Z - Universiteit Utrecht
Z - Universiteit Utrecht
Z - Universiteit Utrecht
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
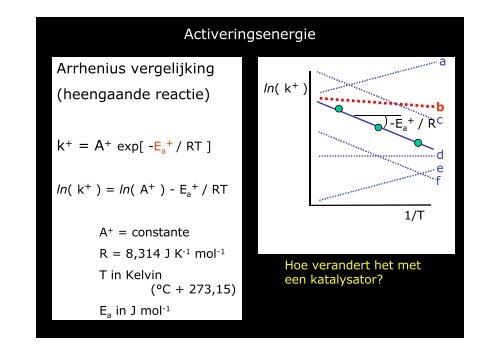
Activeringsenergie<br />
Arrhenius vergelijking<br />
(heengaande reactie)<br />
k + = A + exp[ -E + a<br />
/ RT ]<br />
ln( k + ) = ln( A + ) - E a<br />
+<br />
/ RT<br />
ln( k + )<br />
a<br />
b<br />
-E<br />
+<br />
a<br />
/ Rc<br />
d<br />
e<br />
f<br />
1/T<br />
A + = constante<br />
R = 8,314 J K -1 mol -1<br />
Hoe verandert het met<br />
T in Kelvin<br />
een katalysator?<br />
(°C + 273,15)<br />
E a<br />
in J mol -1