E t 50- a F 5 - International Joint Commission
E t 50- a F 5 - International Joint Commission
E t 50- a F 5 - International Joint Commission
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1977<br />
Editors: H. SHEAR - A. f, I! WATSON
The statements and views presented in these proceedings are<br />
those of the authors and of the workshop participants, and do not<br />
necessarily represent the views or policies of the <strong>International</strong> <strong>Joint</strong><br />
<strong>Commission</strong>, Research Advisory Board and Pollution from Land Use<br />
Activities Reference Group. Mention of trade names or commercial<br />
products does not constitute endorsement or recommendation for use.<br />
ii
1.<br />
2.<br />
3.<br />
k E S OF SEDIMUsr<br />
Natural Sheet and Channel Erosion of Unconsolidated Source<br />
Material (Geomorphic Control, Magnitude and Frequency of<br />
Transfer Mechanisms)<br />
D. E. Walling<br />
University of Exeter<br />
UNITED KINGDOH<br />
Influence of Land Use on Erosion and Transfer Process of<br />
Sediment<br />
T. Dickinson and G. Wall<br />
University of Guelph<br />
GUELPH. Ontario<br />
Anthropogenic Influences of Sediment Quality at a Source<br />
a) Pesticides and PCB's<br />
R. Miles<br />
Agriculture Canada<br />
LONM)N, Ontatio<br />
b) Nutrients: Carbon, Nitrogen and Phosphorus<br />
c) Metals<br />
M. Miller<br />
University of Guelph<br />
GUELPH, Ontario<br />
G. K. Rutherford<br />
Queen's University<br />
KINGSTON, Ontario<br />
R. Frank<br />
Ontario Ministry of<br />
Agriculture 6 Food<br />
GUELPH, Ontario<br />
PAGE<br />
1<br />
5<br />
11<br />
37<br />
69<br />
81<br />
95
4. Regional Overview of the Impact of Land Use on Water Quality 105<br />
CHAPTER 2<br />
TRANSFURT OF SEDIMENT<br />
A. D. McElroy<br />
Mdwest Research Institute<br />
KANSAS CITY, Mo.<br />
1. Influence of Flow Characteristics on Sediment Transport<br />
with Emphasis on Grain Size and Mineralogy<br />
J. Culbertson<br />
U.S. Geological Survey<br />
RESTON, Va.<br />
2. Sediment Storage and Remobilization Characteristics of<br />
Watersheds<br />
J. Knox<br />
University of Wisconsin<br />
MADISON, Wisconstn<br />
C. Nordin<br />
U.S. Geological Survey<br />
LAKEWOOD. Colorado<br />
3. Forms and Sediment Associations of Nutrients (C.N. and P.)<br />
Pesticides and Metals<br />
a) Nutrients - P 157<br />
H. L. Golterman<br />
Limnologisch Instituut<br />
NETHERLANDS<br />
b) Nutrients - N 181<br />
c) Pesticides<br />
T. Logan<br />
Ohio State University<br />
COLUMBUS. Ohio<br />
H. B. Pionke<br />
U.S. Department of Agriculture<br />
Agricultural Research Service<br />
UNIVERSITY PARK, Pennsylvania<br />
iv<br />
117<br />
137<br />
151<br />
T. Cahill<br />
Resource Management Associates<br />
WEST CHESTER, Pennsylvania<br />
199
d) Trace Metals<br />
Ulrich F6rstner<br />
University of Heidelberg<br />
4. Geochemistry of Sediment/Water Interactions of Nutrients,<br />
Pesticides and Metals, Including Observations on Availability<br />
a) Nutrients<br />
b) Pesticides<br />
c) Metals<br />
D. R. Bouldin<br />
Cornel1 University<br />
ITHACA, New York<br />
IWLICATIONS<br />
FOR TI-E GREAT LAKES<br />
1. Physical Processes<br />
2. Nutrients<br />
J. B. Weber<br />
North Carolina State University<br />
RALEIGH, North Carolina<br />
I. Jonasson<br />
Energy Mines and Resources<br />
OTTAWA, Ontario<br />
J. R. Vallentyne<br />
Environment Canada<br />
OTTAWA, Ontario<br />
G. H. DUKY<br />
University of Wisconsin<br />
MADISON, Wisconsin<br />
V<br />
PAGE<br />
219<br />
235<br />
245<br />
255<br />
275<br />
279<br />
285<br />
291
3. Pesticides<br />
4. Metals<br />
GwrER 5<br />
~KSHOP ORGANIZERS AND AITNDEES<br />
vi<br />
PAGE<br />
293<br />
295<br />
299
In April 1972 the United States and Canada signed the Great Lakes<br />
Water Quality Agreement. The responsibility for the implementation of this<br />
Agreement was assigned to the <strong>International</strong> <strong>Joint</strong> <strong>Commission</strong>. The <strong>International</strong><br />
<strong>Joint</strong> <strong>Commission</strong> was established pursuant to the Boundary Waters Treaty of<br />
1909. Its operating concept assumes that solutions to boundary waters problems<br />
should be sought by joint deliberations of a permanent tribunal of three<br />
Canadians and three Americans.<br />
Under the terms of the Agreement a Research Advisory Board was<br />
established to advise the <strong>International</strong> <strong>Joint</strong> <strong>Commission</strong> on a continuing<br />
basis on matters relating to ongoing research and research needs related to<br />
Great Lakes Water Quality. Another group, with a defined lifespan of five<br />
years was also established - the <strong>International</strong> Reference Group on Great<br />
Lakes Pollution from Land Use Activities (PLUARG). The PLUARG was charged<br />
with answering the following questions:<br />
(1) Are the boundary waters of the Great Lakes System<br />
being polluted by land drainage (including ground and<br />
surface runoff and sediments) from agriculture, forestry,<br />
urban and industrial land development, recreational and<br />
park land development, utility and transportation systems<br />
and natural sources?<br />
(2) If the answer to the foregoing question is in the<br />
affirmative, to what extent, by what causes, and in what<br />
localities is the pollution taking place?<br />
(3) If the <strong>Commission</strong> should find that pollution of the<br />
character just referred to is taking place, what remedial<br />
measures would, in its judgement, be most practicable and<br />
what would be the probable cost thereof?<br />
The <strong>Commission</strong> is requested to consider the adequacy of existing<br />
programs and control measures, and the need for improvements thereto,<br />
relating to:<br />
(a) inputs of nutrients, pest control products, sediments,<br />
and other pollutants from the sources referred to above;<br />
(b) land use;<br />
(c) land fills, land dumping, and deep well disposal practices;<br />
1
(d) confined livestock feeding operations and other animal<br />
husbandry operations; and<br />
(e) pollution from other agricultural, forestry and land<br />
use sources.<br />
In carrying out its study the <strong>Commission</strong> should identify deficiencies<br />
in technology and recommend actions for their correction.<br />
The PLUARG, in its Study Plan of 1974 established a series of pilot<br />
watershed studies and other special land use studies in the United States<br />
and Canada to assess the impact of land on water use quality as related to<br />
river loadings to the Great Lakes. It was recognized that a variety of<br />
nutrients and contaminants are transported both by mineral and organic<br />
sediment. A better understanding of sediment-associated nutrient and<br />
contaminant transport in streams in time and space was needed to assess<br />
their impact on the Great Lakes.<br />
The PLUARG therefore referred this matter to the Research Advisory<br />
Board as the IJC's principal advisor on Great Lakes research. An evaluation<br />
was requested of the state-of-the-art of this topic together with recommendations<br />
for further research. The Board in turn decided to sponsor this workshop to<br />
synthesize current research and to identify research needs on nutrient and<br />
contaminant transport by sediment within fluvial systems. Clarification was<br />
sought on the interrelationships of source, in-channel storage, resuspension<br />
and transport mechanisms with long-term, seasonal and single-event flows,<br />
and including an examination of the interaction of sediment and water chemistry<br />
on key nutrients and contaminants.<br />
The Research Advisory Board anticipated positive recommendations<br />
from this workshop not only in terms of research needed, but of how the<br />
research that has been done can be put to use to solve Great Lakes water<br />
quality problems. These recommendations moreover, would be sufficiently<br />
succinct for clear-cut decisions by the <strong>Commission</strong> to advise appropriate<br />
Governmental action.<br />
It is intended that the recommendations in these proceedings will<br />
have an immediate effect on PLUARG studies and a longer term influence on<br />
the Great Lakes research community.<br />
2<br />
Dr. Harvey Shear<br />
Dr. Andrew E. P. Watson<br />
<strong>International</strong> <strong>Joint</strong> Commissio<br />
Great Lakes Regional Office<br />
100 Ouellette Avenue<br />
WINDSOR, Ontario
For the City of Kitchener:<br />
Mrs. Edith MacIntosh<br />
Mayor of Kitchener<br />
Good Morning. It is my pleasure to welcome you here today. I<br />
bring greetings on behalf of Kitchener to all of you who are involved,<br />
interested and concerned in this workshop on pollution from land use activities.<br />
I was looking over your program and I note that your purpose, and<br />
I am quoting, "is to synthesize current research and to identify research<br />
needs on nutrient and contaminant transport by sediment within fluvial<br />
systems". Further, you will be "clarifying the interrelationships of source,<br />
in-channel storage, resuspension and transport mechanisms with long term,<br />
seasonal and single-event flows, including an examination of the interaction<br />
of sediment and water chemistry on key nutrients and contaminants". I looked<br />
at that and I wondered what it all meant. Well, to me it meant, that,<br />
for instance, ten years ago at our cottage on the Bruce Penninsula, I could<br />
walk down to the shoreline, dip a pail into the and water take a drink.<br />
Today I can't do that. My hope is that with your expertise it will be<br />
possible in the not too distant future to drink Georgian Bay water again.<br />
I brought with me our latest report on the bacterial examination<br />
of drinking water and as I mentioned, ten years ago we could drink the water.<br />
Whenever we had it tested it came back A number 1. Today, it has a very<br />
high coliform bacteria count. It is rather disturbing.<br />
During my term as National President of the Consumer's Association<br />
of Canada, the soap bubble conditions in the Great Lakes was area a major<br />
issue. Canadian consumers fought for the ban on phosphorus in detergents in<br />
cooperation with the <strong>International</strong> <strong>Joint</strong> <strong>Commission</strong>. I have been told that<br />
this is still a problem. I understand that the Grand River watershed is<br />
one of the three major Canadian watersheds selected for intensive study<br />
under the PLUARG program. You, as invited experts, will be seeing first<br />
hand I hope, the work being done in the Grand. You may be wondering why I<br />
was carting around these two huge volumes. Here are 700 pages of the very<br />
latest information just tabled this week on what is being done in the Grand<br />
River Basin.<br />
Mr. Chairman, delegates, I would like to issue a very warm welcome<br />
to all of you. I know you have come from different countries and from<br />
across the province and from other provinces. I hope you have a very successful<br />
workshop here and we are glad you chose Kitchener. Again, welcome to<br />
everyone.<br />
5
For PLUARG<br />
Dr. R. L. Thorns<br />
Director<br />
Great Lakes Biolimnology Laboratory<br />
Department of Fisheries and the Environment<br />
Fisheries and Marine Service<br />
Canada Centre for Inland Waters<br />
Bur Zington, Ontario<br />
Thank you very much MKS. MacIntosh. A number of us present at<br />
this meeting, have recently returned from a symposium in Amsterdam on the<br />
interaction of sediment and fresh water. The organizer was Dr. Golterman<br />
who is going to talk to us later in this workshop on nutrients. The symposium<br />
was a significant event in limnological studies because it emphasized<br />
the fact that the role of sediments in the lacustrine environment is now<br />
starting to come to the fore. It is very gratifying to me personally<br />
and to many of us engaged on sediment studies at the Canada Centre for<br />
Inland Waters. The role and the significance of sediments is thus becoming<br />
very apparent, particularly with regard to toxic trace metals and persistent<br />
organic contaminants that are becoming all too apparent in our environment.<br />
The PLUARG which is charged with an assessment of the impact on<br />
the boundary waters of the Great Lakes of pollutants from diffuse sources,<br />
designed its programs to incorporate a significant number of sediment<br />
oriented studies. These studies are now in progress. Close to two years<br />
ago we decided to hold this workshop in order to bring together many of<br />
the internationally known experts in the field of fluvial sediment. We<br />
wanted to assure ourselves that the work we were doing was correct. We<br />
wanted an opportunity to discuss OUT problems; to assist in the interpretation<br />
of our data, and, if the experts have come up with new knowledge or techniques<br />
we want to know, so that we may, even at this stage, make modifications<br />
to OUK programs. Consequently, I feel that this is a highly significant<br />
workshop; to the reference group, to the <strong>International</strong> <strong>Joint</strong> <strong>Commission</strong><br />
and to us participants and wish it every success in achieving its objectives.<br />
6
For the Research Advisory Board<br />
Dr. A. R. LeFeuvre<br />
Director<br />
Canada Centre for Inland Waters<br />
BurZington, ktario<br />
I am very happy to be with you this morning at the beginning of<br />
this important workshop. As the Canadian chairman of the Research Advisory<br />
Board, I am very happy that we are able to lend our support and sponsorship<br />
to this workshop.<br />
I think that it is very timely, as Dr. Thomas has pointed out.<br />
The topic of the workshop is very important to our understanding of the<br />
several mechanisms that are at work in bringing pollutants and nutrients<br />
from the diffuse sources on the watershed into the river systems and then<br />
eventually into the boundary waters of the Great Lakes. This, of course,<br />
is why it is a reference under the <strong>International</strong> <strong>Joint</strong> <strong>Commission</strong> and<br />
provided for under the Canada-U.S. Water Quality Agreement. The Research<br />
Advisory Board, as you may know, is that part of the structure established<br />
under the Canada-U.S. Water Quality Agreement which has the responsibility<br />
for advising the Governments through the <strong>Commission</strong> on the needed research,<br />
and those research areas which could profitably be accomplished by joint<br />
effort, and provide this advice to the Governments in the of form annual<br />
reports and special reports.<br />
At our most recent reporting experience which was July, last<br />
the Research Advisory Board tabled a report on Research Needs. This was<br />
the compilation of identified research needs with priorities that identified<br />
for the Governments and their agencies those areas that required additional<br />
research effort and those areas which were of priority interest. All the<br />
workshops that are sponsored by the Research Advisory Board have of as one<br />
their objectives the identification of research needs. We hope that<br />
in your deliberations over the next several days you will be able to delineate<br />
and identify more precisely some of the continuing research needs<br />
in this important area. I think that we all realize that while we have<br />
learned a considerable amount about the processes involved, a great deal<br />
is still to be learned in this important area. We certainly encourage you<br />
to give conscious thought to the need for additional research as well as<br />
to the results of research that has already been undertaken.<br />
Please be conscious of the advice, guidance and recommendations<br />
that can come to the final report of PLUARG because of your deliberation<br />
here. You have a two fold task: one is to assist PLUARG in developing<br />
its recommendations with regard to pollution coming from land use activities.
It is important that you keep this in mind a as very positive and immediate<br />
output from your deliberations. The other point to consider is the identification<br />
of research needs that still exist and that may be addressed both<br />
through government research agencies and through university research.<br />
We are certainly very pleased to bring these words to you this<br />
morning and hope that in your deliberations you will have a very successful<br />
workshop in this important area, and we wish you the best of luck in your<br />
deliberations this morning and the days to follow.
