an <strong>in</strong>crease <strong>in</strong> cytoplasmic sta<strong>in</strong><strong>in</strong>g, with<br />
<strong>in</strong>creased CapG around the nuclear membrane, but CapG<br />
did not accumulate <strong>in</strong> the nucleus. Subcellular fractionation<br />
further validated this f<strong>in</strong>d<strong>in</strong>g, show<strong>in</strong>g <strong>in</strong>creased<br />
CapG <strong>prote<strong>in</strong></strong> levels <strong>in</strong> the cytoplasmic fraction after dox<br />
treatment. We observed <strong>in</strong>creased motility, suggest<strong>in</strong>g that<br />
elevated cytoplasmic CapG may be sufficient for <strong>in</strong>creased<br />
<strong>cancer</strong> cell motility.<br />
CapG has been shown to <strong>in</strong>teract with microtubuledependent<br />
organelles dur<strong>in</strong>g the cell cycle [25] , possibly<br />
mediat<strong>in</strong>g cross-talk between the act<strong>in</strong> cytoskeleton and<br />
1958 April 21, 2011|Volume 17|Issue 15|