Tabelsamling for spektroskopi / cm - LMFK
Tabelsamling for spektroskopi / cm - LMFK
Tabelsamling for spektroskopi / cm - LMFK
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
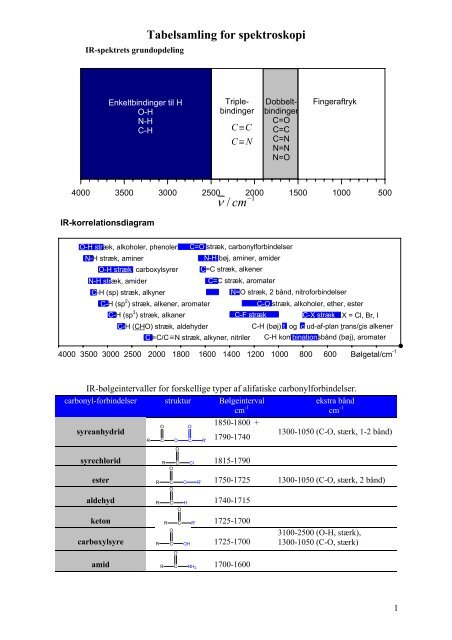
IR-spektrets grundopdeling<br />
<strong>Tabelsamling</strong> <strong>for</strong> <strong>spektroskopi</strong><br />
Enkeltbindinger til H<br />
O-H<br />
N-H<br />
C-H<br />
C≡C<br />
C≡<br />
N<br />
Triplebindinger<br />
Dobbeltbindinger<br />
C=O<br />
C=C<br />
C=N<br />
N=N<br />
N=O<br />
Fingeraftryk<br />
4000<br />
3500<br />
3000<br />
2500<br />
ν / <strong>cm</strong><br />
2000<br />
−1<br />
1500<br />
1000<br />
500<br />
IR-korrelationsdiagram<br />
O-H stræk, alkoholer, phenoler<br />
N-H stræk, aminer<br />
O-H stræk, carboxylsyrer<br />
N-H stræk, amider<br />
C-H (sp) stræk, alkyner<br />
C=O stræk, carbonyl<strong>for</strong>bindelser<br />
N-H bøj, aminer, amider<br />
C=C stræk, alkener<br />
C=C stræk, aromater<br />
N=O stræk, 2 bånd, nitro<strong>for</strong>bindelser<br />
C-H (sp 2 ) stræk, alkener, aromater<br />
C-O stræk, alkoholer, ether, ester<br />
C-H (sp 3 ) stræk, alkaner<br />
C-F stræk C-X stræk X = Cl, Br, I<br />
C-H (CHO) stræk, aldehyder<br />
C ≡C/C ≡N stræk, alkyner, nitriler<br />
C-H (bøj) t og c ud-af-plan trans/cis alkener<br />
C-H kombinationsbånd (bøj), aromater<br />
4000 3500 3000 2500 2000 1800 1600 1400 1200 1000 800 600 Bølgetal/<strong>cm</strong> -1<br />
IR-bølgeintervaller <strong>for</strong> <strong>for</strong>skellige typer af alifatiske carbonyl<strong>for</strong>bindelser.<br />
carbonyl-<strong>for</strong>bindelser struktur Bølgeinterval<br />
<strong>cm</strong> -1<br />
syreanhydrid<br />
R<br />
R<br />
R<br />
O<br />
C<br />
R<br />
O<br />
O<br />
C<br />
O<br />
C<br />
O<br />
C<br />
O<br />
H<br />
O<br />
R C<br />
O<br />
carboxylsyre R<br />
C<br />
OH 1725-1700<br />
O<br />
C<br />
Cl<br />
R'<br />
R'<br />
R'<br />
1850-1800 +<br />
1790-1740<br />
syrechlorid 1815-1790<br />
ekstra bånd<br />
<strong>cm</strong> -1<br />
1300-1050 (C-O, stærk, 1-2 bånd)<br />
ester 1750-1725 1300-1050 (C-O, stærk, 2 bånd)<br />
aldehyd 1740-1715<br />
keton 1725-1700<br />
amid 1700-1600<br />
R<br />
O<br />
C<br />
3100-2500 (O-H, stærk),<br />
1300-1050 (C-O, stærk)<br />
NH 2<br />
1
NMR-tabeller<br />
1 H-NMR korrelationsdiagram<br />
PhOH<br />
ROH<br />
RCHO, RCOOH<br />
Aromatisk, Ar-H<br />
CHCl 3<br />
C=C-H<br />
CH 2<br />
Cl 2<br />
Additiv eff.<br />
R 2<br />
CH-O-CO-R<br />