BHiJFVEKl Q<br />
SOURCES OF SEDIMENT
INTRODUCTION<br />
Natural Sheet and Channel Erosion of Unconsolidated Source<br />
Material (Geomorophic Control, Magnitude and Frequency of<br />
Transfer Mechanisms)<br />
D. E. Walting<br />
This brief discussion of erosion processes and origins, the<br />
sources and delivery characteristics of sediment within the fluvial system<br />
aims to provide an introductory background some to of the questions associated<br />
with a discussion of sediment-associated contaminants and nutrients.<br />
It is concerned essentially with suspended sediment and more particularly<br />
the wash load portion of total sediment load, because fine grained sediment<br />
is in general the most chemically active. Consideration of the topic can<br />
be usefully structured under four headings: first, the general geomorphological<br />
context; secondly, the process mechanisms involved; thirdly, the<br />
integration of these processes into the watershed and the spatial characteristics<br />
of catchment response, and lastly, the resultant in terms of the<br />
temporal pattern of sediment delivery from a watershed.<br />
THE GENERAL GEWCRPKILOGICAL CONTEXT<br />
The magnitude of sediment flux within the fluvial system must<br />
reflect the general intensity of the denudation processes and in this<br />
respect it is useful to briefly consider the global pattern. Over the past<br />
twenty or more years, and particularly as a result of the impetus provided<br />
by the <strong>International</strong> Hydrological Decade (1965-74), measurements of the<br />
sediment yield of rivers in many parts of the world have become available.'<br />
Analysis of this information can provide an insight into the range of<br />
values exhibited at the world level and some indication of dominant controlling<br />
factors. The classic work by Langbein and Schumm,3 based on<br />
rivers in the United States, suggests that a global-scale relationship<br />
be established between annual precipitation and sediment yield and that the<br />
can<br />
form of the relationship can be accounted for in terms of the opposing<br />
influences of vegetation cover and precipitation energy (Figure 1). Maximum<br />
yields correspond to an optimum combination of these influences at a mean<br />
annual precipitation of 300 nun. A similar pattern was found by Douglas4 in<br />
an evaluation of the relationship between sediment yield and annual runoff<br />
at the global level, although the inclusion of information from tropical<br />
areas demonstrated a tendency for yields to increase again in areas of high<br />
runoff (Figure 1). These curves are inevitably very generalised as they<br />
take little or no account relief, of the environmental energy and other<br />
physiographic factors. Attempts have also been made to define the controls<br />
according to major vegetation types' and to develop multivariate equations.6<br />
In so far as it is possible to apply these general curves, we<br />
might expect sediment yields in the Great Lakes region to be relatively low<br />
and to amount to approximately 100 tonnesfkm'. This conforms to a<br />
11
Y<br />
L<br />
N' 800- A<br />
F<br />
-rc<br />
3<br />
-<br />
600-<br />
9 - / \<br />
Q<br />
I \<br />
~400- / \ \<br />
Y<br />
L<br />
ry'<br />
F<br />
* 1<strong>50</strong>-<br />
L<br />
9 -<br />
100-<br />
-<br />
c<br />
E<br />
i! <strong>50</strong>-<br />
$ -<br />
0 <strong>50</strong>0 lo00 1<strong>50</strong>0 2000<br />
Effective Precipitation (mm)<br />
B<br />
@ 01 I<br />
I r 1 I<br />
0 Mean Annual 400 Runoff 800 (mm) 12<br />
Figure 1.<br />
General global relationships between sediment yield and annual<br />
precipitation and runoff proposed by Langbein and Schum (1958)<br />
and Douglas (1967).<br />
12
ecent Canadian survey7 which suggests that levels of 20-100 tonnes/km2 are<br />
characteristic of this area. The same survey provides an average value of<br />
mean suspended sediment concentration of <strong>50</strong>-200 mg/l. (Figure 2). A U.S.<br />
Geological Survey compilation’ depicts the area of the United States tributary<br />
to the Great Lakes as exhibiting mean concentrations of less than<br />
300 mg/l (Figure 2) which, for an annual runoff of 300 mm corresponds to<br />
sediment yields of less than 90 tonnes/km2. In the geomorphological context,<br />
it can be inferred that the area under discussion is characterized by somewhat<br />
subdued erosion processes. Furthermore, it should be stressed that in<br />
such an area it will not always be immediately obvious precisely what<br />
processes are involved, and where the dominant sources of sediment are<br />
located. Generalized figure; on stream loads inevitably conceal considerable<br />
variations in sediment yield in both space and time and in order to<br />
appreciate these more fully, the processes involved must be discussed.<br />
EROSION F’ROCESSES<br />
In considering the processes involved in sediment detachment and<br />
entrainment, a useful distinction can be made between two major sediment<br />
sources, namely, the land surface and the channel system. The first<br />
reflects the action of rainsplash and surface runoff, often termed sheet<br />
erosion, and includes the development of rills where surface flow becomes<br />
concentrated. The second reflects the erosive power of concentrated channel<br />
flow and includes streambank scour, streambed degradation, and floodplain<br />
scour. Gully erosion is to some extent a link between the two in that it<br />
could be viewed as a development of sheet and rill erosion or a headward<br />
extension of channel erosion.<br />
Much work on sheet erosion has been carried out by agricultural<br />
engineers concerned with soil erosion and the influence of various cultivation<br />
practices and conservation measures, and the erosion plot and<br />
laboratory sprinkler have become well-used research tools. A distinction<br />
can usefully be made between the four major contributing processes, namely,<br />
splash detachment, splash transport, runoff detachment and runoff transport.<br />
Many empirical and theoretical relationshi s have been developed for<br />
total soil loss’ and for the component processes,Po based on rainfall and<br />
flow properties, topography and soil character (Tables 1 and 2). Soil<br />
erodibility has been related to various physical and chemical properties”<br />
and factors such as the particle size characteristics, structure, permeability,<br />
organic matter content, dispersion and cation exchange capacity<br />
must be taken into account.<br />
The Universal Soil Loss Equation” (U.S.L.E.) developed by the<br />
United States Department of Agriculture from over 10,000 plot years of<br />
records has been widely applied in many environments and its form provides<br />
a summary of the major controls on sheet erosion (Table 1). However, it<br />
should be recognized that the equation was developed from data collected<br />
from small plots and soil loss from a plot cannot necessarily be equated<br />
with sediment yield from a drainage basin. If sheet erosion or loss soil<br />
is to be estimated for individual events, rather than on a seasonal basis,<br />
detailed consideration of temporal variation in sediment availability both<br />
during storms and between events is required.’ Erodibility measures<br />
should then be thought of as dynamic rather than static indices and removal<br />
13
TABLE I<br />
Some examples of equations describing the Sheet Erosion Process. 47<br />
St =<br />
x 1.73<br />
. tan 1.35 B Kirkby (1969)<br />
A = E 1.35<br />
0.35<br />
s cs' sp - Ls . '30<br />
THE UNIVERSAL SOIL LOSS EQUATION (U.S.L.E.)<br />
E = R.K.L.S.C.P.<br />
DEVELDPMENTS OF THE U.S.L.E.<br />
b<br />
G = a(Q .q K.L.S.C.P.<br />
P<br />
A = (aA<br />
Where:<br />
St =<br />
*r<br />
+ bcQ. qp' ) K.L.S.C.P.<br />
Soil transport<br />
x = Slope length<br />
L =<br />
B = Slope gradient<br />
s =<br />
A = Soil loss<br />
c =<br />
E =<br />
S<br />
cs =<br />
Soil erodibility factor<br />
Soil cover factor<br />
P<br />
G<br />
=<br />
=<br />
s<br />
P<br />
Ls<br />
= Slope (per cent)<br />
= Slope length<br />
Q<br />
qp<br />
=<br />
=<br />
P -<br />
30 - 2 year 30 minute rainfall<br />
Ar =<br />
E = Soil loss<br />
A =<br />
R = Rainfall factor<br />
a,b =<br />
c = coefficient<br />
15<br />
1'75 Musgrave (1947)<br />
K =<br />
Wischmeier and Smith (1965)<br />
Williams (1975)<br />
Foster et a1 (1973)<br />
Soil erodibility factor<br />
Slope length factor<br />
Slope steepness factor<br />
Cropping factor<br />
Conservation factor<br />
Sediment yield<br />
Runoff volume<br />
Peak flow rate<br />
Drainage area<br />
Soil loss<br />
Weighting parameters
TABLE 2<br />
Some examples of equations describing various components of the<br />
Sheet Erosion Process.4g<br />
RAINFALL DETACHMENT<br />
Dr = Sdr.<br />
2<br />
Ai. 1 Meyer & Wischmeier (1969)<br />
Id = C.COs S (CosS.P2 - C,.d.P) Fleming & Fahmy (1973)<br />
2<br />
SS = f(KE. M . Vt )<br />
Negev ( 1967)<br />
RUNOFF DETACHMENT<br />
Df = Sdf . Ai (SQ. Qp )<br />
Rd = K. X.y.S<br />
TRANSPORT CAPACITY OF RAINFALL<br />
Meyer & Wischmeier (1969)<br />
Fleming & Fahmy (1973)<br />
T = St,. S I Meyer & Wischmeier (1969)<br />
It = T.P.Tan S Fleming & Fahmy (1973)<br />
TRANSWRT CAPACITY OF RUNOFF<br />
INTERRILL TRANSPORT CAPACITY<br />
T , = C . (Si + C ) W. L. (‘2.1)<br />
Cl s1 so<br />
Where:<br />
Dr =<br />
‘dr =<br />
Ai =<br />
I =<br />
I =<br />
d<br />
c =<br />
s =<br />
P =<br />
c1 =<br />
d =<br />
ss =<br />
Soil detached by rainfall<br />
Soil effect factor<br />
Area of increment<br />
Rainfall intensity<br />
Soil detachment rate<br />
Soil type parameter<br />
Angle of slope<br />
Rainfall intensity<br />
Soil parameter<br />
Particle size<br />
Soil splash<br />
Meyer & Wischmeier (1969)<br />
Bennett (1974)<br />
Foster & Meyer (1975)<br />
K = Soil parameter<br />
8 = Fluid specific gravity<br />
Y = Flow depth<br />
Tr = Splash transport capacity<br />
‘tr =<br />
Soil effect constant<br />
It = Impact transport rate<br />
T = Parameter<br />
T -<br />
f -<br />
s =<br />
tf<br />
Tc =<br />
Runoff transport capacity<br />
Soil effect constant<br />
Flow transport capacity<br />
so = Local bed slope<br />
ICE= Kinetic energy<br />
M = RaindroD .~ mass<br />
Vt = Terminal velocity of raindrops<br />
T . = Interrill transport capaci’<br />
Cl<br />
C . = Soil tranSDOrtabilitv cosi<br />
efficient<br />
Si = Interrill slope steepness<br />
Df = Soil detached by runoff Cso = Transport capacity at zero<br />
Sdf = Soil effect constant<br />
U =<br />
slope<br />
Excess rainfall rate<br />
Q =<br />
Rd =<br />
Flow rate L<br />
Rate of soil detachment<br />
i<br />
c<br />
=<br />
=<br />
Interrill<br />
constant<br />
slope length<br />
16
of accumulated loose material must be distinguished from the inherent<br />
erodibility of the soil. Scope exists for replacement of the rainfall term<br />
in the U.S.L.E. with a runoff parameter,14 (Table 1) and research could be<br />
directed at providing an index of snowmelt runoff, in order to make the<br />
approach more applicable to spring melt conditions.” Results from erosion<br />
plots at Sherbrooke, Quebec,I6 Sandusky, Ohio,” and Guelph,” have referred<br />
to annual soil loss rates equivalent to up to 126, 280 and nearly 4,000<br />
tonnes/km2 respectively and demonstrate that these processes could be a<br />
significant sediment source in this region.<br />
Little attention is usually attached to subsurface erosion processes<br />
in terms of land surface sediment sources, but it should be recognized<br />
that water flow within the soil may carry clay particles and colloidal<br />
material. In this context it is difficult to distinguish between suspended<br />
and dissolved transport. PipingIg may also develop at horizons in the soil<br />
where throughflow is concentrated and where significant hydraulic gradients<br />
exist and this mechanism is doubtless significant for sediment removal,<br />
although little work has as yet been carried out on its relative importance.<br />
In contrast to sheet erosion, much less attention, both in terms<br />
of experimental measurement and theoretical analysis has been applied to<br />
processes of channel erosion and many of the mechanisms are poorly understood,<br />
Where channels are cut in non-cohesive materials, scour will occur<br />
along a reach if the transport capacity of the flow is not satisfied and<br />
equilibrium transport relationships and regime theory” could be employed<br />
to evaluate the extent of erosion. Several studies have focused on the<br />
factors influencing the erosion resistance of cohesive materials and the<br />
effect of moisture content” but there is considerable scope €or application<br />
to the field situation. A pioneering field investigation by Wolman<br />
in 1959,” pointed to various mechanisms of bank erosion. He found severe<br />
erosion in winter months when high flows attacked banks that were previously<br />
wet and he noted significant erosion from combinations of cold periods, wet<br />
hanks, frost action and rises in stage, and that freeze-thaw action also<br />
produced some erosion without a change of stage. Subsequent work has<br />
pointed to the importance of pre-conditioning of the banks, particularly by<br />
wetting, hcfore significant erosion occurs‘ and Carson &.” have<br />
developed a model of bank sediment entrainment for the spring flood in the<br />
Eaton Basin, Quebec. This is based on simulation of changes in bank erodibility,<br />
with sediment entrainment decreasing with increased duration of<br />
flow past a point and the rate of initial entrainment closely related to<br />
the time elapsed since that point was last submerged.<br />
Gully development is generally associated with an imbalance in<br />
the fluvial system such as occurs with a severe climatic event, improper<br />
land use or changes in base level. Their growth combines the action of<br />
concentrated surface water, alternate freeze-thaw of exposed banks and<br />
sides and sloughing due to undercutting and waterfall retreat. Several<br />
empirical studies of factors influencing gully growth have been carried<br />
out2* but in general the precise mechanics of gully development are poorly<br />
understood.<br />
THE WATERSHED RESPONSE TO EROSION PROCESSES<br />
In order to understand the sediment yield of a drainage basin,<br />
the processes outlined above must be integrated into the catchment and<br />
17
evaluated in terms of the overall drainage basin dynamics and hydrology.<br />
Not all the sediment eroded from a watershed will be transported out of the<br />
basin and the ratio of gross or total erosion (T) to sediment yield (Y) is<br />
Y<br />
generally referred to as the delivery ratio (D), i.e. D = 7, which is<br />
usually expressed as a percentage. The delivery ratio may vary in from<br />
excess of 90% to approaching zero and in a relatively homogeneous catchment<br />
it will tend to decrease with increasing basin area. Several workers have<br />
established quantitative relationshi s between the ratio and drainage area<br />
and other catchment characteristics,P6 and a generalized relationship<br />
between delivery ratio and catchment area based on such work is presented<br />
in Figure 3. Considerations of channel routing and in-channel and flood<br />
plain deposition and storage are involved in this characteristic of sediment<br />
delivery but little attention has been given to the precise process mechanisms<br />
involved.<br />
The relative efficacy of the various erosion processes in contributing<br />
to the sediment yield is also best evaluated at the basin level.<br />
Sheet erosion has primarily been studied on plots of bare or poorly vegetated<br />
soil, often under intense artificial rainfall, and the question of<br />
its importance under natural conditions in humid areas is closely related<br />
to discussion of the extent to which overland flow is a significant process<br />
in storm hydrograph production*’ and the relative importance of throughflow<br />
and surface runoff. Kirkby” demonstrated that rainfall intensities rarely<br />
exceed natural infiltration capacities in humid areas with good vegetation<br />
cover and suggested that throughflow is an important mechanism in storm<br />
hydrograph generation, with surface runoff only occurring from saturated<br />
areas near the slope base. The concept of widespread overland flow, resulting<br />
from rainfall intensity exceeding infiltration capacity, popularized by<br />
Horton” belongs essentially to semi-arid areas.<br />
Furthermore, the partial-area and variable source area models of<br />
storm runoff production, which have gained wide acceptance in years, recent 30 have very important implications for sediment source areas. If surface<br />
runoff is generated from a small portion of the basin close to the channel<br />
network, the area of the watershed significant for sediment yield will be<br />
limited to this contributing area, (Figure 4). The stream network must<br />
also be viewed as a dynamic element of the drainage basin and will in many<br />
cases expand and contract in response to variations in catchment moisture<br />
status and storm magnitude. 31 Acceptance of the dynamic character of both<br />
material erodibility and of the contributing area and drainage networks<br />
within a catchment necessarily means that sediment entrainment is very<br />
time-variant.<br />
The question of the precise sources of the sediment transported<br />
from a drainage basin is one that still requires detailed research. When a<br />
good vegetation cover is present and in the absence of direct evidence of<br />
overland flow, it is attractive to ascribe the dominant source to the<br />
channel system. Work by Kirkby3’ in an upland catchment in Scotland<br />
suggested that 93% of the sediment came from erosion of river bluffs and the<br />
study by Carson &. 33 of the Eaton Basin in the Quebec Appalachians<br />
concluded that the suspended sediment load originated primarily from scour<br />
of the banks of the channel network. However, calculation of sediment<br />
budgets can indicate that the rate of channel enlargement is totally inadequate<br />
to account for the volume of sediment represented by the sediment<br />
yield and alternative sources must be sought. Study of the clay mineralogy<br />
18
I-<br />
W<br />
.<br />
Based on:<br />
\ Roehl (1 962)<br />
Maner (1 958)<br />
Gottschalk and Brune (19<strong>50</strong>)<br />
2 100-<br />
Maner and Barnes (1 953)<br />
2<br />
t <strong>50</strong>-<br />
E<br />
a<br />
20-<br />
*r, 10t<br />
F 5-<br />
$<br />
@ 2-<br />
1<br />
0.0 1<br />
1 1<br />
0-05 0.1<br />
I I<br />
0.5 1.0<br />
I<br />
5<br />
I<br />
10<br />
1 I<br />
<strong>50</strong> 100<br />
I<br />
<strong>50</strong>0 1000<br />
Drainage Area (Square miles)<br />
Figure 3. A generalized relationship between drainage area and sediment<br />
delivery ratio based on the findings of several U.S. workers.
N<br />
0<br />
2 Year Return Period 10 Year Return Period<br />
area<br />
Figure 4 . A hypothetical example of the variation of the storm runoff<br />
contributing area and the stream network within a catchment for<br />
flood events of different magnitude.
of sediments may provide information on source areas. " Techniques of<br />
'chemical fingerprinting' may also help to provide an answer and it is<br />
noteworthy that the increased attention recently being paid to the chemical<br />
quality of sediment may indirectly provide valuable evidence to as sediment<br />
sources.<br />
Despite some debate as to precise processes and source areas<br />
involved in sediment yield and their relation to storm runoff dynamics,<br />
several studies have successfully related catchment sediment yields to<br />
hydrometeorological variables, both on an annual and on a storm-period<br />
basis, 35 and to land use and other catchment characteristics. 36 Soil loss<br />
equations can also be adapted for catchment if use a routing algorithm is<br />
introduced. 3 7 Some researchers have, however, noted differences in the<br />
pattern of response to hydrometeorological conditions according to catchment<br />
size,3R with yields from plots and small catchments reflecting the<br />
seasonal distribution of rainfall energy and those from large catchments<br />
reflecting the runoff regime. Explanation of such contrasts must be<br />
sought in considerations of sediment routing, delivery ratios, and the<br />
partial-area concept. Following from the early work of Nege~,'~ progress<br />
has also been made in the field of computer simulation of erosion and<br />
sediment yields at the catchment level," although the dependence on<br />
existing runoff models and the use of optimized parameters can to call<br />
question the extent to which such models represent reality. Some attempts<br />
have been made to develop more deterministic models for individual hillslopes<br />
and small catchments," but progress must be limited until our<br />
knowledge of sediment processes and source areas improves and allows more<br />
realistic conceptual models to be formulated.<br />
TEMPORAL PATTERNS OF SEDIMENT I~LIVERY<br />
Temporal and spatial integration of the processes of erosion and<br />
sediment yield within a drainage basin result in a complex of pattern<br />
variation in sediment yield at the catchment outlet. A knowledge of the<br />
magnitude and timing of sediment concentration and loads is essential if<br />
the problems of sediment-associated contaminants and nutrients to are be<br />
evaluated. Sediment discharge (S) / streamflow ((1) or sediment concentration<br />
(C) / streamflow (Q) relationships or rating curves have been used<br />
by many workers to characterize the general range of variation of sediment<br />
transport rates and to demonstrate the tendency for increased concentrations<br />
or loads to be assoc'ated with high flows. In general a relationship of<br />
the form S or C = aQ' can be established (Figure 5). The close dependence<br />
of sediment concentration on discharge should not be viewed as indicative<br />
of a transport capacity effect, because the wash load is essentially a noncapacity<br />
load, but rather that the erosion processes causing sediment<br />
entrainment generally operate most effectively during periods of high flow.<br />
In most cases, considerable scatter about the simple rating relationship is<br />
apparent and this can be related to seasonal and hysteretic effects, the<br />
detailed timing of the concentration graph relative to the flow hydrograph,<br />
and an exhaustion effect operating both during a single event and through a<br />
series of events. '* An example from a British catchment of some of these<br />
controls and in particular the exhaustion effect is presented in Figure 6.<br />
Several researchers have suggested that the slope (b) and intercept (a)<br />
values of a rating relationship can be used to infer source areas and the<br />
general sediment dynamics of a catchment. 43<br />
21
1 000<br />
i<br />
\<br />
9,<br />
E<br />
- - - . - - -<br />
Daily Mean Discharge (cfs)<br />
22
W<br />
N<br />
Figure 6. Variation of suspended sediment concentration during a series<br />
of storm runoff events on the River<br />
Dart, Devon, England (46 km’). This clearly demonstrates the complexity of the relationship<br />
between concentration and discharge.
The seasonal distribution of yield from a catchment will reflect<br />
the interaction of precipitation and flow regimes and sediment availability.<br />
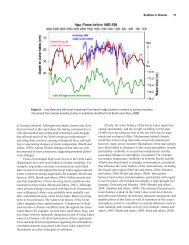
In the Great Lakes region the seasonal yield pattern is dominated by the<br />
period of the spring melt, (Figure 7) and Dickinson &.'4 calculated that<br />
about 55% of the sediment load of rivers in southern Ontario is carried<br />
during March and April. Duration or frequency analysis can also be usefully<br />
employed to demonstrate that a large proportion of the sediment load<br />
of a river is carried during a short period of time. In small catchments<br />
in East Devon, England, for example, approximately 78 percent of the annual<br />
suspended sediment load was carried by flows which equalled or exceeded 1%<br />
of the time or within an overall duration equivalent to 3.5 days. Similar<br />
analysis for several rivers tributary to the Great Lakes is presented in<br />
Figure 8. In the case of the Cass River, Michigan, approximately 80 percent<br />
of the load was carried in 5 percent of the year. If the data available<br />
are sufficiently detailed, analysis can be extended to individual storms in<br />
order to evaluate the relative importance of storms of varying magnitude in<br />
sediment production. 'I5 Long periods of record will also enable relative<br />
importance of extreme events to be assessed and anlysis of the significance<br />
of events of varying return period could be usefully combined with an assessment<br />
of the extent and character of the associated contributing areas and<br />
of thresholds in hydrologic response. Techniques of measuring suspended<br />
sediment concentrations and loads are now well documented46 but careful<br />
evaluation of patterns of temporal variation in sediment transport is necessary<br />
if sampling programmes are to provide reliable estimates of sediment<br />
yield.<br />
Any statement of research needs depends closely on the perception<br />
of the purposes involved and on the subjective views of the writer. In the<br />
opinion of this writer, who is concerned with the geomorphological and hydrological<br />
background, future research on erosion processes, sediment sources<br />
and sediment yields should be focused on the drainage basin as a unit of<br />
study. Since the suspended load of a stream is primarily a non-capacity<br />
load, attention should be directed towards sediment sources and entrainment<br />
processes within the basin rather than channel transport hydraulics. Detailed<br />
evaluation of the erosion processes operating on a slope or a small plot<br />
may be of limited value if the eroded material does not enter a stream. The<br />
fate of the sediment as it is routed down the channel network remains another<br />
large area of uncertainty but this is outside the scope of this discussion.<br />
Worthwhile areas of study could include:<br />
1) In terms of process dynamics, detailed investigation of the<br />
mechanisms and significance of sediment entrainment by subsurface<br />
runoff and of bank erosion is required. Plot and reach studies will<br />
be necessary but the results should be integrated within the overall<br />
watershed response.<br />
2) Development of sediment budgets in order to demonstrate the<br />
relative importance of various processes.<br />
24
C<br />
4 30<br />
RIVER CASS (2196 km2)<br />
1966-70<br />
a < 30<br />
401<br />
x<br />
20<br />
$<br />
5 10<br />
s<br />
P O<br />
BIG CREEK (591 km2)<br />
1967-73<br />
Figure 7. The average annual distribution of monthly sediment loads for<br />
several rivers draining to the Great Lakes.<br />
25
/<br />
/<br />
/<br />
/<br />
/<br />
/<br />
/<br />
/<br />
/<br />
/<br />
/ /<br />
/<br />
/<br />
/<br />
/<br />
/<br />
/<br />
I 1 I I I I I 1 I 1 I 1 I 1<br />
0.1 0.2 0.5 1 2 5 10 20 30 40 <strong>50</strong> 60 70 8C<br />
Cumulative Percentage of Time<br />
Figure 8. Duration characteristics of sediment yield from four catchments<br />
draining to the Great Lakes. Data from two other U.S. rivers<br />
are also included for comparative purposes.<br />
26
3) Delimitation of major source areas in order to evaluate the<br />
spatially distributed nature of erosion processes.<br />
4) Integration of source and budget studies with realistic<br />
concepts of storm runoff production, particularly the partial-area<br />
and variable source area concepts.<br />
5) Detailed monitoring of individual storm runoff events in order<br />
that the spatial and temporal integration of sediment dynamics can be<br />
understood.<br />
6) Development of physically-based models of erosion and sediment<br />
yield as a long term objective.<br />
LITERATURE CITED<br />
follow.<br />
1.<br />
2.<br />
3.<br />
4.<br />
5.<br />
6.<br />
7.<br />
8.<br />
References to elaborate points raised in the preceding discussion<br />
(Numbers refer to numbers in parentheses in the text)<br />
Task Committee on Preparation of Sedimentation Manual (1962).<br />
Sediment Transportation Mechanics: Introduction and Properties<br />
of Sediment, Proc. A.S.C.E. J. Hyd. Div. E, HY4, 77-107.<br />
For example: U.N.E.S.C.O., (1974). Gross Sediment Transport to<br />
the Oceans, U.N.E.S.C.O. Sc. 74lWSl33.<br />
Holeman, J. N., (1968). The Sediment Yield of Major Rivers of<br />
the World, Water Resources Research,<br />
4, 737-47.<br />
Langbein. W. B. and Schumm, S. A., (1958). Yield of Sediment in<br />
Relation to Mean Annual Precipitation, Trans. Amer. Geophys. Union,<br />
- 39, 1076-84.<br />
See also Wilson, L., (1973). Variations in Mean Annual Sediment<br />
Yield as a Function of Mean Annual. Precipitation, Amer. J. Sci.<br />
273, 335-349.<br />
Douglas, I., (1967). Man, Vegetation and the Sediment Yield of<br />
Rivers, Nature, 215, 925-8.<br />
Fleming, G., (1969). Design Curves for Suspended Load Estimation,<br />
Proc. Inst. Civ. Em., 9, 1-9.<br />
Jansen, J. M. L. and Painter, R. B., (1974). Predicting Sediment<br />
Yield from Climate and Topography, J. Hydrol. 11, 371-80.<br />
Stichling, W.. (1973). Sediment Loads in Canadian Rivers, in Fluvial<br />
Processes and Sedimentation, Proc. 9th Hydrology Symposium, Edmonton.<br />
Alberta, National Research Council, 39-72.<br />
Rainwater, F. H., (1962). Stream Composition in the Conterminous<br />
United States, U.S.G.S. Hydr. Inv. Atlas HA61.<br />
27
9. For example: Musgrave, G. W., (1947). The Quantitative Evaluation<br />
of Factors in Water Erosion, a First Approximation, J. Soil and<br />
Water Conservation 2, 133-8.<br />
Zingg, A. W. (1940) Degree and Length of Land Slope as it Affects<br />
Soil Loss in Runoff, Agricultural Engineering<br />
2, 59-64.<br />
Wischmeier, W. H. and Smith, D. D. (1958) Rainfall Energy and its<br />
Relation to Soil Loss, Trans. Amer. Geophys. Union, 2, (2). 285-91.<br />
Mirtskhoulava, T. E. (1974) Prediction of Erosion Intensification<br />
by Man, Int. Assoc. Hydr. Sci. Pub. 113, 78-82.<br />
10. For example: Meyer, L. D. and Wischmeier, W. H. (1969)<br />
Mathematical Simulation of the Processes of Soil Erosion by<br />
Water, Trans. Am. SOC. Ag. Engrs. 12, (6), 754-8, 762.<br />
Foster, G . R. and Meyer, L. D. (1975) Mathematical Simulation<br />
of Upland Erosion by Fundamental Erosion Mechanics, in Present<br />
and Prospective Technology for Predicting Sediment Yields and<br />
Sources, U.S.D.A. ARS-S-40 190-207<br />
Free, G. R. (1960) Erosion Characteristics of Rainfall, Ag.<br />
Engineering, 41, (7) 447-9, 455.<br />
Rowlison, D. L. and Martin, G. L. (1971) Rational Model Describing<br />
Slope Erosion, Proc. A.S.C.E. J. Irrig. Dr. Div. 97 (IRl), 39-<strong>50</strong>.<br />
Kiling, M. and Richardson, E. V. (1973) Mechanics of Soil Erosion<br />
from Overland Flow Generated by Simulated Rainfall, Colorado<br />
State University Hydrology Papers, 63.<br />
Carson, M. A. and Kirkby, M. J. (1972) Hillslope Form and Process<br />
Ch. 8., Surface Water Erosion.<br />
11. For example: Barnett, A. P. and Rogers, 3. S. (1966) Soil Physical<br />
Properties Related to Runoff and Erosion from Artificial Rainfall<br />
Trans. A.S.C.E. 2, 123 et al.<br />
Wischmeier, W. H. and Mannering, J. V. (1969) Relation of Soil<br />
Properties to its Erodibility, Proc. Soil Sci. SOC. America, 3,<br />
(1) , 131-37.<br />
Wischmeier, W. H. &. (1971) A Soil Erodibility Nomograph for<br />
Farmland and Construction Sites, J. Soil and Water Conservation<br />
- 26, 189-93.<br />
Bryan, R. B. (1969) The Development, Use and Efficiency of<br />
Indices of Soil Erodibility, Geoderma,<br />
2, (l), 5-25.<br />
12. Wischmeier, W. H. and Smith, D. D. (1965) Predicting Rainfall-<br />
Erosion Losses from Cropland East of the Rocky Mountains,<br />
Agriculture Handbook, 282, USDA, ARS.
13.<br />
14.<br />
15.<br />
16.<br />
17.<br />
18.<br />
1.9.<br />
20.<br />
21.<br />
22.<br />
For example: Emmett, W. W. (1970) The Hydraulics of Overland<br />
Flow on Hillslopes, U.S.G.S. Prof. Paper 662-A.<br />
Ellison, W. D. (1945) Some Effects of Raindrops and Surface<br />
Flow on Soil Erosion and Infiltration, Trans. Amer. Geophys. Union<br />
- 26, 415-29.<br />
Williams, J. R. (1975) Sediment Yield Prediction with Universal<br />
Equation Using Runoff Energy Factors, in Present and Prospective<br />
Technology for Predicting Sediment Yields and Sources, USDA,<br />
ARS-S-40, 244-52.<br />
Wigham, J. M. and Stolte, W. J. (1973) Sediment Yield Estimates<br />
from an Index of Potential Sediment Production, in Fluvial Processes<br />
and Sedimentation, Proc. 9th Hydrology Symposium, Edmonton, Alberta,<br />
208-16.<br />
Clement, P. and Gadbois, P. (1971) Comparison et Interpretation<br />
des Resultats de Deux Parcelles Experimentales d'Erosion 1969-70<br />
(Sherbrooke, Quebec). Proc. 2nd Guelph Symp. Geomorphol., 266-27<br />
Schwab, G. 0. et&., (1972) Chemical and Sediment Movement from<br />
Agricultural Land into Lake Erie Rept. Water Resources Center,<br />
Ohio State University.<br />
Ketcheson, J. K. &., (1973) Potential Contribution of<br />
Sediment from Agricultural Land, in Fluvial Processes and<br />
Sedimentation, Proc. 9th Hydrology Symposium, Edmonton, Alberta,<br />
208-16.<br />
Jones, A. (1971) Soil Piping and Stream Channel Initiation<br />
Water Resources Research, 1, 602-10.<br />
For example: Lane, E. W. (1955) The Importance of Fluvial<br />
Morphology in Hydraulic Engineering, Proc. A.S.C.E. 3. Hyd. Div.,<br />
- 81, 1-17.<br />
Blench, T. (1957) Regime Behaviour of Canals and Rivers,<br />
Butterworths.<br />
For example: Smerdon, E. T. and Beasley, R. P. (1959) Tractive<br />
Force Theory Applied to Stability of Open Channels in Cohesive<br />
Soils. Research Bull. 715, Univ. Missouri Ag. Expt. Station,<br />
Columbia, Mo.<br />
Grissinger, E. H. (1966) Resistance of Selected Clay Systems<br />
to Erosion by Water, Water Resources Research, g,(l), 131-8.<br />
Partheniades, E. (1971) Erosion and Deposition of Cohesive<br />
Materials, in H. W. Shen (ed.) River Mechanics, 2.. 251-91.<br />
Wolman, M. G. (1959) Factors Influencing Erosion of a Cohesive<br />
River Bank, Amer. J. Sci., 257, 204-16.<br />
29
23.<br />
24.<br />
25.<br />
26.<br />
27.<br />
28.<br />
29.<br />
30.<br />
Knighton, A. D. (1973) Riverbank Erosion in Relation to Streamflow<br />
Conditions, River Bollin-Dean, Cheshire, East Midland Geographer,<br />
- 5, ( E), 416-26.<br />
Harrison, S. S. (1970) Note on the Importance of Frost Weathering<br />
in the Disintegration and Erosion of Till in East-Central Wisconsin,<br />
Bull. Geol. SOC. Am., 3, 3407-10.<br />
Carson, M. J., d., (1973) Sediment Production in a Small<br />
Appalachian Watershed During Spring Runoff: The Eaton Basin,<br />
1970-72, Canadian J. Earth Sciences, E, (12), 1707-34.<br />
For example: Thompson, J. R. (1964) Quantitative Effect of<br />
Watershed Variables on the Rate of Gully Head Advancement. Trans.<br />
Am. SOC. Ag. Engrs., 1, (I), 54-55.<br />
Beer. C. E. and Johnson, H. P. (1965) Factors Related to Gully<br />
Growth in the Deep Loess Area of Western Iowa, Proc. (1963)<br />
Federal Interagency Sedimentation Conference, USDA Misc. Pub.<br />
970. 37-43.<br />
Heede, B. H. (1974) Stages of Development of Gullies in the Western<br />
United States of America, Zeits. Geomorph..<br />
Is, (3), 260-71.<br />
For example: Roehl, J. W. (1962) Sediment Source Areas, Delivery<br />
Ratios and Influencing Morphological Factors, Internat. Assoc.<br />
Hyd. Sci. Pub., 2, 202-13.<br />
Maner, S. B. (1958) Factors Affecting Sediment Delivery Rates<br />
in the Red Hills Physiographic Area, Trans. Amer. Geophys. Un.,<br />
39, 669-75.<br />
-<br />
Renfro, G. W. (1975) Use of Erosion Equations and Sediment-<br />
Delivery Ratios for Predicting Sediment Yield, in Present and<br />
Prospective Technology for Predicting Sediment Yields and<br />
Sources, USDA, ARS-S-LO, 33-45.<br />
For example: Pierce, R. S. (1967) Evidence of Overland Flow<br />
on Forest Watersheds: and<br />
Hewlett, J. D. and Hibbert, A. R. (1967) Factors Affecting<br />
the Response of Small Watersheds ot Precipitation in Humid<br />
Areas, both in:<br />
Sopper, W. E. and Lull, H. W. (eds.) Int. Symp. Forest Hydrology<br />
(Pergarnon Press), 247-51 and 275-90.<br />
Kirkby, M. J. (1969) Infiltration, Throughflow and Overland Flow,<br />
in R. J. Chorley (ed.) Water Earth and Man, 215-28.<br />
Horton, R. E. (1933) The Role of Infiltration in the Hydrologic<br />
Cycle, Trans. Amer. Geophys. Un., 14 446-60.<br />
For example: Dunne, T. and Black, R. D. (1970) Partial Area<br />
Contributions to Storm Runoff in a Small New England Watershed, Water<br />
Resources Research, 5, 1269-1311.<br />
Ragan, R. M. (1967) An Experimental Investigation of Partial Area<br />
Contributors. Int. Assoc. Hyd. Sci. Pub., 3, 241-9.<br />
30
Nutter, W. L. and Hewlett, 3. D. (1971) Stormflow Production<br />
From Permeable Upland Basins, in Monke, E. J. (Ed.) Biological<br />
Effects in the Hydrological Cycle, Proc. Third Int. Seminar for<br />
Hydrology Professors, 248-58.<br />
Engman, W. T. (1974) Partial Area Hydrology and its Application<br />
to Water Resources, Water Resources Bulletin (3), 512-21.<br />
Dunne, T., Moore, T. R. and Taylor, C. R. (1975) Recognition<br />
and Prediction of Runoff-Producing Zones in<br />
Ass. Hyd. Sci. Bull. 0, 305-27.<br />
Humid Regions. Int.<br />
31. For example: Gregory, K. J. and Walling, D. E. (1968) The<br />
Variation of Drainage Density Within a Catchment. Int.<br />
Sci. Bull. 2, 61-8.<br />
Ass. Hyd.<br />
Blyth, K. and Rodda, J. C. (1973) A Stream Length Study. Water<br />
Resources Research 2, (5) 1454-61.<br />
32. Kirkby, M. J. (1967) Measurement and Theory of Soil Creep,<br />
J. Geol. 3, 359-78.<br />
33. Carson, M.J. gtt (1973) (see ref. 24).<br />
34. For example: Wall, G. J. and Wilding, L. P. (1976) Mineralogy<br />
and Related Parameters of Fluvial Suspended Sediments in Northwestern<br />
Ohio. J. Environ. Qual. 2, 168-73.<br />
Klages, M. G. and Hsieh, Y. P. (1975) Suspended Solids Carried<br />
by the Gallatin River of Southwestern Montana: 11. Using<br />
Mineralogy for Inferring Sources. J. Environ. Qual. 4. 68-73.<br />
35. For example: Dragoun, F. .J. (1962) Rainfall Energy as Related<br />
to Sediment Yield, J. Geophys. Research, 67, (4), 1495-1<strong>50</strong>1.<br />
Williams, J. R. (1971) Prediction of Sediment Yields from<br />
Small Watersheds, Trans. Am. SOC. Ag. Engrs., E, (6), 1158-62.<br />
Guy, H. P. (1964) An Analysis of Some Storm Period Variables<br />
Affecting Stream Sediment Transport, U.S.G.S. Prof. Paper, 462E.<br />
Walling, D. E. (1974) Suspended Sediment and Solute Yields from<br />
a Small Catchment Prior to Urbanization, Inst. Br. Geogr. Spec.<br />
Pub. 6, 169-191.<br />
36. For example: Hindall, S. M. (1976) Prediction of Sediment<br />
Yield in Wisconsin streams. Proc. Third Federal Interagency<br />
Sedimentation Conference, 1205-18.<br />
Shown, L. M. (1970) Evaluation of a Method for Estimating Sediment<br />
Yield, U.S.G.S. Prof. Paper, 700-B, 245-9.<br />
Flaxman, E. M. (1972) Predicting Sediment Yield in the Western<br />
United States, Proc. A.S.C.E. J. Hyd. Div., 98. (HY12); 2073-85.<br />
31
37.<br />
38.<br />
39.<br />
40.<br />
41.<br />
42.<br />
43.<br />
44.<br />
Williams, J. R. (1975) Sediment Routing for Agricultural<br />
Watersheds. Water Resources Bulletin, 11. 965-74.<br />
McGuiness. J. L. & (1971) Relation of Rainfall Energy and<br />
Streamflow to Sediment Yield from Small and Large Watersheds,<br />
J. Soil and Water Cons., 26, 2087-98.<br />
Dickinson, W. T. g& (1975) Fluvial Sedimentation in Southern<br />
Ontario, Canadian J. Earth Sciences, 12, 1813-9.<br />
Negev, M. (1967) A Sediment Model on a Digital Computer. Rept.<br />
76, Stanford University, California.<br />
For example: Fleming, G. and Leytham, K. M. (1976) The<br />
Hydrologic and Sediment Processes in Natural Watershed Areas.<br />
Proc. Third Federal Interagency Sedimentation Conference, 1,<br />
233-46.<br />
Gupta, S. K. (1974) A Distributed Digital Model for Estimation<br />
of Flows and Scdiment Load from Large Ungauged Watersheds, Doctoral<br />
Thesis, University of Waterloo, Dept. Civil Engineering.<br />
For example: Foster, G. R. and Meyer, L. D. (1975) Mathematical<br />
Simulation of Upland Erosion by Fundamental Erosion Mechanics, in<br />
Present and Prospective Technology for Predicting Sediment Yields<br />
and Sources, USDA ARS-S-40, 190-207.<br />
Meyer, L. D. and Wischmeier, W. H. (1969) Mathematical Simulation<br />
of the Process of Soil Erosion by Water, Trans. Am. SOC. Ag.<br />
Engrs., 8, (4) 572-7, 580.<br />
Li Ruh-Ming 5 & (1976) Water and Sediment Routing from Small<br />
Watersheds, Proc. Third Federal Interagency Sedimentation Conference,<br />
- 1, 193-204<br />
Walling, D. E. and Teed, A. (1971) A Simple Pumping Sampler for<br />
Research into Suspended Sediment Transport in Small Catchments,<br />
J. Hydtol., z, 325-37.<br />
Porterfield, G . (1973) Computation of Fluvial-Sediment Discharge,<br />
U.S.G.S. Techniques of Water Resources Investigations, C3, (3).<br />
Bauer, L. and Tille, W. (1967) Regional Differentiations of the<br />
Suspended Sediment Transport in Thuringia and their Relation to<br />
Soil Erosion. Int. Ass. Hyd. Sci. Pub., 75, 367-77.<br />
Flaxman, E. M. (1975) The Use of Suspended Sediment Load<br />
Measurements and Equations for Evaluation of Sediment Yield in<br />
the West, in Present and Prospective Technology for Predicting<br />
Sediment Yields and Sources, USDA ARS-S-40, 46-56.<br />
Dickinson, W. T. eta2 (1975) Fluvial Sedimentation in Southern<br />
Ontario, Canadian Journal of Earth Sciences, 1 2, 1813-9.<br />
32
45. Piest, R. F. (1965) The Role of the Large Storm as a Sediment<br />
Contributor, Proc. (1963) Federal Interagency Sedimentation<br />
Conference, USDA Misc. Pub. 970, 97-108.<br />
46. For example: Guy, H. P. and Norman, V. W., (1970). Field Methods<br />
for Measurement of Fluvial Sediment, U.S.G.S. Techniques of<br />