CH 3<br />
-O-CO-R<br />
CH 3<br />
-OR , R-CH 2<br />
-Cl, R-CH 2<br />
-Br<br />
R-CH 2<br />
-CO-Ar<br />
R-CH 2<br />
-Ar<br />
CH 3<br />
-CO-R<br />
R-CH 2<br />
-R<br />
CH 3<br />
-R<br />
Alifatisk sp 3 hybridiseret C-H<br />
12<br />
10<br />
8<br />
6<br />
4<br />
2<br />
0<br />
δ Η<br />
/ppm<br />
CH 3 -protoner δ Η /ppm CH 2 -protoner δ Η /ppm CH-protoner δ Η /ppm<br />
CH 3 -R 0.9 R-CH 2 -R 1.4 R 2 -CH-R 1.5<br />
CH 3 -C-O 1.3 R-CH 2 -C-O 1.9 R 2 -CH-C-O 2.0<br />
CH 3 -C-NO 2 1.6 R-CH 2 -C-NO 2 2.0 R 2 -CH-C-NO 2 2.0<br />
CH 3 -C=C 1.6 R-CH 2 -C=C 2.1 R 2 -CH-C=C 2.3<br />
CH 3 C≡C 1.8 R-CH 2 -C≡C 2.2 R 2 -CHC≡C 2.6<br />
CH 3 -CO-OR 2.0 R-CH 2 -CO-OR 2.2 R 2 -CH-CO-OR 2.5<br />
CH 3 -CO-N 2.0 R-CH 2 -CO-N 2.2 R 2 -CH-CO-N 2.4<br />
CH 3 -CN 2.0 R-CH 2 -CN 2.3 R 2 -CH-CN 2.7<br />
CH 3 -CO-OAr 2.1 R-CH 2 -CO-OAr 2.3 R 2 -CH-CO-OAr 2.7<br />
CH 3 -S 2.1 R-CH 2 -S 2.4 R 2 -CH-S 3.2<br />
CH 3 -CO-R 2.2 R-CH 2 -CO-R 2.4 R 2 -CH-CO-R 2.7<br />
CH 3 -N 2.3 R-CH 2 -N 2.5 R 2 -CH-N 2.8<br />
CH 3 -Ar 2.3 R-CH 2 -Ar 2.7 R 2 -CH-Ar 3.0<br />
CH 3 -CO-Ar 2.6 R-CH 2 -CO-Ar 2.9 R 2 -CH-CO-Ar 3.3<br />
CH 3 -N-Ar 2.8 R-CH 2 -N-Ar 3.1 R 2 -CH-N-Ar 3.0<br />
CH 3 -N-CO-R 2.9 R-CH 2 -N-CO-R 3.2 R 2 -CH-N-CO-R 4.0<br />
CH 3 -I (spec) (2.2) R-CH 2 -I 3.2 R 2 -CH-I 4.3<br />
CH 3 -O-R 3.3 R-CH 2 -O-R 3.4 R 2 -CH-O-R 3.7<br />
CH 3 -Br (spec) (2.7) R-CH 2 -Br 3.5 R 2 -CH-Br 4.3<br />
CH 3 -Cl (spec) (3.0) R-CH 2 -Cl 3.6 R 2 -CH-Cl 4.2<br />
CH 3 -OH (spec) 3.4 R-CH 2 -OH 3.6 R 2 -CH-OH 3.9<br />
CH 3 -O-CO-R 3.7 R-CH 2 -O-CO-R 4.1 R 2 -CH-O-CO-R 4.8<br />
CH 3 -O-Ar 3.8 R-CH 2 -O-Ar 4.3 R 2 -CH-O-Ar 4.5<br />
CH 3 -NO 2 (spec) (4.3) R-CH 2 -NO 2 4.4 R 2 -CH-NO 2 4.7<br />
CH 3 -F (spec) (4.3) R-CH 2 -F 4.5 R 2 -CH-F 4.8<br />
Tabel med kemiske skift <strong>for</strong> methyl (CH 3 ), methylen (CH 2 ) og methin (CH) protoner.<br />
R = alkyl, Ar = aromat, phenyl. Mærket (spec) betyder, at værdien kun gælder <strong>for</strong> en specifik<br />
<strong>for</strong>bindelse.<br />
2
indingstype <strong>for</strong>bindelse δ H -interval<br />
ppm<br />
O-H alkoholer 1-5<br />
phenoler 4-10<br />
carboxylsyrer 9-13<br />
N-H aminer 1-5<br />
amider 5-12<br />
Tabel med δ H <strong>for</strong> protoner i OH og NH.<br />
13 C-NMR korrelationsdiagram<br />
C-Br<br />
C-Cl<br />
C-I<br />
keton<br />
aldehyd<br />
carboxylsyre<br />
Carbonyl, C=O<br />
ester, amid og syrechlorid<br />
C=C<br />
Aromatisk<br />
-CH 3<br />
-CH 2<br />
-<br />
C-O<br />
C-N<br />
Alifatisk sp 3 -hybridiseret C<br />