Water Resource Investigations, g, (3).<br />
47. Kirkby, M. J. (1969) Erosion by Water on Hillslopes, in R. J.<br />
Chorley (ed.) Water, Earth and Man.<br />
Musgrave, G. W. (1947) Quantitative Evaluation of Factors in<br />
Water Erosion, a First Approximation, J. Soil and Water<br />
Conservation, 2, 133-8.<br />
Wischmeier, W. H. and Smith, D. D. (1965) Rainfall Erosion<br />
Losses from Cropland East of the Rocky Mountains, U.S.D.A. Agr.<br />
Handbook, 282.<br />
Williams, J. R., (1975). - See Ref. 14.<br />
Foster, G. R. 9 &. (1973) Erosion Equation Derived from<br />
Modelling Principles. ASAE Paper 73-25<strong>50</strong>.<br />
48. Meyer, L. D. and Wischmeier, W. H., (1969). See Ref. 41.<br />
Fleming, G. and Fahmy, M. (1973) Some Mathematical Concepts<br />
for Simulating the Water and Sediment Systems of Natural Water-<br />
shed Areas, Univ. Strathclyde Dept. Civ. Eng. Rept. H0-73-26.<br />
Negev, M. (1967). See Ref. 39.<br />
Bennett, J. P. (1974) Concepts of Mathematical Modelling of<br />
Sediment Yield, Water Resources Research,<br />
lo, 485-92.<br />
Foster, G. R. and Meyer, L. D., (1975). - See Ref. 41.<br />
33
DISCUSS ION<br />
Mr. T. CahiZZ: Dr. Walling, the application of partial area hydrology is<br />
complicated in parts of the Great Lakes Basin, especially in Lake Erie by<br />
the existence of extensive under-drainage systems in the agricultural<br />
regions. Do you know, or have you had any exposure to any work investigating<br />
partial area applications, or analysis in basins with heavy underdrainage<br />
networks?<br />
Dr. D. ValZing: I think the short answer to this is no. The concept of<br />
partial area and variable source area is rather complicated. It is difficult<br />
enough to get this working as a realistic deterministic model, let alone<br />
building in underdrainage. The two are obviously very closely linked.<br />
Dr. D. Baker: You used a figure showing three different storms with equal<br />
runoff but greatly differing sediment yields associated with those storms.<br />
I could not read the time base. To what extent might variations in source<br />
areas and ground cover affect the sediment yields at the outlet of your<br />
watershed, or is it washing out of accumulated sediments in the channel<br />
itself? What is the source of the variations?<br />
Dr. D. Wuv'aZv'aZing: The storms were over about a three day period. The watershed<br />
size is about 30 km'. The rainfall characteristics were essentially<br />
similar for those three storms. My own feeling is that one has got both<br />
storage in the channel and on the land surface, and one has got these<br />
important exhaustion effects. Once the first sediment is removed from the<br />
land surfare, you get back to the inherent erodibility of the soil and the<br />
sediment production rates then drop quite markedly.<br />
DrS. ?'. Uickinson: You made a comment with regard to the bank erosion that<br />
you called preconditioning. I might say that initial conditions are important.<br />
I suggest that in fact the initial conditions are important for any of the<br />
erosion processes and perhaps that is what you are saying and you are also<br />
saying source areas are important and the whole timing is important. I think<br />
there has been a fair bit of experience in the modelling exercises if that<br />
one can make a good guess at the initial conditions, soil conditions, moisture<br />
conditions, cropping conditions, or whatever, one be may able to make a<br />
reasonable estimate of what is going to come off, whether its the bank or the<br />
field or whatever. The major problem is making a good guess of how those<br />
conditions vary. Would you agree with that?<br />
35
Dr. D. Walling: Yes, entirely. One wants some continuous accounting process<br />
whereby one can watch the conditions as they vary through time. A very good<br />
point.<br />
Dr. E. &gley: I have one observation to make regarding what you have<br />
said. From the point of view of impact on such a large area as the Great<br />
Lakes, it is obviously not possible to do the kinds of detailed studies one<br />
would like to have for such a broad area other than in small areas to obtain<br />
a feeling for the variables involved or the intensity of the processes. I<br />
think one of the things we need to address ourselves to, perhaps in the<br />
working session, is how do we extend the knowledge that we can gain from<br />
small areas to assessing impact over broad areas. This is a major problem<br />
when you are looking at something the size of the Great Lakes area.<br />
36
Influence of Land Use on Erosion and Transfer Process<br />
of Sediment<br />
T. Dickinson and G. Wall<br />
The average sediment yield from the landscape and the condition of<br />
stream channels tend to change with advancing forms of man's land use activities,<br />
as summarized in Table 1. Land uses which can cause severe erosion<br />
and sediment problems (e.g. highway and urban construction, timber harvesting,<br />
strip mining operations) have been identified; and land uses which tend to<br />
stabilize the landscape (e.g. reforestation, permanent pastures, stabilized<br />
urban settings) have been noted in the literature. Yet it remains difficult<br />
to quantitatively estimate changes in erosion and/or sediment yield due to<br />
land use changes - even in regions where sediment data are available, let<br />
alone in areas where extrapolation is being attempted.<br />
Although persons have observed and measured soil erosion and de-<br />
position phenomena in nature and have ascribed general reasons for these<br />
occurrences, the interplay of the forces, causes and the boundary conditions<br />
pertaining to erosion and sediment movement are less obvious.<br />
An investigation of the influence of land use on erosion and transport<br />
processes (as indeed an investigation of the processes themselves) also<br />
reveals that apparent land use effects are closely related to the of scale<br />
the studies undertaken. The dominant forces and boundary conditions governing<br />
erosion and sediment transport vary with landscape scale (i.e. the<br />
processes predominant on a plot are quite different from those determining<br />
conditions on a large field). Although the matter of scale has been empirically<br />
handled by simple extrapolation and by means of the delivery ratio approach,<br />
the real influence of scale on the processes and on apparent land use effects<br />
is not well understood.<br />
In light of the present state of knowledge regarding erosion and<br />
transport processes and the part scale has played in affecting statements on<br />
land use effects, the following discussion is focused on approaches which<br />
have been taken on different landscape scales - identifying items learned and<br />
apparent gaps.<br />
PLOT SCALE<br />
Plot studies have been used extensively by agriculturalists and<br />
hydrologists to study soil and water losses under a variety of climatic,<br />
physiographic, land use, and soil management situations. Plots became the<br />
unit of experimentation in these studies since they provided a quick and<br />
37
inexpensive means of obtaining the needed data in a short period of time.<br />
The basic premise common to all plot studies has been that by locating plots<br />
in a similar environment, all variables can be controlled except the one to<br />
be studied. Differences in results are therefore attributed to the differences<br />
in treatments. Generally, all plot studies have assumed that results<br />
were applicable to a greater or lesser degree beyond the limits of the plot.<br />
A review of the literature has indicated that there is no standard<br />
design for plot equipment (Hayward, 1971). Plot sizes range from a few<br />
square feet in laboratory studies to several acres in field studies. The<br />
most common plot size was 1/40 to 1/<strong>50</strong> of an acre. In general plots were 6,<br />
10, or 12 feet wide and 35, 70 or 72.6 ft. long. Plot width is often determined<br />
by drill size and tractor use within the plot. Plot length is usually<br />
determined by the local recommended terrace interval or the available length<br />
of uniform slope. Mutchler (1963) has given details of plot design and<br />
construction.<br />
The experimental design of soil loss plot experiments should permit<br />
the measurement of the difference between two or more treatments well as as<br />
furnish criteria by which the degree of confidence that can be placed in the<br />
result may be judged (Brandt, 1941). The two requisites of such a design are<br />
replication and randomisation. The purpose of replication is to increase<br />
precision and provide an estimate of error while randomisation ensures that<br />
the estimate of error is valid. It is significant to note that while the<br />
majority of plot studies reported in the literature did not replicate or<br />
randomize treatments, results are presented quantitatively (Hayward, 1971).<br />
The lack of proper design in these studies may mean that the results are<br />
either obscure or misleading (Brandt, 1941).<br />
Plot studies have been effectively employed to isolate factors<br />
which influence soil loss and runoff. Among the factors found to be most<br />
closely related to soil loss and runoff include: plant cover and mulches<br />
(Dickson, 1929); Kittredge. 1954; Duley. 1952). cropping practices and<br />
tillage (Van Doren and Bartelli, 1956; Meyer and Mannering, 1961), rainfall<br />
variables (Wischmeier, 1959; Rogers, &. , 1964) and slope parameters (Neale,<br />
1937; Zingg, 1940; Barnett and Rogers, 1966). In addition plot studies have<br />
been employed to evaluate the effectiveness of remedial measures proposed to<br />
reduce soil and water loss (Smith, 1941; Van Doren and Bartelli, 1956; Mannering<br />
and Meyer, 1961). However, there is an apparent lack of plot research in the<br />
literature that was designed to assess the importance of soil factors on<br />
erosion and runoff. Similarly, few studies have been conducted to study<br />
erosional processes.<br />
The findings from small plot studies have shown conclusively that a<br />
myriad of variables affect erosion. However, the extrapolation of plot data<br />
beyond the plot remains a problem. While many authors of plot research have<br />
freely assumed that their data had an application beyond the plot, it is<br />
generally admitted there is little evidence to support the direct aerial<br />
expansion of plot data.<br />
Several attempts have been made to relate plot data to field conditions<br />
through rational or empirical equations (Zingg, 1940; Musgrave, 1947;<br />
Smith, 1941; Van Doren and Bartelli, 1956). However, the universal soil loss<br />
equation (Wischmeier and Smith, 1965) is currently the empirical equation in<br />
38
LAND USE<br />
A. Natural forest<br />
or grassland<br />
B. Heavily grazed<br />
areas<br />
C. Cropping<br />
D. Retirement of land<br />
from cropping<br />
E. Urban construction<br />
F. Stabilization<br />
C. Stable urban<br />
TABLE 1.<br />
EFFECT OF LAND-USE SEQUENCE ON RELATIVE<br />
SEDIMENT YIELD AND CHANNEL STABILITY<br />
(after Guy, 1970 - a modification from Wolman, 1967)<br />
SEDIMENT YIELD<br />
Low<br />
Low to moderate<br />
Moderate to heavy<br />
Low to moderate<br />
Very heavy<br />
Moderate<br />
Low to moderate<br />
39<br />
CHANNEL STABILITY<br />
Relatively stable<br />
with some bank<br />
erosion<br />
Somewhat less<br />
stable than A<br />
Some aggradation and<br />
increased bank erosion<br />
Increasing stability<br />
Rapid aggradation and<br />
some bank erosion<br />
Degradation and severe<br />
bank erosion<br />
Relatively stable
greatest use. This statistical regression model was based upon soil loss<br />
records from test plots in U.S.A. the and takes the form A = RKLSCP where A<br />
is the average annual soil loss in tons per acre a specific from field, R is<br />
the rainfall factor, K is the soil erodibility factor, L is the slope length,<br />
S is the slope gradient, C is the cropping management factor and P is the<br />
existing conservation factor.<br />
The universal soil loss equation was designed to predict average<br />
annual soil losses from fields and construction sites by sheet and rill<br />
erosion. In order to obtain some estimate of the confidence limits of the<br />
equation, average annual soil losses were computed on 189 plots for which<br />
about 2300 plot years of records were available (Wischmeier, 1973). The plot<br />
predictions deviated from the corresponding measured soil losses by an<br />
average of 12% of the 11.3 ton overall average soil loss for the sample.<br />
The universal soil loss equation has been used for the prediction<br />
of watershed sediment yields but its capabilities and limitations for this<br />
use must be recognized (Shen, 1976). Universal soil loss equation predictions<br />
provide gross erosion levels and are not designed to predict deposition of<br />
sediments. It does not include sediment losses OK gains between the field<br />
and the stream OK reservoir. In order to use the universal soil loss equation<br />
to estimate sediment yield from a watershed it is necessary to introduce a<br />
weighted factor that takes into account deposition of eroded sediments. The<br />
sediment delivery ratio (defined as the change per unit area of downstream<br />
sediment movement from the source to any given measuring point) has been used<br />
for this purpose (Roehl 1962; Speraberry and Bowie, 1969; Renfro, 1972).<br />
Williams (1972) has modified the universal soil loss equation for predicting<br />
storm sediment yields by substituting the product of storm runoff amount and<br />
rate for the rainfall factor (R).<br />
Studies of erosion and sediment transport on watersheds have involved<br />
two approaches. On the one hand, sediment loads (primarily suspended<br />
solids) have been regularly sampled at the watershed outlet and inferences<br />
have been drawn regarding the sources of the sediment and the effects of land<br />
use. On the other hand, conceptual models of erosion and sedimentation<br />
processes have been hypothesized and used to estimate sediment yields at<br />
selected watershed outlet points. Where possible, these estimates have been<br />
compared with monitored results - OK monitored data have been used to "calibrate"<br />
the models. Each approach is considered below with regard to inherent<br />
strengths and weaknesses.<br />
(5) Sediment Load Monitoring<br />
Suspended sediment load data have been sparse in relation to those<br />
available for other hydrologic variables such as rainfall and streamflow. In<br />
Canada, it is only during the last two decades that continuous sampling of<br />
sediment loads for selected streams has been undertaken. Not only has<br />
sampling been sparse over area, but also it has been relatively infrequent in<br />
time .<br />
40
Although many of the sediment data have been carefully collected,<br />
and there have been pressing needs to make good use of this information,<br />
conclusions drawn from many of the monitored sediment load data regarding<br />
land use effects should be given careful consideration. Problems in this<br />
regard are noted below.<br />
Sediment load monitoring has customarily been time- rather than<br />
event-oriented (i.e. samples have been collected at regular time intervals<br />
rather than focused on storm events). As a consequence, peak concentrations<br />
and loads have not often been well defined. Since the loads occurring during<br />
peak flow periods account for the bulk of the total sediment load transported<br />
from a watershed (Dickinson, g., 1975) it is imperative that peak events<br />
be adequately sampled.<br />
Sediment samples taken during the year on a time-oriented base that<br />
misses many of the major sediment events do not provide either useful estimates<br />
of annual suspended loads or indices of such loads. Further, sample loads<br />
collected during periods of low flow, and land use effects inferred from low<br />
flow data, are not necessarily indicative of the annual suspended sediment<br />
load picture. This sampling problem poses a sufficient dilemma, that at the<br />
present time there is a need to examine the relative importance of peak flow<br />
events in order to design improved sampling schemes.<br />
In the northeastern United States and eastern Canada, the stream<br />
sediment load is controlled by source conditions rather than by downstream<br />
flow conditions. It is very difficult to "look upstream" from downstream<br />
data to identify important sources and land use effects without detailed<br />
information on source erosion and transport areas.<br />
(b) Watershed Models<br />
Watershed models of sediment yield have incorporated concepts<br />
whereby rainfall and runoff detach soil from field surfaces, transport it<br />
overland to drainageways, and move it downstream. One group of models<br />
applies the Universal Soil Loss Equation to land units (e.g. fields or groups<br />
oof fields) and routes the eroded material to other land and/or units downstream<br />
by means of empirical delivery ratios or transport factors (Kling and<br />
Olson, 1974; Frere, 1973). A second group couples erosion and sediment<br />
delivery concepts to deterministic hydrologic models (Negev, 1967; Fleming,<br />
1972; Simons &., 1975). Although some of the hydraulics-based aspects of<br />
these latter models are reasonably sophisticated, many of the concepts pertaining<br />
to the erosion of material and its transport are as simplistic and<br />
empirical as the delivery ratio approach.<br />
There is no doubt that* such models can be fitted to specific sets<br />
of input and output data, as numerous examples now illustrate. However, as<br />
the components and parameters are conceptual rather than physical in nature,<br />
it is extremely risky to apply the models to input conditions outside the<br />
range of conditions fitted - and to interpret model results in terms of<br />
land use effects. Predicted effects are not more meaningful than the individual<br />
and collective concepts included in the model.<br />
41
SWY NOTES<br />
1.<br />
2.<br />
3.<br />
4.<br />
5.<br />
6.<br />
Gaps in existing conceptual models include:<br />
lack of an adequate characterization of the dynamic development<br />
of the drainage system in a basin;<br />
lack of identification of the relative importance and physical<br />
significance of soil moisture storage concepts;<br />
lack of description of depositional processes in microdrainage<br />
systems; and<br />
insufficient emphasis on the role of large storms (year-to-year<br />
and storm-to-storm hydrologic differences often account for<br />
larger variations in sediment yield than most other factors!)<br />
Although plot studies have contributed information about factors<br />
influencing soil loss and runoff, the lack of statistical rigour<br />
in the design of these studies makes it difficult to interpret<br />
.ind/or use the results in a quantitative manner.<br />
?imple extrapolation of plot results to larger areas (often implied<br />
by the units used for reporting soil loss, i.e. tons/acre) is not<br />
valid.<br />
As plot data are of more qualitative than quantitative value, the<br />
use of them as a base for quantitative deterministic models of land<br />
use effects is questionable.<br />
Plot studies have not delineated sources of soil loss and associated<br />
land use effects.<br />
Land use effects inferred from time-oriented stream sediment sampling<br />
programs may be in error both qualitatively and quantitatively.<br />
lxisting watershed modelling approaches - although convenient - have<br />
not resolved our lack of understanding of erosion and transport<br />
processes, and of the effect of land use changes on these processes.<br />
Neither the plot nor the watershed approaches have addressed the<br />
problem of erosion sources or the problems of delivery and deposition<br />
between sources and downstream locations. In order to understand the processes<br />
and to draw meaningful conclusions about land use effects, focus must<br />
be given to sources and movement from sources to outlet. Event-oriented<br />
sampling studies from sources downstream, and @e application of dynamic<br />
contributing area concepts appear to be two of the most promising new approaches<br />
to the problem.<br />
Ackerman, W. C., and K. L. Corinth, (1962). An Empirical<br />
Equation for Reservoir Sedimentation. Publication 59,<br />
<strong>International</strong> Association of Scientific Hydrology, <strong>Commission</strong><br />
of Land Erosion. pp. 359-366.<br />
42
Adams, J. E., R. C. Henderson, R. M. Smith, (1959). Interpretations of<br />
Runoff and Erosion from Field Scale Plots on Texas Blackland<br />
Soil. Soil Sei. x, pp. 232-238.<br />
American Society of Civil Engineering, (1975). Sedimentation<br />
Engineering. (ed.) V. A. Vanouri. ASCE, Manuals and Reports<br />
on Engineering Practice. No. 54.<br />
Anderson, H. W., (1949). Flood Frequencies and Sedimentation from<br />
Forest Watersheds. Transactions, American Geophysical Union,<br />
- 30, (4), Washington, D. C., pp. 567-583.<br />
Archer, R. J., (1960). Sediment Discharge of Ohio Streams During<br />
Floods of January-February 1959. Ohio Department of Natural<br />
Resources, Division of Water, Columbus, Ohio.<br />
Baird, R. W., (1964). Sediment Yields from Blackland Watersheds.<br />
Transactions, American Society of Agricultural Engineers, 1.<br />
(4), St. Joseph, Michigan, pp. 454-456.<br />
Barnett, A. P., J. S. Rogers, J. H. Holladay, A. E. Dooley, (1965).<br />
Soil Erodibility Factors for Selected Soils in Georgia and<br />
South Carolina. Trans. Am. SOC. Agric. Engr., 2, pp. 393-395.<br />
Barnett. A. P., J. S. Rogers, (1966). Soil Physical Properties<br />
Related to Runoff and Erosion from Artificial Rainfall.<br />
Ibid. 2, 123-125 and 128.<br />
Baver. L. D., (1937). Rainfall Characteristics of Missouri in Relation<br />
to Runoff and Erosion. Soil Sci. SOC. Am. Proc., ll, 533-536.<br />
w.,<br />
Beale, 0. W., G. B. Nutt, T. C. Peele, (1955). The Effects of Mulch<br />
Tillage on Runoff, Erosion, Soil Properties and Crop Yields.<br />
19, 244-7.<br />
-<br />
Beer, C. E., and H. P. Johnson, (1965). Factors Related to Gully<br />
Growth in the Deep Loess Area of Western Iowa. Proceedings of<br />
the 1963 Federal Interagency Conference on Sedimentation, United<br />
States Department of Agriculture, Miscellaneous Publication<br />
970, pp. 37-43.<br />
Bennett, H. H., (1933). The Quantitative Study of Erosion Technique<br />
and Some Preliminary Results. Geogr. Rev., 2, pp. 423-432.<br />
Bethlahmy, N., W. J. Kidd, (1966). Controlling Soil Movement From<br />
Steep Road Fills. U. S. For. Serv. Res. Note I.N.T. 45.<br />
Blench, T., (1957). Regime Behavior of Canals and Rivers. Butteworth<br />
Scientific Publications, London, England.<br />
Blow, F., (1955). Quantity and Hydrological Characteristics of Litter<br />
Under Upland Oak Forests in Eastern Tennessee. J. For., 53<br />
190-195.<br />
Bonnard, D., and .I. Bruschin. Transports Solides en Suspension dans<br />
les Rivieres Suisses - Lonza, Grand-Eau, 1966-1969. Ecole<br />
Polytechnique Federale de Lausanne, Lausanne, Switzerland, (1970).<br />
Borland, W. M., (1961). Sediment Transport of Glacier-Fed Streams in<br />
Alaska. Journal of Geophysical Research, 66, (10).<br />
43
Borst, H. L., R. Woodburn, (1940). Rain Simulator Studies of the<br />
Effect of Slope on Erosion and Runoff. U.S. Dept. Agric.<br />
Soil Conserv. Serv. TP 36.<br />
Borst. H. L., R. Woodburn, (1942). The Effect of Mulching and Methods<br />
of Cultivation on Runoff and Erosion from Muskegon Silt Loam.<br />
Agric. Engng., St. Joseph, Mich., 3, pp. 19-22.<br />
Brandt, A. E., (1941). The Design of Plot Experiments for Measurement<br />
of Runoff and Erosion. 9. 2, pp. 429-432 and 436.<br />
Brice, J. C., (1966). Erosion and Deposition in the Loess-mantled<br />
Great Plains, Medicine Creek Drainage Basin, Nebraska. Professional<br />
Paper 352-H, United States Geological Survey, Washington, C. D.<br />
Brill, G. D., 0. R. Neal, (19<strong>50</strong>). Seasonal Occurrence of Runoff and<br />
Erosion from a Sandy Soil in Vegetable Production. Agron.<br />
J., 42, pp. 192-5.<br />
Brown, C. B., (19<strong>50</strong>). Sediment Transportation in Engineering<br />
Hydraulics, €I. Rouse (ed.) John Wiley and Sons, Inc., New<br />
York. N.Y., p. 771.<br />
Browning, G. M., C. L. Parish, J. Glass, (1947). A Method for<br />
Determining the Use and Limitations of Rotation and Conservation<br />
Practices in the Control of Soil Erosion in Iowa. J. Am. SOC.<br />
Agron., 3, pp. 65-73.<br />
Brune, G. B., (1948). Proceedings, Federal Interagency Conference on<br />
Sedimentation, Denver, Colo.<br />
Brune, G. B., (1953). Collection of Basin Data on Sedimentation, United<br />
States Department of Agriculture, Soil Conservation Service,<br />
Milwaukee, Wisc.<br />
Cameron, D. G.. (1952). Studies in Soil Conservation at Cowra Research<br />
Station 1941-45. J. Soil Conser. Serv. N.S.W., 5, pp. 158-168.<br />
Campbell, F. B., H. A. Bauder, (1940). A Rating Curve Method for<br />
Determining Silt Discharge of Streams. Transactions, American<br />
Geophysical Union, Part 2, Washington D. C., pp. 603-607.<br />
Carter, C. E., F. E. Dendy, C. W. Doty. (1966). Runoff and Soil<br />
Loss from Pastured Loess Soils in North Mississippi. Mississippi<br />
Water Resources Conference, Jackson, Miss.<br />
Carreker, J. R., (1946). Proper Cropping Practices Strengthen Terraces<br />
on Sloping Ground. Agric. Engng., St. Joseph, Mich., 27, pp. 311-<br />
312, 315.<br />
Carreker, J. R., (1948). Submission to Committee on Agricultural<br />
Hydrology. Am. SOC. Agric. Engr. w. (29), pp. 114-116.<br />
Carreker, J. R., (1954). The Effects of Rainfall, Land Slope and<br />
Cropping Practices on Runoff and Soil Losses. J. Soil Wat. Conserv.,<br />
- 9, pp. 115-119.<br />
44
Carreker, J. R., A. P. Barnett, (1949). Runoff and Soil Loss<br />
Measurements by Cropping Periods. Agric. Engng., St. Joseph,<br />
Mich. 2. pp. 173-176.<br />
Clark, C. O., (1945). Storage and the Unit Hydrograph Transactions<br />
American Society of Civil Engineers,<br />
110, pp. 1419-1488.<br />
Colby, B. R., (1956). Relationship of Sediment Discharge of Strean-<br />
flow. U.S. Geol. Survey Open-file Report, 170 pp.<br />
Colby, B. R., (1963). Fluvial Sediments - A Summary of Source<br />
Transportation, Deposition and Measurement of Sediment Discharge.<br />
U.S. Geol. Survey Bull., 1181-A, 49 pp.<br />
Colby, B. R., and C. H. Hembree, (1955). Computations of Total<br />
Sediment Discharge, Niobrara River near Cody, Nebraska. Water-<br />
Supply Paper 1357, United States Geological Survey, Washington,<br />
D.C.<br />
Collier, C. R., (1963). Sediment Characteristics of Small Streams in<br />
Southern Wisconsin, 1954-1959. Water-Supply Paper 1669-B,<br />
United States Geological Survey, Washington, D. C.<br />
Collier, C. R., et&., (1964) Influences of Strip Mining on the<br />
Hydrologic Environment of Beaver Creek Basin, Kentucky, 1955-<br />
1959. Professional Paper 427-B. United States Geological<br />
Survey, Washington, D. C.<br />
Collier, C. R., personal communication, (1969).<br />
Collier, C. R., et&., (1971). Influence of Strip Mining on the<br />
Hydrologic Environment of Parts of Beaver Creek Basin, Kentucky,<br />
1955-1966. Professional Paper 427-C. United States Geological<br />
Survey, Washington, D. C.<br />
Conrey, G. W., E. M. Burrage, (1937). Soil Losses of Fertility<br />
Experiment Plots. Soil Sci. SOC. Am. Proc., 2, pp. 547-554.<br />
Costin, A. B., D. J. Wimbush, D. Kerr, (1960). Studies in Catchment<br />
Hydrology in the Australian Alps. I1 - Surface Runoff and Soil<br />
Loss. CSIRO Div. Pl., Ind. Tech. Pap. No. 14.<br />
Cox, R. K., (19<strong>50</strong>). The Land Use Factor in Wheat Land Erosion<br />
Control. J. Soil Conserv. Serv. NSW, 6, pp. 159-170.<br />
Crawford, N. H. and R. K. Linsley, (1966) Digital Simulation in<br />
Hydrology: Stanford Watershed Model IV. Tech. Rept., No. 39,<br />
Department of Civil Engineering, Stanford University, California.<br />
Curtis, W. F., J. K. Culbertson, E. B. Chase, (1973). Fluvial Sediment<br />
Discharge to the Oceans from the Conterminous United States.<br />
U.S. Geol. Survey Circular 67, 17 pp.<br />
Davis, W. W., (1937). A New Method for Measurement of Erosion from<br />
Experimental Plots. Soil Sci. SOC. Am. Proc., 2, pp. 579-583.<br />
45
Dawdy, D. R., (1967) Knowledge of Sedimentation in Urban EnVirOmentS.<br />
Journal of the Hydraulics Division, ASCE, 2, (HY6), Proc. Paper<br />
5595, pp. 235-254.<br />
Dawdy. D. R., R. W. Litchy, and J. M. Bergmann, (1972). A Rainfall-<br />
Runoff Simulation Model for Estimation of Flood Peaks for Small<br />
Drainage Basins. U.S. Geological Survey Professional Paper <strong>50</strong>6-B.<br />
Dickinson, R. E., (1929). The Results and Significance of the Spur<br />
(Texas) Runoff and Erosion Experiments. J. Am. SOC. Agron.,<br />
- 21, pp. 415-422.<br />
Dickinson. W. T., A. Scott, and G. Wall, (1975). Fluvial Sediment-<br />
ation in Southern Ontario. Can. Jour. Earth Sciences, 12,<br />
(ll), pp. 1813-1819.<br />
Dickinson, W. T., and G. J. Wall, (1976). Temporal Pattern of Erosion<br />
and Fluvial Sedimentation in the Great Lakes Basin. Geoscience<br />
Canada, 3, pp. 158-163.<br />
Dils, R. E., Soil Loss Plots. Personal Communication.<br />
Diseker, E. G., and J. T. McGinnis, (1967). Evaluation of Climatic<br />
Slope and Site Factors on Erosion from Unprotected Roadbanks.<br />
Transactions, American Society of AgKiCUltUKd Engineering,<br />
- 10, pp. 9-14.<br />
Dodge, A. F., (1935). Measurement of Runoff as Influenced by Plant<br />
Cover Density, Symposia Commemorating Six Decades of the Modern<br />
Era in Botanical Science, (Iowa State College), 1. pp. 185-193.<br />
Dortignac, E. J., W. C. Hickey, (1963). Surface Runoff and Erosion<br />
as Affected by Soil Ripping. Proc. Fed. Interagency Sediment<br />
Conf. 1963. U.S. Dept. Agric. Misc. Publ. 970, pp. 156-165.<br />
Dragoun, F. J., (1962). Rainfall Energy as Related to Sediment Yield.<br />
Journal of Geophysical Research, American Geophysical Union,<br />
- 67, (4). Washington, D. C.<br />
Duley, F. L. and F. G. Ackerman, (1934). Runoff and Erosion from<br />
Plots of Different Lengths. Journal of Agricultural Research,<br />
- 48, (6).<br />
Duley, F. L., (1939). Surface Factors Affecting the Rate of Intake of<br />
Water by Soils. Soil Sci. SOC. Am. PKOC., fi, pp, 60-64.<br />
Duley, F. L., (1952). Relationship Between Surface Cover and Water<br />
Penetration, Runoff, and Soil Losses. Proc. Int. Grassld.<br />
Cong., 5, pp. 942-946.<br />
Duley, F. L., (1956). The Effect of Synthetic Soil Conditions (HPAN)<br />
on Intake Runoff and Erosion. Soil Sci. SOC. Am. PKOC., 0,<br />
pp. 420-421.<br />
Duley, F. L., F. G. Ackerman, (1934). Runoff and Erosion from Plots<br />
of Different Lengths. Agric. Res. Wash., 48, pp. <strong>50</strong>5-510.<br />
46
Duley, F. L., 0. E. Hays, (1932). The Effect of the Degree of<br />
Slope on Runoff and Soil Erosion. a. 65, pp. 349-360.<br />
Dunford, E. G., (1954). Surface Runoff and Erosion from Pine Grass-<br />
lands of the Colorado Front Range. J. For., 52, pp. 923-927.<br />
Einstein, H. A., (19<strong>50</strong>). The Bed Load Function for Sediment<br />
Transportation in Open Channel Flows. Technical Bulletin<br />
1026, United States Department of Agriculture, Soil<br />
Conservation Service.<br />
Feagon, W. T., J. Tedmond, (1955). Planning of Hydrology Experiments<br />
of Forested Catchments in Australia. Aust. N. 2. Ass. Adv.<br />
Sci., Sec. K., Melbourne.<br />
Fleming, G., (1968). The Stanford Sediment Model I: Translation.<br />
Bulletin <strong>International</strong> Association of Scientific Hydrology,<br />
XI1 e, Annee 2, pp. 108-125.<br />
Fleming, G., (1969). Design Curves for Suspended Load Estimation,<br />
Proceedings, Institution of Civil Engineers, Paper 7185, No.<br />
43, London, England, pp. 1-9.<br />
Fleming, G. (1972). Sediment Erosion-Transport-Deposition Simulation:<br />
State-of-the-art. Paper Presneted at the Sediment Yield Workshop,<br />
U.S.D.A. Sed. Lab., Oxford, Mississippi, November 28-30.<br />
Flint, R. F., (1972). Fluvial Sediment in Salem Fork Watershed West<br />
Virginia, U.S. Geol. Survey Water Supply Paper 1798-K.<br />
Forest Service, Fort Collins, Col., (1965). Notes on Sedimentation<br />
Activities, United States Bureau of Reclamation.<br />
Foster, G. R., and L. D. Meyer, (1975). Mathematical Simulation<br />
of Upland Erosion Using Fundamental Erosion Mechanics.<br />
Publication ARS-S-40, United States Department of Agriculture,<br />
Agricultural Research Service.<br />
Foster, G. R., and W. H. Wischmeier, (1973). Evaluating Irregular<br />
Slopes for Soil Loss Prediction. Paper 73-227, Presented at Meeting<br />
of American Society of Agricultural Engineers, Lexington, Kentucky.<br />
Free, G. R., (1960). Research Tells How Much Soil We Can Afford to<br />
Lose, Crops and Soils, 2, p. 21.<br />
Frere, M. H., (1973). "Adsorption - Transport of Agricultural Chemicals<br />
in Watersheds", Transactions of the ASAE,<br />
16, pp. 569-577.<br />
Garcia, G., W. C. Hickey, E. J. Dortignac, (1963). An Inexpensive<br />
Runoff Plots. U.S. For. Serv. Res. Note R.M. 12.<br />
Garde, L. E., C. A. Van Doren, (1949). Soil Losses as Affected by<br />
Cover Rainfall and Slope. Soil Sci. SOC. Am. Proc., 16, pp. 374-8.<br />
47
Geiger, A. F., and J. N. Holeman, (1959). Sedimentation of Lake<br />
Barcroft, Fairfax County, Va. SCS-TP-136, United States<br />
Department of Agriculture, Soil Conservation Service.<br />
George, J. R., (1963). Sedimentation in the Stony Brook Creek Basin,<br />
New Jersey, U.S. Geol. Survey Open File Report, 71 pp.<br />
Gilmore, D. A., (1965). Hydrological Investigations of Soil and<br />
Vegetation Types in the Lower Cotter Catchment. M. Ag. Sci.<br />
Thesis., Aust. Nat. Univ., Canberra.<br />
Glymph, L. M., (1951). Relation of Sedimentation to Accelerated Erosion<br />
in the Missouri River Basin. Technical Paper 102, United States<br />
Department of Agriculture, Soil Conservation Service.<br />
Glymph, L. M. (1954). Studies of Sediment Yields from Watersheds. Publication<br />
No. 36 de L'Association <strong>International</strong> D'Hydrologie,<br />
<strong>International</strong> Union of Geodesy and Geophysics, pp. 178-191.<br />
Gottschalk, L. C., and G. M. Brune, (19<strong>50</strong>). Sediment Design Criteria<br />
for the Missouri Basin Loess Hills. SCS-TP-97, United States<br />
Department of Agriculture, Soil Conservation Service.<br />
Gray, D. M.. (1968). Snow Hydrology of the Prairie Environment.<br />
Proceedings of the Workshop Seminar, Sponsored by the Canadian<br />
National Committee for the <strong>International</strong> Hydrologic Decade<br />
and the Univ. of N.B., Feb. 28 and 29.<br />
Grissinger, E. H., (1966). Resistance of Selected Clay Systems to<br />
Erosion by Water. Water Resources Research, 2, (1).<br />
Guy, H. P., (1964). An Anlysis of Some Storm-Period Variables Affecting<br />
Stream Sediment Transport. Professional Paper 462-E, United<br />
States Geological Survey, Washington, D. C.<br />
Guy, H. P., (1965). Residential Construction and Sedimentation at<br />
Kensington, Maryland, Proceedings of 1963 Federal Interagency<br />
Conference on Sedimentation, United States Dept. of Agriculture,<br />
Miscellaneous Publication 970.<br />
Guy, H. P., (1969). Laboratory Theory and Methods for Sediment Analysis:<br />
Techniques of Water Resources Investigations of the U.S. Geol. Survey,<br />
Book 5, Chapter C1.<br />
Guy, H. P., (1970). Fluvial Sediment Concepts: Techniques of Water<br />
Resources Investigations of the U.S. Geol. Survey, Book 3, Chapter<br />
Cl.<br />
Guy, H. P., (1970). Sediment Problems in Urban Areas: U.S. Geol. Survey<br />
Circular 601-E.<br />
Guy, H. P., and G. E. Ferguson, (1962). Sediment in Small Reservoirs<br />
due to Urbanization. Journal of the Hydraulics Division, ASCE,<br />
- 86, HY2, Proc. Paper 3070., pp. 27-37.<br />
40
Guy, G. P., and G. E. Ferguson, (1970). Stream Sediment: an Environment<br />
Problem: Jour. of Soil and Water Conservation, Soil<br />
Conservation Society of America, 5, (6), pp. 217-221.<br />
Guy, H. P., and W. Norman, (1970). Field Methods for Measurement of<br />
Fluvial Sediment: Techniques of Water Resources Investigation<br />
of the U.S. Geol. Survey, Book 3, Chapter C2.<br />
Happ, S. C., (1944). Effect of Sedimentation on Floods in the Kickapoo<br />
Valley, Wisconsin. Journal of Geology, G, (1).<br />
Happ, S. C., (1968). Valley Sedimentation in North-Central Mississippi.<br />
Proceedings of Third Mississippi Water Resources Conference,<br />
April 9-10.<br />
Happ, S. C., G. Rittenhouse, and G. C. Dobson, (1940). Some Principles<br />
of Accelerated Stream and Valley Sedimentation. Technical<br />
Bulletin 695, United States Dept. of Agriculture (original<br />
source, Rittenhouse, G ., and Holt, R. D. unpublished; copies<br />
in SCS and ARS libraries).<br />
Harley, B. M., F. E. Perkins, and P. S. Eagleson, (1970). A Modular<br />
Distributed Model of Catchment Dynamics. MIT, Dept. of Civil<br />
Engineering, Hydrodynamics Lab. Report No. 133.<br />
Hayward, J. A., (1971). A Review of Soil Loss Plot Studies, Unpublished<br />
paper obtained from author. Tussock Grasslands and Moutain<br />
Lands Institute, New Zealand.<br />
Heidel, S. S., (1956). The Progressive Lag of Sediment Concentration<br />
with Flood Waves. American Geophysical Union Transactions 37,<br />
(I), P. 56.<br />
Heineman, H. G. and R. F. Piest, (1975). Soil Erosion-Sediment Field<br />