C≡C<br />
220<br />
200<br />
180<br />
160<br />
140<br />
120<br />
100<br />
80<br />
60<br />
40<br />
20<br />
0<br />
δ C<br />
/ppm<br />
MS-tabeller<br />
A A+1 A+2<br />
Type<br />
Atom masse % masse % masse %<br />
H 1 100 2 0.015 3 A<br />
C 12 100 13 1.1 14 A+1<br />
N 14 100 15 0.37 16 A<br />
O 16 100 17 0.04 18 0.20 A<br />
F 19 100 20 21 A<br />
Si 28 100 29 5.1 30 3.4 A+2<br />
P 31 100 32 33 A<br />
S 32 100 33 0.79 34 4.4 A+2<br />
Cl 35 100 36 37 32.0 A+2<br />
Br 79 100 80 81 97.3 A+2<br />
I 127 100 128 129 A<br />
Isotoptabel <strong>for</strong> de mest almindelige grundstoffer.<br />
3
Antal C % M % M+1 % M+2<br />
1 100 1,1<br />
2 100 2,2<br />
3 100 3,3<br />
4 100 4,4<br />
5 100 5,5 0,12<br />
10 100 11,0 0.54<br />
M +•<br />
antal N lige<br />
0,2,4.. masse<br />
antal N ulige<br />
1,3,5... masse<br />
Tabel <strong>for</strong> nitrogenregel.<br />
Tabellen viser den additive effekt af flere C-<br />
atomer i molekylet<br />
funktionel gruppe m/z basis ion m/z ionserie<br />
amin 30<br />
+<br />
CH 2 =NH 2 30, 44, 58, 72, 86, 100<br />
alkoholer, ethere 31 CH 2 =OH + 31, 45, 59, 73, 87, 101<br />
aldehyder, ketoner 29 CHO + 29, 43, 57, 71, 85, 99,<br />
alifatiske alkaner 15<br />
+<br />
CH 3 15, 29, 43, 57, 71, 85, 99<br />
alkener 27<br />
+<br />
C 2 H 3 27, 41, 55, 69, 83, 97<br />
carboxylsyrer 45<br />
+<br />
CHO 2 45, 59, 73, 87<br />
estere 43 C 2 H 3 O + 43, 57, 71, 85, 99<br />
amider 44 CH 2 NO + 44, 58, 72, 86<br />
nitro<strong>for</strong>bindelser 30 NO + 30, 46, 60, 74, 88<br />
aromater 39 (CH) n 38-40, 50-52, 63-66, 75-79<br />
phenylalkyler 77<br />
+<br />
C 6 H 5 77, 91, 105, 119, 133<br />
Tabellen viser ionserier <strong>for</strong> en række funktionelle grupper<br />
m/z fragment-ion <strong>for</strong>tolkning<br />
30 H 2 C<br />
+<br />
NH 2<br />
Aminer, primære, sekundære og<br />
tertiære<br />
43<br />
O<br />
methylketon, alkylethanoat<br />
O<br />
+<br />
CH 3<br />
C<br />
Z CH 3<br />
Z = alkyl, alkoxy etc<br />
77 H<br />
+<br />
H<br />
Substituerede benzener<br />
X<br />
H<br />
H<br />
X = halogen, alkyl, alkoxy mm.<br />
91<br />
H<br />
+<br />
CH 2<br />
R<br />
105<br />
tropylium-ion<br />
O<br />
O<br />
C+ C<br />
Z<br />
Z = alkyl, alkoxy etc<br />
O<br />
O<br />
149 Fra Phthalater (blødgører i<br />
+ O H<br />
O<br />
R = alkyl<br />
O<br />
R<br />
O<br />
O<br />
R<br />
plastik)<br />
Tabellen viser karakteristiske ioner, som ofte <strong>for</strong>ekommer i massespektre<br />
1