Research in Progress. EOS-Trans AGU, 56, (3), pp.<br />
149-157.<br />
Henderson, F. M., (1966). Open Channel Flow. Macmillan, New York.<br />
Hill, A. L., (1965). Rates of Sediment Production in the Kansas River<br />
District. Report of Kansas City District, United States Corps<br />
of Engineers.<br />
Hickey, W. C., E. J. Dortignac, (undated). An Evaluation of Soil<br />
Ripping and Soil Pitting on Runoff and Erosion in the Semi<br />
Arid South West. Int. Assn. Scient. Hydrol., Publ. 65, pp.<br />
22-23.<br />
Holeman, J. N., (1968). The Sediment Yield of Major Rivers of the<br />
World. Water Resources Research, 5, (4), pp. 737-747.<br />
Holtan, H. N., N. C. Lopez, (1971). USDAHL-70 Model of Watershed<br />
Hydrology. USDA, Agricultural Research Service, Tech. Bulletin<br />
1435.<br />
49
Horton, R. E., (1938). An Interpretation and Application of Runoff<br />
Plot Experiments with Reference to Soil Erosion Problems.<br />
Soil Sci. SOC. Am. Proc., 2, pp. 340-349.<br />
Horner, G. M., (1960). Effect of Cropping Systems on Runoff, Erosion<br />
and Wheat Yields. Agron. J., 52, pp. 342-344.<br />
Homer, G. M., A. G. McCall, F. G. Bell, (1944). Investigations of<br />
Erosion Control and Reclamation of Eroded Land at the Palouse<br />
Conservation Experiment Station, Pullman, Wash. 1931-42. U.S.<br />
Dept. Agric. Tech. Bull. 860.<br />
Interagency Task Force, (1967). Upper Mississippi River Comprehensive<br />
Basin Study. Fluvial Sediment, Appendix G, United States Corps<br />
of Engineers.<br />
Ireland, H. A., C. F. Sharp, D. H. Eargle, (1939). Principles of<br />
Gully Erosion in the Piedmont of South Carolina. Technical<br />
Bulletin 633, United States Dept. of Agriculture.<br />
James, L. D., (1970). An Evaluation of Relationships Between Streamflow<br />
Patterns and Watershed Characteristics Through the Use Opset: of<br />
A Self-calibrating Version of the Stanford Watershed Model. Water<br />
Resources Institute Research Report 36, University of Kentucky,<br />
Lexington.<br />
Jansen, J. M. L., R. B. Pamter. (1974). Predicting Sediment Yield<br />
from Climate and Topography. Journ. of Am. SOC. of Civil Engr.<br />
Jour. of Hydrology, g, pp. 371-380.<br />
Jones, B. L., (1966). Effects of Agricultural Conservation Practices<br />
on the Hydrology of Corey Creek Basin, Pennsylvania, 1954-1960.<br />
Water-Supply Paper 1532-C, United States Geological Survey,<br />
Washington, D. C.<br />
Jones, H. R., (1961). Runoff and Soil Loss Studies at Wagga Research<br />
Station. J. Soil Conserv. Serv. NSW, 17 pp. 156-169.<br />
Kennedy, A. L., (1941). Equipment for Runoff Measurement. Agric.<br />
Engng, St. Joseph, Mich., 22, pp. 218 and 220.<br />
Ketcheson, J. W., W. T. Dickinson, P. S. Chisholm, (1973).<br />
Potential Contributions of Sediment from Agricultural Land.<br />
Proc. 9th Canadian Hydrology Symposium, Edmonton.<br />
Kilinc, M. Y., (1972). Mechanics of Soil Erosion from Overland Flow<br />
Generated by Simulated Rainfall. Dissertation, Submitted to<br />
the Dept. of Civil Engineering, Colorado State University,<br />
Fort Collins, Colorado.<br />
Kittredge, J., (1954). Influences of Pine and Grass on Surface Runoff<br />
and Erosion. J. Soil Wat. Conserv., 9, pp. 179-185, 193.<br />
Kling, G. F., G. W. Olson, (1974). "Sediment Transport Computer<br />
Model" Cornel1 Agronomy Mimeo, pp. 74-11.<br />
<strong>50</strong>
Krusekpof, H. H., (1943). The Effect of Slope on Soil Erosion.<br />
Missouri Agricultural Experiment Station Research Bulletin<br />
363.<br />
Krusekpof, H. H., (1958). Soils of Missouri--Genesis of Great Soil Groups.<br />
Soil Science, 3, pp. 19-27.<br />
Kulp, J. L., (1961). Geologic Time Scale. Soil Science, 133<br />
(3459), pp. 1105-1114.<br />
Kunkle, S. H. and G. H. Comer, (1972). Suspended, Bed, and Dissolved<br />
Sediment Loads in the Sleepers River, Vermont. ARS 41-188,<br />
United States Dept. of Agriculture, Agricultural Research<br />
Service.<br />
Lamb, J., G. R. Free, H. H. Wilson, (1944). The Seasonal Occurrence<br />
of Soil Erosion in New York as Related to Rainfall Intensities.<br />
J. Am. SOC. Agron., 36, pp. 37-45.<br />
Lamy, D. L., (1949). Some Effects of Summer Storms at Wagga Soil<br />
Conservation Research Station, 1947-48. J. Soil Conserv. Serv.<br />
NSE, 5, pp. 39-43.<br />
Lane, E. W., (1955). Design of Stable Channels. Transactions, ASCE,<br />
120, Paper No. 2776, pp. 1234-1260.<br />
Lane, E. W., (1955). The Importance of Fluvial Morphology in Hydraulic<br />
Engineering. Proceedings, ASCE, a, Paper No. 745.<br />
Lane, E. W., W. M. Borland, (1951). Estimating Bed Load. Transactions,<br />
American Geophysical Union, Washington, D. C.<br />
Langbein, W. B., S. A. Schumm, (1958). Yield of Sediment in<br />
Relation to Mean Annual Precipitation. Transactions, American<br />
Geophysical Union, 2, Washington, D. C., pp. 1076-1084.<br />
Leaf, C. F., (1970). Sediment Yield from Central Colorado Snow Zone.<br />
ASCE Jour. of Hydraulic Div., 95, pp. 87-93.<br />
Leopold, L. B., (1968). Hydrology for Urban Planning--a Guidebook<br />
on the Hydrological Effects of Urban Land Use. U.S. Geological<br />
Survey, Circular 554.<br />
Leopold, L. B., W. W. Emmett, R. M. Myrick, (1966). Channel and<br />
Hillslope Processes in a Semiarid area in New Mexico. Professional<br />
Paper 3524, United States Geological Survey, Washington, D. C.<br />
Leopold, L. B., T. Maddock, (1953). The Hydraulic Geometry of<br />
Stream Channels and Some Physiographic Implications. Professional<br />
Paper 252, United States Geological Survey, Washington, D. C.<br />
Leopold, L. B., M. G. Wolman, J. P. Miller, (1964). Fluvial Processes<br />
in Geomorphology, W. H. Freeman and Co., San Francisco, Calif.<br />
328 pp.<br />
51
Li, R. M., (1972). Sheet Flow Under Simulated Rainfall. Thesis<br />
Submitted to the Dept. of Civil Engineering, Colorado State<br />
University, Fort Collins, Colorado.<br />
Li, R. M., H. W. Shen, (1973). Effect of Tall Vegetation on Flow<br />
and Sediment. Journal of Hydraulics Division, American Society<br />
of Civil Engineers, 9, (HY5). Proc. Paper 9748, pp.<br />
793-814.<br />
Li. R. M., D. B. Simons, (1973). Discussion of "Sediment Yield<br />
Computed with Universal Equation" by J. R. Williams and H. D.<br />
Berndt. Journal of the Hydraulics Division, American Society<br />
of Civil Engineers, 99, (HYll), Proc. Paper 10109,<br />
pp. 2145-2147.<br />
Li, R. M., D. B. Simons, M. A. Stevens, (1975). Nonlinear Kinematic<br />
Wave Approximation for Water Routing. Water Resources Research,<br />
- 11, (2). pp. 245-252.<br />
Lillard, J. H., (1941). Effects of Crops and Slopes on Rates of Runoff<br />
and Total Soil Loss. Agric. Engng., St. Joseph, Hich., 22,<br />
pp. 396-398 and 406.<br />
Logan, J. M., (1960). Runoff and Soil Loss Studies at Wellington<br />
Research Station, Part I - Runoff. J. Soil Conserv. Serv. NSE,<br />
3, pp. 95-111.<br />
Logan, J. M., (1960). Runoff and Soil Loss Studies at Wellington Research<br />
Station, Part I1 - Soil Loss. u., 16, pp. 214-227.<br />
Longley, A. J., E. J. Bondy, (1963). Reducing Soil Loss in Kansas.<br />
Jour. Soil Wat. Conserv., e, pp. 160-161.<br />
Love, S. K., (1936). Suspended Matter in Several Small Streams. Trans-<br />
actions, 17th Annual Meeting, American Geophysical Union,<br />
Washington, D. C.<br />
Love, L. D., B. C. Goodell, (1960). Watershed Research on the Frazer<br />
Experimental Forest. J. For., 58, pp. 272-275.<br />
Lowdermilk, W. C., (1931). Studies of the Role of Forest Vegetation<br />
in Surficial Runoff and Soil Erosion. Agric. Engng, St. Joseph,<br />
Mich., 12, pp. 107-112.<br />
Lowdermilk, W. C., (1935). Certain Aspects of the Role of Vegetation<br />
in Erosion Control. Symposia Commemorating Six Decades of the<br />
Modern Era in Botanical Science (Iowa State College), 1, pp. 123-132.<br />
Lowdermilk, W. C., H. L. Sundling, (19<strong>50</strong>). Erosion Pavement to<br />
Formation and Significance. Trans. Am. Geophys. Un., 31,<br />
pp. 96-100.<br />
Lull, H. W., K. G. Reinhart, (1963). Logging and Erosion on Rough<br />
Terrain in the East. Proceedings of 1963 Federal Interagency<br />
Conference on Sedimentation, United States Dept. of Agriculture,<br />
Miscellaneous Publication 970, pp. 43-47.<br />
52
Lsuby, G. C., (1963). Causes of Variation in Runoff and Sediment Yield<br />
from Small Drainage Basins in Western Colorado. Miscellaneous<br />
Publication 970, U.S. Dept. of Agriculture.<br />
Lsuby, G. C., (1970). Hydrologic and Biota Effects of Grazing Versus Non-<br />
Grazing Near Grand Junction, Colorado. Professional Paper<br />
700-B, United States Geological Survey., Washington, D. C., pp.<br />
232-236.<br />
Lustig, L. K., (1965). Sediment Yield of the Castaic Watershed,<br />
Western Los Angeles County, California - A quantitative Geomorphic<br />
Approach. Professional Paper 422-F, U.S. Geological Survey,<br />
Washington, D. C.<br />
Mallory, C. W., J. J. Boland, (1970). A System Study of Storm<br />
Runoff Problems in a New Town. Water Resources Bull., 6,<br />
(6), pp. 980-89.<br />
Maner, S. B., (1958). Factors Affecting Sediment Delivery Rates in the<br />
Red Hills Physiographic Area, American Geophysical Union Trans-<br />
action, 3, (4). pp. 669-674.<br />
Maner, S. B., L. H. Barnes, (1953). Suggested Criteria for<br />
Estimating Gross Sheet Erosion and Sediment Delivery Rates<br />
for the Blackland Prairies Problem Area in Soil Conservation.<br />
U.S. Dept. of Agriculture, Soil Conservation Service<br />
Publication.<br />
Mannering, J. V., L. D. Meyer, (1961). The Effects of Different Methods<br />
of Corn Stalk Residue Management on Runoff and Erosion as Evaluated<br />
by Simulated Rainfall. Soil Sci. SOC. Am. Proc., 5, pp. <strong>50</strong>6-510.<br />
Mannering, J. V., (1963). The Effects of Various Rates of Surface Mulch on<br />
Infiltration and Erosion. Ibid. 27, pp. 84-86.<br />
Mansue, L. J., A. B. Commings, (1974). Sediment Transport by Streams<br />
Draining into the Delaware Estuary. U.S. Geol. Survey Water Supply<br />
Paper 1532-H.<br />
Marston, R. B., (1952). Ground Cover Requirements for Summer Storm Runoff<br />
Control on Aspen Sites in Northern Utah. J. For., x, pp. 303-7.<br />
McCully, W. G., W. J. Bowmer, (1969). Erosion Control on Roadsides<br />
in Texas. Research Report 67-8F, Texas Agricultural and Mechanical<br />
University, College Station, Tex.<br />
McKay, G. E., (1968). Problems of Measuring and Evaluating Snowcover;<br />
Proceedings of the Workshop Seminar. Canadian Nat. Comm. for<br />
Internat. Hydr. Decade and Univ. of N.B., Feb. 28-29.<br />
Menard, H. W., (1961). Some Rates of a Regional Erosion. Journal of<br />
Geology, 69, pp. 154-161.<br />
Meyer, L. D.. (1965). Mathematical Relationships Governing Soil<br />
Erosion by Water. Journal of Soil and Water Conservation,<br />
- 7-0, (4).<br />
53
Meyer, L. D. (1971). Soil Erosion by Water on Upland Areas. Chapter 27,<br />
Vol. 11, River Mechanics edited by H. W. Shen, Colorado State<br />
University, Fort Collins, Colorado.<br />
Meyer, L. D., G. R. Foster, M. J. M. Romkens, (1975). Sources of<br />
Soil Eroded by Water from Upland Slopes. Publication ARS-S-40,<br />
U.S. Dept. of Agriculture, Agricultural Research Service.<br />
Meyer, L. D., E. J. Monke, (1965). Mechanics of Soil Erosion by<br />
Rainfall and Overland Flow. Transactions, American Society of<br />
Agricultural Engineers, A, (4), St. Joseph, Michigan<br />
Meyer, L. D., L. A. Kramer, (1968). Relation Between Land-Slope<br />
Shape and Soil Erosion. Paper Presented at the 1968 Winter Meeting<br />
of ASAE, Chicago, Illinois.<br />
Meyer, L. D., E. J. Monke, W. H. Wischmeier, (1969). Mathematical<br />
Simulation of the Process of Soil Erosion by Water. Transactions,<br />
American Society of Agricultural Engineers, 12, St. Joseph,<br />
Michigan.<br />
Meyer, L. D., J. V. Mannering, (1961). Minimum Tillage for Corn: Its<br />
Effect on Infiltration and Erosion. Agric. Engng., St. Joseph,<br />
Michigan, 42, pp. 72-75, 86, 87.<br />
Miller, C. R., (1951). Analysis of Flow-Duration, Sediment-Rating Curve<br />
Method of Computing Sediment Yield. United States Bureau of<br />
Reclamation, Hydraulics Bureau.<br />
Miller, C. R., R. Woodburn, H. R. Turner, (1962). Upland Gully<br />
Sediment Production. <strong>International</strong> Association of Scientific<br />
Hydrology Publication No. 59.<br />
Moore, W. R., C. E. Smith, (1968). Erosion Control in Relation<br />
to Watershed Management. Am. SOC. of Civil Engineers, Proc.<br />
- 94, (IR3), pp. 321-331.<br />
Moldenhauer, W. C., W. H. Wischmeier, (1960). Soil and Water Losses<br />
and Infiltration Rates on Ida Silt Loam as Influenced by Cropping<br />
Systems, Tillage Practices and Rainfall Characteristics. Soil<br />
Sci. SOC. Am. Proc., 2, pp. 409-413.<br />
Mundroff, J. C., (1964). Fluvial Sediment in Kiowa Creek Basin, Colorado,<br />
U.S. Geol. Survey Water Supply Paper 1798-A.<br />
Murota, A., M. Hashino, (1969). Studies of a Stochastic Rainfall<br />
Model and its Application to Sediment Transportation. Tech,<br />
Rept., Osaka University, 9, pp. 231-247.<br />
Musgrave, G . W., (1947). The Quantitative Evaluation of Factors in Water<br />
Erosion, a First Approximation. Journal of Soil and Water<br />
Conservation, 2, pp. 133-138.<br />
Mutchler, C. K., (1963). Runoff Plot Design and Installation for<br />
Soil Erosion Studies. Agricultural Research Service Report<br />
NO. 41-79, U.S. Dept. of Agriculture.<br />
54
Neale, 0. R., (1937). The Effect of the Degree of Slope and Rainfall<br />
Characteristics on Runoff and Soil Erosion. Soil Sci. SOC.<br />
Am. Proc., 2, pp. 525-32.<br />
Neale, 0. R., (1939). Some Concurrent and Residual Effects of Organic<br />
Matter Additions on Surface Runoff. g. 5, pp. 420-25.<br />
Neal, J. H., (1945). Soil and Water Losses as Affected by Rainfall<br />
Characteristics. Agric. Engng., St. Joseph, Michigan, 3,<br />
pp. 463-464.<br />
Neal, 0. R., G. D. Brill, (1951). Conservation Effects of Crop<br />
Rotation on a Sandy Soil in Vegetable Production. J. Soil<br />
Wat. Conserv., 5, pp. 187-190 and 199.<br />
Neff, E. L., (1967). Discharge Frequency Compared to Long-Term Sediment<br />
Yields. Symposium on River Morphology, <strong>International</strong> Union of<br />
Geodesy and Geophysics, pp. 236-242.<br />
Negev, M., (1967). A Sediment Model on a Digital Computer. Technical<br />
Report 76, Stanford University, Stanford, California.<br />
Newman, J. E., (1970). Climate in the 1970's. Crops and Soils,<br />
-<br />
22, pp. 9-12.<br />
O'Loughlin, C. L. Private communication.<br />
Olson, T. C., W. H. Wischmeier, (1963). Soil Erodibility Evaluations<br />
for Soils on the Runoff and Erosion Stations. Soil Sci. Soc.<br />
Am. Proc., 27, pp. 590-592.<br />
Onstad, C. A., G. R. Foster, (1974). Erosion and Deposition<br />
Modeling on a Watershed. Scientific Journal Series 8537,<br />
Minnesota Agricultural Experiment Station.<br />
Packer, P. E., (1951). An Approach to Watershed Protection Criteria.<br />
J. For., 9. pp. 639-644.<br />
Parsons, D. A.. (1954). Coshocton-Type Runoff Samplers, Laboratory<br />
Investigations. SCS-TP-124. Soil Conservation Service, U.S.<br />
Dept. of Agriculture, Washington, D.C.<br />
Patric, J. H., (1959). Increasing Water Yield in Southern California<br />
Mountains. Jour. Am. Water Works Assoc., 51, pp. 474-480.<br />
Peel, T. C., (1937). The Relation of Certain Physical Characteristics<br />
to the Erodibility of Soils. Soil Sci. Soc. Am. Proc., 11,<br />
pp. 97-100.<br />
Peter, J. C., (1967). Effects on a Trout Stream of Sediment from<br />
Agricultural Practices. Journ. of Wildlife Management,<br />
- 31, (4).<br />
55
Piest, R. F., (1963). Long Term Sediment Yields from Small Watershed.<br />
<strong>International</strong> Association of Sci. Hydrology, General Assembly<br />
of Berkley, (1963), 11 (2) - Transport of sediment pp. 121-140.<br />
Piest, R. F., (1965). The Role of the Large Storm as a Sediment Contributor<br />
PKOC. of 1963 Federal Interagency Conference on Sedimentation,<br />
Miscellaneous Pub. 970. U.S. Dept. of Agriculture, pp. 97-108.<br />
Piest, R. F., H. G. Heineman, (1965). Experimental Watersheds<br />
Contribute Useful Sedimentation Facts. Internat. Assoc. of<br />
Sci. Hydr., 1. (2), p. 372.<br />
Piest, R. F., R. G. Spomer, (1968). Sheet and Gully Erosion in<br />
the Missouri Valley Loessial Region. Transactions, American<br />
Society of Agricultural Engineers, 11, St. Joseph,<br />
Michigan, pp. 8<strong>50</strong>-853.<br />
Piest, R. F., J. M. Bradford, R. G. Spomer, (1975). Mechanisms of<br />
Erosion and Sediment Movement from Gullies. Publication ARS-<br />
S-40, Agricultural Research Service, U.S. Dept. of Agriculture.<br />
Piest, R. F., M. Bradford, G. M. Wyatt, (1975). Soil Erosion and<br />
Sediment Transport. ASCE Hydraulic Div., p. 65.<br />
Portfield. G., (1972). Computation of Fluvial Sediment Discharge<br />
Techniques of Water Resources Investigations of U.S. Geol. Survey,<br />
Book 3, Chapter C3.<br />
Rainwater, F. E., (1962). United States Geological Survey Hydrologic<br />
Investigations Atlas. HA-61.<br />
Rantz, S. E., (1964). Snowmelt Hydrology of a Sierra Nevada Stream.<br />
U.S. Geol. Survey Paper 1779-R.<br />
Reed, L. A., (1971). Hydrology and Sedimentation of Corey Creek and<br />
Elk Run Basins, North Central Pennsylvania, U.S. Geol. Survey<br />
Water Supply Paper 1532-E.<br />
Reed, L. A., (1976). Sediment Characteristics of Five Streams Near<br />
Harrisburg, Pennsylvania Before Highway Construction. USGS<br />
Water Supply Paper 1798”.<br />
Renard, K. G., (1969). Sediment Rating in Ephemeral Streams.<br />
ASAE Transaction, p. 80-85.<br />
Renard, K. G., L. J. Lane, (1972). Sediment Yield as Related to a<br />
Stochastic Model of Ephermeral Runoff. Paper Presented at the<br />
Sediment Yield Workshop, USDA Sed. Lab., Oxford, Mississippi.<br />
Rendon-Herrero, O., (1974). Estimation of Washload Produced on Certain<br />
Small Watersheds. Journal of the Hydraulics Div., American<br />
Society of Civil Engineers, 100, (HY7), PKOC. Paper 10638,<br />
pp. 835-848.<br />
56
Renfro, G. W., (1972). Use of Erosion Equations and Sediment Delivery<br />
Ratios for Predicting Sediment Yield, Paper Presented at the<br />
Sediment Yield Workshop, USDA Sed. Lab., Oxford, Mississippi.<br />
Renfro, G. W., J. W. Adair, (1968). Engineering and Watershed<br />
Planning Unit Technical Guide No. 12. Soil Conservation Service,<br />
U.S. Dept. of Agriculture, Fort Worth, Texas.<br />
Renfro, G. W., (1972). Use of Erosion Equations and Sediment Delivery<br />
Ratios for Predicting Sediment Yield. USDA Sed. Lab. Oxford,<br />
Mississippi.<br />
Rice, R. M., G. T. Foggin, (1971). Effect of High Intensity Storms<br />
on Soil Slippage on Mountanous Watersheds in South California.<br />
Water Resources Research, 1, (6).<br />
Ritter, J. R., (1974). The Effects of Hurricane Agnes Flood on Channel<br />
Geometry and Sediment Discharge of Selected Streams in Susquehanna<br />
River Basin, Pennsylvania. U.S. Geol. Survey Jour. of Research<br />
-<br />
2, (6), pp. 753-761.<br />
Rockwood, D. M., (1964). Program Description and Operating Instructions,<br />
Streamtlow Synthesis and Reservoir Regulation. Tech. Bulletin No.<br />
22, U.S. Army Engineering Uivision, North Pacific, Portland, Oregon.<br />
Rodriguez-Iturbe, I., C. F. Nordin, (1968). Time Series Analysis<br />
of Water and Sediment Discharges. IASH, 13, (2),<br />
pp. 69-86.<br />
Roehl, J. W., (1962). Sediment Source Areas, Delivery Ratios, and<br />
Influencing Morphological Factors. Publication 58, IASH, pp.<br />
202-203.<br />
Rogers, J. S., A. P. Barnett, C. Cobb, (1964). An Evaluation of Factors<br />
Affecting Runoff and Soil Loss from Simulated Rainfall. Trans.<br />
Am. SOC. Agric. Engr., I, pp. 457-459.<br />
Rosenbrock, H. H., (1960). An Automatic Method for Finding the Greatest<br />
or Least Value of a Function. The Computer Journal, 3,<br />
pp. 175-184.<br />
Rowlison, D. L., 6. L. Martin, (1971). Rational Model Describing<br />
Slope Erosion. Journal of the Irrigation and Drainage Division,<br />
ASCE, 97, (Irl), Proc. Paper 7981, pp. 39-<strong>50</strong>.<br />
Ruhe, R. V., R. B. Daniels, (1965). Landscape Erosion - Geologic<br />
and Historic. Journal of Soil and Water Conservation.<br />
Ruhe, R. V,, R. Meyer, W. H. Scholtes, (1957). Late Pleistocene<br />
Radiocarbon Chronology in Iowa. American Journal of Science,<br />
I 225, pp. 671-689.<br />
57
Sayre. W. W., H. P. Guy, A. R. Chamberlain, (1963). Uptake and<br />
Transport of Radionuclides by Stream Sediments. U.S. Geol.<br />
Survey Professional Paper 433-A.<br />
Scheidegger, A. E., (1961). Theoretical Geomorphology, Prentice-<br />
Hall, Inc., Englewood Cliffs, N.J.<br />
Schumm, S. A., (1956). The Role of Creep and Rainwash on the Retreat<br />
of Badland slopes. American Journal of Science, 254,<br />
pp. 693-706.<br />
Schumm, S. A., (1965). Quanternary Paleohydrology. The Quanternary<br />
of the United States.<br />
Searcy, J. K., (1959). Flow Duration Curves. Water Supply Paper<br />
1542-A, U.S. Geological Survey, Washington,<br />
D. C.<br />
Seginer, I., (1966). Gully Development and Sediment Yield. Research<br />
Report No. 13, The Israel Ministry of Agriculture, Soil Conser-<br />
vation Division.<br />
Shen, H. W., R. M. Li. (1973). Rainfall Effect on Sheet Flow Over<br />
Smooth Surface. Journal of the Hydraulics Division, American<br />
Society of Civil Engineers, E.. (HY5). Proc. Paper 9733.<br />
pp. 771-792.<br />
Shen, H. W., R. W. Li, (1976). Watershed Sediment Yield, 2, Stochastic<br />
Approaches to Water Resources. Edited and published by H. W. Shen<br />
for Collins Colorado.<br />
Simons, D. B., R. M. Li, M. A. Stevens, (1975). Development of<br />
Models for Predicting Water and Sediment Routing and Yield<br />
from Storms on Small Watershed. Colorado State Univ. Report<br />
CER74-75DBS-RML"AS24. Prepared for USDA Forest Service, Rocky<br />
Mountain Forest and Range Experimental Station, Flagstaff, Arizona.<br />
Smith, D. D., D. M. Whitt, (1948). Evaluating Soil Losses from Field<br />
Areas. E. 2, pp. 394-396 and 398.<br />
Smith, D. D., W. H. Wischmeier, (1957). Factors Affecting Sheet and<br />
Rill Erosion. Trans. Am. Geophys. Un., 38, pp. 889-896.<br />
Smith, D. D., (1962). Rainfall Erosion. Adv. Agron., 14, pp. 109-148.<br />
Smith, D. D., (1941). Interpretation of Soil Conservation Data for<br />
Field Use. Agricultural Engineering, 22, pp. 173-175.<br />
Smith, D. D., (1966). Broad Aspects of Erosion Research Presented at<br />
the 1966 American Society of Agricultural Engineers Winter meeting.<br />
Smith, D. D., D. M. Whitt. (1947). Estimating Soil Losses from<br />
Field Areas of Claypan Soils. Proceedings, Soil Science Society<br />
of America, 12, Madison, Wisc., pp. 485-490.<br />
Smith, D. D., W. H. Wischmeier, (1957). Factors Affecting Sheet<br />
and Rill Erosion. Trans. AGU, 3, pp. 889-896.<br />
Smith, D. D., W. H. Wischmeier. (1958). Evaluation of the Factors<br />
in Soil Loss Equation. Agricultural Engineer, 2, pp. 458-<br />
462.<br />
58
Smith, B. M., st. (1967). Renewal of Desurfaced Austin Clay.<br />
Soil Science, 103.<br />
Smith, R. M., W. L. Stamey, (1965). Determining the Range<br />
of Tolerable Erosion. Soil Science, 100, (6).<br />
Soil Conservation Service, United States Department of Agriculture,<br />
Procedures for Determining Rates of Land Damage, Land Depreciation,<br />
and Volume of Sediment Produced by Gully Erosion. Technical<br />
Release No. 32, Geology, July (1966).<br />
Soons, J. M., (1966). Some Observations of Microclimate and Erosion<br />
Processes in Cass Basin in the Southern Alps. Paper delivered N. Z.<br />
Hydrological Society Symposium on Precipitation and Erosion (1966).<br />
(cyclostyled).<br />
Speraberry, J. A., A. J. Bowie, (1969). Predicting Sediment Yields<br />
from Complex Watersheds. Trans. ASAE, 12, pp. 188, 201.<br />
Spomer, R. G., H. G. Heinemann, R. F. Piest, (1971). Consequences<br />
of Historic Rainfall on Western Iowa Farmland. Water Resources<br />
Research, L, (31, pp. 524-535.<br />
Springer, D. K., C. B. Breinig, M. E. Springer, (1963). Predicting Soil<br />
Losses in Tennessee. 3. Soil Wat. Conserv., 2, pp. 157-158.<br />
Stall, J. B., L. J. Bartelli, (1959). Correlation of Reservoir Sedimentation<br />
and Watershed Factors: Springfield Plains, Illinois, Illinois<br />
State Water Survey, Report of Investigations 37.<br />
Swanson, N. P., A. R. Dedrick, (1965). Protecting Soil Surfaces Against<br />
Water Erosion with Organic Mulches. Paper Delivered Am. Soc.<br />
Agron., Ohio, (1965). (cyclostyled).<br />
Swanson, N. P., A. R. Dedrick, H. E. Weakly, (1965). Soil Particles and<br />
Aggregates Transported in Runoff from Simulated Rainfall. Trans.<br />
Am. SOC. Agric. Engr., &, pp. 437-440.<br />
Swanson, N. P.. A. R. Dedrick, H. E. Weakly, H. R. Haise, (1965).<br />
Evaluation of Mulches for Water Erosion Control. w. 8, pp.<br />
438-440.<br />
Swanson, N. P., A. R. Dedrick, (1966). Simulation of Increased Slope<br />
Length on Small Runoff Plots. Paper Presented at Am. SOC. Agric.<br />
Engr. (cyclostyled).<br />
Taylor, R. E., 0. E. Hays, E. Bay, R. M. Dizon, (1964). Corn Stover<br />
Mulch for Control of Runoff and Erosion on Land Planted Corn after<br />
pp. 123-125.<br />
Corn. Soil Sci. SOC. Am. Proc., 8,<br />
Thompson, J. R., (1964). Quantitative Effect of Watershed Variables on<br />
the Rate of Gully Head Advancement. Transactions, American Society<br />
of Agricultural Engineers, 2, (A), St. Joseph, Michigan.<br />
pp. 54-55.<br />
59
Thoreson, A. S., J. K. Maddy, (1963). Using the Soil Loss Equation in<br />
Iowa. J. Soil Wat. Conserv., la, pp. 159-160.<br />
Todorovic, P., V. Yevjevich, (1969). Stochastic Process of<br />
Precipitation. Colorado State University Hydrology Paper 35.<br />
Turner, G. T., E. J. Dortignac, (1954). Infiltration Erosion and<br />
Herbage Production of Some Mountain Grasslands in Western<br />
Colorado. J. For., 52, pp. 858-860.<br />
Uhland, R. E., (1935). The Effect of Plant Cover on Soil and Water<br />
Losses. Symposia Commemorating Six Decades of the Modern<br />
Era in Biological Science (Iowa State College), 1, pp. 115-122.<br />
United States Department of Agriculture. Summary of Reservoir Sediment<br />
Disposition Surveys Made in the United States through 1970.<br />
Miscellaneous Publication 1226, Compiled Under the Auspices of<br />
the Sedimentation Committee, Water Resources Council, (1973).<br />
United States Geological Survey, National Reference List of Water<br />
Quality Stations, Water Year 1971, Water Resources Division,<br />
Washington, D. C., (1971).<br />
United States Geological Survey Water Supply Papers. Quality of<br />
Surface Water of the United States, (published) and annual<br />
summaries (unpublished) Washington, D. C., (1959-1967).<br />
University of Missouri, Agricultural Experiment Station, (1962).<br />
Missouri Soil and Water Conservation Needs Inventory.<br />
Ursic, S. J., F. E. Dendy, (1965). Sediment Yields from Small Watersheds<br />
Under Various Land Uses and Forest Covers. Proceedings of<br />
(1963) Federal Interagency Conference on Sedimentation, Miscellaneous<br />
Publication 970, United States Dept. of Agriculture, pp. 47-52.<br />
USBR., (1960). Investigation of Meyer-Peter, Muller Bedload Formulas.<br />
Sedimentation Section, Hydrology Branch, Division of Project<br />
Investigation, U.S. Dept. of the Interior, Bureau of Reclamation.<br />
U.S. Dept. of Agriculture, (1975). Erosion and Sedimentation. Soil<br />
Conservation Service, Great Lakes <strong>Commission</strong>, Appen. 18.<br />
U.S. Dept. of Housing and Urban Development, (1970). Proceedings of the<br />
National Conference on Sediment Control, Washington, D. C.<br />
September 14-16, 1969, May 1970.<br />
U.S. Environmental Protection Agency, (1975). Control of Water Pollution<br />
from Cropland. Vol. 1, USDA ARS H-S-1 and EPA 600/2-75-0269.<br />
Van Doren, C. A., R. S. Stauffer, E. H. Kidder, (19<strong>50</strong>). Effect of<br />
Contour Fanning onSsoil Loss and Runoff. Soil Sci. SOC. Am.<br />
Proc., 2, pp. 413-417.<br />
Van Doren, C. A., L. J. Bartelli, (1956). A Method for Forecasting<br />
Soil Losses. Agricultural Engineering, x, pp. 335-341.<br />
60
Van Vliet, L. J. P., G. J. Wall, W. T. Dickinson. The Effect<br />
of Agricultural Land Use on Sheet Erosion Losses in Southern<br />
Ontario. Can. Jour. Soil Science (in press).<br />
Vice, R. B., G. E. Ferguson, H. P. Guy, (1968). Erosion from<br />
Suburban Highway Construction. Journal of the Hydraulics<br />
Division, ASCE, 94. (HY l), Proc. Paper 5742, pp. 347-348.<br />
Vice, R. B., H. P. Guy, G. E. Ferguson, (1969). Sedimentation Movement<br />
in an Area of Suburban Highway Construction, Scott Run Basin,<br />
Fairfax County, Virginia, 1961-64. U.S. Geol. Survey Water Supply<br />
Paper 1591-E.<br />
Walker, P. H., (1966). Postglacial Environments in Relation to Landscape<br />
and Soils on the Cary Drift, Iowa. Agriculture and Home Economics<br />
Experiment Station Bulletin 549, Iowa State University.<br />
Whitaker, F. D. H. G. Heinemann. The Effects of Soil Management<br />
Variables on Soil Losses (to be published in University of Missouri<br />
Experiment Station Bulletin).<br />
Whitaker, F. D., V. C. Jameson, V. F. Thornton, (1961). Runoff and Erosion<br />
Losses from Mexico Silt Loam in Relation to Fertilization and<br />
Other Management Practices. Soil Sci. SOC. Am. Proc., 2, pp. 401-403.<br />
Williams, J. R., (1972). Sediment Yield Prediction with Universal Equation<br />
Using Runoff Energy Factor. Paper Presented at the Sediment Yield<br />
Workshop, USDA Sed. Lab., Oxford Mississippi.<br />
Williams, J. R., (1975). Sediment Routing for Agricultural Watershed.<br />
Water Resources Bulletin, 11, (5), pp. 965-970.<br />
Williams, J. R., H. D. Berndt, (1972). Sediment Yield Computed with<br />
Universal Equation. Journal of Hydraulics Division, American<br />
Society of Civil Engineers, 98, (HYlZ), Proc. Paper<br />
9426, pp. 2087-2098.<br />
Williams, J. R., H. D. Berndt, (1972). Sediment Yield Computed with<br />
Universal Equation. Journal of the Hydraulics Division, ASCE,<br />
- 98, (HYlZ), Proc. Paper 9426. pp. 2087-2098<br />
Wilson, L., (1972). Seasonal Sediment Yield Pattern of U.S. Rivers,<br />
Water Resources Research, 8 (6), pp. 1470.<br />
Wilson, L., (1973). Variation in Mean Annual Sediment Yield as a Function<br />
of Mean Annual Precipitation, Am. Jour. of Sci. (in Press).<br />
273, (4). pp. 334-349.<br />
61
Wiltshire, C. R., (1947). Runoff Plots and Standard Runoff and Soil<br />
Loss Measuring Equipment Used by the New South Wales Soil<br />
Conservation Service. J. Soil Conserv. Serv. N.S.W., 3,<br />
pp. 171-178.<br />
Wiltshire, C. R., (1948). The Measurement of Runoff and Soil Loss from Plot<br />
Experiments. u. ft, pp. 40-44.<br />
Wischmeier, W. H., (1960). Cropping-Management Factor Evaluations for<br />
a Universal Soil Loss Equation. Soil Sci. SOC. Am. Proc., 26,<br />
pp. 322-326.<br />
Wischmeier, W. H., (1959). A Rainfall Erosion Index for a Universal<br />
Soil Loss Equation. Soil Sci. SOC. Am. Proc., 2, pp. 246-249.<br />
Wischmeier, W. H., (1966). Relation of Field-Plot Runoff to Management and<br />
Physical Factors. H. 2, pp. 272-277.<br />
Wischmeier, W. H., D. D. Smith, (1958). Rainfall Energy and its<br />
Relationship to Soil Loss. Trans. Am. Geophys. Un., 3, pp.<br />
285-291.<br />
Wischrneier, W. H., D. D. Smith, R. E. Uhland, (1958). Evaluation of<br />
Factors in the Soil Lose Equation. Agric. Engng., St. Joseph<br />
Michigan, 2, pp. 458-462 and 474.<br />
Wischmeier, W. H., (1962). Storms and Soil Conservation, Jour. of Soil<br />
and Water Conservation. x, (2).<br />
Wischmeier, W. H., (1962). Rainfall Erosion Potential, Agric. Engineerinl<br />
- 43, (4). pp. 212.<br />
Wischmeier, W. H., (1972). Estimating the Cover and Management Factor<br />
for Undisturbed Areas. Paper Presented at the Sediment Yield<br />
Workshop, USDA Sed. Lab. Oxford, Mississippi.<br />
Wischmeier, W. H., D. D. Smith, (1958). Rainfall Energy and its<br />
Relationship to Soil Loss, Am. Geophys. Union Trans., 3,<br />
- 2, pp. 285.<br />
Wischmeier, W. H., D. D. Smith, R. E. Uhland, (1958). Evaluation of<br />
Factors in the Soil-Loss Equation, Jour. of Agric. Engineering.<br />
Wischmeier, W. H., D. D. Smith, (1960). A Universal Soil-Loss Equation<br />
to Guide Conservation Farm Planning. 7th <strong>International</strong> Congress<br />
of Soil Science, Madison, Wisconsin.<br />
Wischmeier, W. H., D. D. Smith, (1965). Predicting Rainfall-Erosion<br />
Losses from Cropland East of the Rocky Mountains. Agriculture<br />
Handbook 282, U.S. Dept. of Agriculture, Agricultural Research<br />
Service.<br />
62
Wischmeier, W. H., C. B. Johnson, B. V. Cross, (1971). A Soil-<br />
Erodibility Nomograph for Farmland and Construction Sites.<br />
Journal of Soil and Water Consevation, a, (5), pp.<br />
189-193.<br />
Wischmeier, W. H., (1973). Upslope Erosion Analysis in River Mechanics,<br />
111, H. W. Shen (ed.)<br />
Woodburn, R., (1945). A Comparison of Erosion Losses After Turning<br />
Under Legumes and Non Legumes. Agric. Engng., St. Joseph,<br />
Michigan, 26, pp. 247-240.<br />
Wolman, M. G., (1964). Problems Posed by Sediment Derived from Construction<br />
Activities in Maryland. Report to Maryland Water<br />
Pollution Control <strong>Commission</strong>, Annapolis.<br />
Wolman, M. G., J. P. Miller, (1960). Magnitude and Frequency of<br />
Forces in Geomorphic Processes. Journal of Geology, 68.<br />
Wolman, M. G., A. P. Schick, (1967). Effect of Construction on<br />
Fluvial Sedimentation in Urban and Suburban Areas in Maryland.<br />
Water Resources Research, 3, (2).<br />
Woolhiser. D. A., P. H. Blinco, (1972). Watershed Sediment Yield-<br />
A Stochastic Approch. Paper Presented at Sediment Yield Work-<br />
shop, USDA Sed. Lab., Oxford, Mississippi.<br />
Woolhiser, D. A.. P. Todorovic, (1974). A Stochastic Model for<br />
Sediment Yield for Ephemeral Streams. Proc. <strong>International</strong><br />
Association for Statistics in Physical Science, Symposium of<br />
Hydrology, Tuscon, Arizona, August 29-September 2, 1971, USDA<br />
Misc. Publication 1275, pp. 295-308.<br />
Yen, B. C., H. G. Wenzel, Jr., (1970). Dynamic Equations for Steady<br />
Spatially Varied Flow. Journal of the Hydraulics Division,<br />
American Society of Civil Engineers, 96, (HY3), pp.<br />
801-814.<br />
Yoon, N. Y., (1970). The Effect of Rainfall on the Mechanics of Steady<br />
Spatially Varied Sheet Flow a on Hydraulically Smooth Boundary.<br />
Ph.D. Thesis, Dept. of Civil Engineering, University of Illinois.<br />
Urbana, Illinois.<br />
Yorke, T. H., (1975). Effects of Sediment Control on Sediment Transport<br />
in the Northwest Branch Anacostia River Basin, Montgomery County,<br />
Maryland, U.S. Geol. Survey, Journ. of Research, 2,(4),<br />
pp. 487-494.<br />
Yorke, T. H., W. J. Davis, (1971). Effect of Urbanization on Sediment<br />
Transport in Bel Pre Creek Basin, Maryland; U.S. Geol. Survey<br />
Prof. Paper 7<strong>50</strong>-B.<br />
Yorke, T. H.. (1972). Sediment Yield of Urban Construction Sources,<br />
Montgomery County, Maryland; Progress Report Rock Creek Anacostia<br />
River Basin, U.S. Geol. Survey Open file Report, p. 39.<br />
63
Young, R. A., C. K. Mutchler, W. H. Wischmeier, (1964). Influence of<br />
Row Direction and Type of Vegetal Cover on the Slope-Soil Loss<br />
Relationship. Trans. Am. SOC. Agric. Engr., 2, pp. 316-317 and 320.<br />
Young, R. A., C. K. Mutchler, (1969). Soil Movement on Irregular<br />
Slopes, Water Resources Research, 5(5), pp. 1084-1089.<br />
Young, R. A., J. L. Wiesrner, (1973). The Role of Rainfall in Soil<br />
Detachment and Transport. Water Resources Research, 9(6),<br />
pp. 1629.<br />
Ziemnicki, S ., C. Jozefaciuk, (1965). Soil Erosion and its Control.<br />
Translated from Erozja, Panstwowe Wydawnictwo Rolnicze i<br />
Lesne, Warszawa.<br />
Zingg, A. W., (1940). Degree and Length of Land Slope as it Affects<br />
Soil Loss in Runoff. Agricultural Engineering, 3, pp.<br />
59-64.<br />
Zingg, A. W., (1941). Soil Movement Within the Surface Profile of Terraced<br />
Lands Unpublished report, U.S. Dept. of Agriculture, Soil<br />
Conservation Service.<br />
64
DISCUSSION<br />
Dr. C. Nordin: I have a comment on your remarks about sediment monitoring.<br />
About forty years ago the Federal Agencies in the United States set up the<br />
interagency sedimentation group to look at sampling problems and to standardize<br />
the methodology in the equipment. The geological survey was quite<br />
heavily involved in this. Paul Benedict, and Bruce Colby in particular<br />
did very extensive work back in the 30's and 40's. At that time they recognized<br />
that sediment sampling had to be event-based; that most of the sediment<br />
could be carried in a single event during the year or at very irregular<br />
intervals. They developed equipment to sample this and methodologies that<br />
give you a very good sample. Now people are not monitoring sediment properly.<br />
It is for one of two reasons: either they don't know what they are doing<br />
and they are unwilling to read the literature, or they are unwilling to<br />
spend the money to do it right. But these things have been known for four or<br />
five decades.<br />
Dr. T. Dickinson: Well, I can only agree with you Carl. I think it is a<br />
good point. Some of these things have been known for years and years, but I<br />
only know that, for instance in Canada, we have not been sampling sediment<br />
for very long. The measurement techniques are known and they are used. In<br />
many cases they are not used well. Now, whether it is because people are not<br />
taking the time to use them well or don't know the appropriate mechanism, I<br />
am not sure. I don't want to go into the detail, but I agree with you that<br />
some of that methodology, or much of is it, already known if we just make<br />
good use of it. I suggest that particularly in small watershed studies<br />
where the events can be very, very short, people are not making the best<br />
use of it and I must say too, that in the <strong>International</strong> <strong>Joint</strong> <strong>Commission</strong><br />
studies, there is a lot of experimentation in doing it and trying to a do<br />
better job. I am not openly criticizing all studies, but I do say many of<br />
the data we have on some of the basins need to be interpreted carefully.<br />
If one looks at the sampling frequency and looks at how they were taken, we<br />
better acknowledge just how that was done and interpret the results accordingly,<br />
and very often the sampling frequency in just time is not anywhere<br />
near where it has to be to give us meaningful results.<br />
Dr. D. Wazling: Just a general comment that concerns the possibility of<br />
using turbidity measurements for documenting sediment output from drainage<br />
basins. One of the traditional views is to dismiss these immediately<br />
and say they have got far too many problems associated with them, I have but<br />
in fact experimented with these in England, and if one uses them for specific<br />
catchment and carries out detailed calibration procedures it seems to me<br />
that they will work and they do provide a very useful means of getting a<br />
continuous record of sediment concentration. The point I really want to<br />
get at is that if one takes that continuous record as being a worthwhile<br />
continuous record, it is extremely interesting to compare individual sampling<br />
programs with a continuous record. When you start to work out error<br />
functions of what individual programs would give compared to what a continuous<br />
record would give, then is it absolutely staggering the errors that<br />
could be creeping in. And I really want to support your point, you could be<br />
4 or <strong>50</strong>0% out with the figures when you compare them with the continuous<br />
record.<br />
65
Dr. T. Pickinson: Two comments. One: we have taken a look at this.<br />
We have been able to find no good relationship on the basins we are<br />
working on right now between turbidity and suspended load that we could<br />
use in a quantitative way. But I quite agree with you that it is a convenient<br />
way. One thing we found convenient about it is that it may be a<br />
way of identifying sources. In other words you can set them up and run them<br />
continously in a number of places in a basin, and at least begin to identify<br />
when there is load coming out of particular areas versus when there is no<br />
load coming out of certain areas; even if we can't say how much load is<br />
coming from here versus how much is coming from there. We have real difficulty<br />
making that step because we have done this in where places we got<br />
measurements. Certainly as an index of helping us sort out sources, we are<br />
finding it very convenient in a couple of the basins.<br />
Dr. D. Simons: I would simply like to make the point in addition to what<br />
has been stated that as we monitor small watersheds, plots, large watersheds;<br />
the data collection, the instrumentation, so and forth require a lot<br />
of time, a lot of amendments of effort. It is expensive and I do think that<br />
it is probably timely to mention that we should take every opportunity to<br />
utilize the physically-based models to determine what the data needs are. I<br />
think in many instances where we are going out and instrumenting watersheds<br />
and plots, we may be gathering data that are trivial OK we may be gathering<br />
during times when they are not important. By utilizing these models and<br />
carrying out sensitivity analyses, I think we can in the future better design<br />
to meet our real data needs to do a better job; get more back for the money<br />
spent in the effort.<br />
Dr. T. Lsickinson: I would certainly agree. I think that the models can be<br />
a great help and play a great learning part and it may be a way of efficiently<br />
spending the dollars we have to try and reach some of the problems faster.<br />
Dr. L. HetZing: Following your talk and the subsequent discussion, I see<br />
three different areas where additional study or research resources could be<br />
put:<br />
1. Modeling and process development including laboratory and/or<br />
field studies directed to the understanding of actual processes.<br />
2. Field measurements over a variety of watersheds directed towards<br />
increasing OUT data base on actual sediment movement.<br />
3. Development of measurement techniques directed towards improving<br />
accuracy and lowering cost. The annual cost of a sediment measurement<br />
station is now $10,000 to $15,000.<br />
Which of these three areas, given $10,000 or $100,000 or $1 million,<br />
would be the best investment considering the present state of the art.<br />
66
Dr. T. Dickinson: I think that is one of the purposes of this workshop. I<br />
think we have got to do them all and I agree you can not do them all on one<br />
particular basin. But hopefully in some of these broader projects like the<br />
IJC one, there is room for some to be done on each one to try to learn from<br />
each one, to complement the other. I think that is the stage we are at. I<br />
don't think we are at the stage where we can say "let's put all our money in<br />
one bag". That begs the question, but I think that is where we are, because<br />
we must have more data to make sense, but on the other hand we have got to<br />
look at some of the processes. I guess the problem I have, is that in terms<br />
of processes 1 think we may have to study them in the field, and in particular<br />
field conditions. At least I think in some cases we have got to do more of<br />
that before we can even go into the laboratory to model them because I am<br />
not sure we even know quite how to set up the laboratory model for the field<br />
situation we want to model. I think that in the laboratory the time scale<br />
we can use is one of the advantages, but we had better find out really what<br />
is the time scale of changes in the field before we go into the laboratory<br />
to try to sort some of that out. I think we have got to do a little bit of<br />
all of it and hopefully in looking at all the resources in the area as a<br />
pool we can come up with a balance that is meaningful, and avoid some of<br />
the pressures that suggest haste and "so and so down the road is doing that".<br />
In fact if "so and so down the road is doing that", we better do something<br />
else and learn in that way.<br />
Dr. E. &gley: I have to draw this session to a close as far as my participation<br />
is concerned. I wish to offer one comment which I would like a<br />
number of you who I know have been working on the relationships between<br />
specific kinds of land use and their effects upon water quality to consider.<br />
If I synthesize correctly what you have said Trevor (Dickinson), in general<br />
our ability to use existing data generating techniques is not sufficiently<br />
discriminatory to enable a distinction to be made amongst various land uses.<br />
I know at least two of you, and I am sure there are many more in this room.<br />
who have done very specific work on attempting to discriminate in terms of<br />
water quality and up-stream land uses, and I would like you to think about<br />
this because it is a question that is going to come out in the working sessions.<br />
67
INTRODUCTION<br />
Anthropogenic Influences of Sediment Quality at a Source.<br />
Pesticides and PCBs<br />
R. Miles<br />
Environmental studies at the London, Ontario, Research Institute of<br />
Agriculture Canada have included investigations of the pollution of streams<br />
and water systems by insecticides used in agriculture. We have analysed<br />
soils in the stream basins, water, suspended sediment, bed load, bed material<br />
and fish for insecticide residue content. In this report I shall discuss the<br />
concentrations of insecticides found in soil and on the various sediments,<br />
the conversion of DDT to its metabolites on sediments, and the relation of<br />
water solubility to insecticide adsorption onto sediments. We studied Big<br />
Creek, Norfolk County, Ontario, which drains 280 square miles including an<br />
important tobacco growing area, and the water system draining the 7,<strong>50</strong>0 acre<br />
Holland Marsh; an intensively cultivated vegetable muck area about 30 miles<br />
north of Toronto. To compare insecticide residues from the two areas in: (a)<br />
soil, (b) suspended sediment, and (c) bed material, samples from Big Creek<br />
contained 4.0, 0.11, and 0.026 ppm DDT, respectively; while corresponding<br />
values from Holland Marsh were 8.0, 29, and 0.30 ppm.<br />
DISCUSSION OF RESULTS<br />
An important sediment is the bed load - the shifting mass of sediment<br />
and detritus which we sampled using a Bogardi Bed Load Sampler. Residues of<br />
total DDT, dieldrin and endosulfan (Thiodan) found in bed material and bed<br />
load of Big Creek in 1973 are listed in Table 1. With one exception (August<br />
14th) the concentrations on the bed load were several times greater than the<br />
concentrations in the bed material. This means that benthic fish and crustaceans<br />
may be exposed to much mre insecticide residue than would be indicated<br />
by analysis of bed material alone.<br />
DDT converts to its metabolite DDE by splitting off HC1. This<br />
conversion to DDE is generally associated with enzyme dehydrochlorination in<br />
insects, mammals and fish. DDT conversion to another metabolite DDD is a<br />
reductive dechlorination and is reported to be favoured by anaerobic conditions.<br />
The ratio of metabolite to parent DDT is a measure of the degradation<br />
of the insecticide residue. In Holland Marsh bed material DDE/DDT<br />
increased from 0.2 (April) to 0.8 (September) in 1972 and from 0.2 (April) to<br />
1.4 (September) in 1973. DDD/DDT increased from 1.6 (April) to 6.7 (September)<br />
in 1972 and from 2.6 (April) to 13.0 (October) 1973. Water is pumped from<br />
the marsh drainage ditch depending on water levels, and very little pumping<br />
occurs during the summer after June. It would appear from the above results<br />
that in the quiet water of the drainage ditch the microorganisms are able to<br />
convert DDT to its metabolites during the period from spring through to<br />
69
autumn. Both DDD and DDE are much less toxic to mammals than the parent DDT<br />
(acute oral LD<strong>50</strong> to male rats > 4,000, 880 and 200 mg/kg respectively) so<br />
the above degradation is a lessening of overall toxicity of DDT residues<br />
although DDE is blamed for the thin eggshell syndrome in birds.<br />
Sediment must require considerable time to move along the of a bed<br />
stream from source to mouth and we theorized that microorganisms would be<br />
able to convert some DDT to its metabolites during the time the sediment<br />
moved downstream in Big Creek, a distance of about 30 miles. We sampled bed<br />
material at 6 locations on the same day over this distance in 1972 and DDE/<br />
DDT increased from 0.2 (upstream) to 1.5 (stream mouth), while DDD/DDT<br />
increased from 0.1 (upstream) to 2.0 (stream mouth). Thus the ratio DDD/DDT<br />
in residues in the bottom sediment increased 20 times in samples from upstream<br />
to the stream mouth! A similar study in 1973 produced the same trend but not<br />
to as significant a degree. Again this represents a considerable reduction<br />
in overall DDT mammalian toxicity.<br />
In analysing water samples for insecticide residue content it is<br />
our normal practice to analyse the whole water sample including suspended<br />
sediment. To determine the amount of insecticide adsorbed on suspended<br />
sediment we filter companion samples and analyse the filtered sediment.<br />
Averaging a large number of samples from Big Creek and Holland Marsh we found<br />
the per cent of the whole warer analysis which was adsorbed on the sediment<br />
to be 61% average for DDT (30-93% range) and 15% average for dieldrin (3-42%<br />
range). These values are in agreement with the water solubility of these<br />
compounds (0.0012 ppm for DDT and 0.186 ppm for dieldrin). In the Holland<br />
Marsh water samples, diazinon (an organophosphorus insecticide) was present<br />
at concentrations greater than the dieldrin but no diazinon was found adsorbed<br />
onto the sediment. These data also agree with the water solubility value (40<br />
ppm) for diazinon. Our laboratory adsorption experiments verify these findings,<br />
i.e. that the more water soluble the chemical, the less it is adsorbed onto<br />
the sediments.<br />
In summary, we have found significantly different insecticide<br />
residue concentrations in soil, suspended sediment, bed load and bed material.<br />
We have noted the conversion on sediment of DDT to its less toxic metabolite<br />
DDD and also to DDE. And we suggest that we might have quite different<br />
environmental pollution with insecticides in the future - the sedimentassociated<br />
organochlorine insecticides settle into the bed material of bays<br />
when a stream enters the receiving lake, but the water-soluble organophosphorus<br />
insecticides which persist will continue on with the water to become widely<br />
distributed in the lake.<br />
70
1973<br />
June 26<br />
July 10<br />
August 14<br />
September 15<br />
October 2<br />
TABLE 1.<br />
INSECTICIDE RESIDUES IN BED MATERIAL AND BED LOAD,<br />
BIG CREEK, NORFOLK COUNTY, ONTARIO (PARTS PER BILLION)<br />
Total - DDT<br />
Bed Mat. Bed Load<br />
30 198<br />
26 45<br />
27 21<br />
35 100<br />
18 30<br />
Dieldrin<br />
Bed Mat. Bed Load<br />
1 6<br />
1 4<br />
2 1<br />
1 8<br />
1 2<br />
18 62 October 16
INTRODUCTION<br />
Anthropogenic Influences of Sediment Quality at a Source.<br />
Pesticides and PCBs<br />
R. Frank<br />
Between 1974 and 1976 samples of suspended solids were collected<br />
at the river mouths of those watercourses on the Canadian side of the Great<br />
Lakes that del.ivered at least 0.5% of the water contributed to that lake by<br />
Canadian land drainage. The suspended solids were removed from stream water<br />
during the high flows in spring by centrifugation and freeze drying. The<br />
field work was carried out by staff of the Canada Centre for Inland Waters,<br />
under PLUARG study Task D. Many suspended solids have still to be<br />
analysed and the findings given herein must be treated as preliminary.<br />
RESULTS<br />
Around Lake Ontario 34 of 53 streams sampled were analysed for<br />
pesticides; along Lakes Erie and St. Clair the number was 14 of 24 streams;<br />
along Lake Huron it was 9 of 21 streams (1974), and 5 of 48 streams (1975),<br />
and along Lake Superior it was 5 of 45 streams.<br />
DDT and/or its metabolites were present in all samples. Mean<br />
residues in 1974 were 138 and 160 ppm in suspended solids entering Lakes<br />
Ontario and Huron, while in 1975 residues were considerably lower at 34.0,<br />
2.9 and 2.3 ppb, respectively, on suspended soljds entering Lakes Erie, St.<br />
Clair, Huron and Superior, In 1974 the largest component in the CDDT was the<br />
parent compound DDT, but in 1975 the largest component was DDE.<br />
Dieldrin was present in <strong>50</strong> and 9% of samples collected at river<br />
mouths along Lakes Ontario and Huron in 1974; these contained mean residues<br />
of 1.5 and 0.2 ppb respectively. All suspended solids entering Lakes Erie-<br />
St. Clair (1975) contained dieldrin; the mean residue being 3.6 ppb. Mean<br />
residues were 0.4 and 0.2 ppb dieldrin in solids entering Lakes Huron and<br />
Superior and were detected in 80% of samples.<br />
Endosulfan was present in 19% of suspended solids entering Lake<br />
Ontario (mean residue 2.3 ppb) but none entered Lake Huron in 1974. In 1975,<br />
endosulfan was present on 79%, 80% and 40% of suspended solids entering Lakes<br />
Erie - St. Clair, Huron and Superior; at the respective mean concentrations<br />
of 5.5, 0.8 and 0.4 ppb.<br />
Heptachlor epoxide and chlordane were not detected on suspended<br />
solids entering Lake Ontario or Lake Huron in 1974 to a limit of 0.5 ppb.<br />
Heptachlor epoxide was present in <strong>50</strong>%, 40% and 20%, respectively, of suspended<br />
solids entering Lakes Erie - St. Clair, Huron and Superior in 1975. The<br />
respective mean residues were 1.2, 0.2 and
Organophosphorus insecticides were not found in any suspended<br />
solids over the two year period to a limit of 1-10 ppb.<br />
PCBs were present in all suspended solids over the 2-year period.<br />
The highest concentrations were in those entering Lake Ontario (194 ppb) and<br />
Lake Huron (84 ppb) in 1974. Mean concentrations were 60, 20 and 20 ppb,<br />
respectively, in 1975 in solids entering Lakes - Erie St. Clair, Huron and<br />
Superior.<br />
A few samples were analysed for HCB in 1974. Two of nine samples<br />
entering Lake Ontario contained HCB at 5 ppb. No HCB was found in two<br />
samples analysed from streams flowing into Lake Huron.<br />
There were enough samples in 1974 to analyse suspended solids<br />
entering Lake Ontario and Lake Huron for s-triazines. None of those streams<br />
flowing into Lake Huron contained s-triazines and 3 only of 34 contained<br />
detectable levels of s-triazines entering Lake Ontario. The mean residue<br />
of the 3 samples was 510 ppb. There was insufficient sample in 1975 to do<br />
s-triazine analyses and more sample is being obtained.<br />
A few streams in Lakes Ontario and Erie - St. Clair were sampled at<br />
monthly intervals throughout the year. Both IDDT and PCB varied greatly trom<br />
month-to-month. This was especially the case among suspended solids from<br />
streams entering Lake Ontario (1974). On the other hand the same parameters<br />
showed only small differences in 1975 from Lakes - St. Erie Clair and Huron.<br />
CONCLUSION CCMNTS<br />
This report is intended to be only preliminary and a more exact<br />
interpretation will have to await completion of analysis and calculations of<br />
actual amounts of pesticide and pollutants being carried to the Great Lakes<br />
by streams.<br />
14
U<br />
VI<br />
Table 1. Pesticides in Suspended Solids Collected at the Mouths of Rivers and Streams on the Canadian Side<br />
of the Great Lakes<br />
Contaminant 1<br />
Samples<br />
Analysed<br />
ZMT<br />
Dieldrin<br />
Endosulfan<br />
Hept. Epox.<br />
Chlordane<br />
PCB<br />
Ontario<br />
(1974)<br />
53<br />
34<br />
34<br />
138<br />
2-2380<br />
18<br />
1.5<br />
0-6<br />
6<br />
2.3<br />
0-35<br />
0<br />
0<br />
34<br />
194<br />
2-1800<br />
Erie 6 St.<br />
Lakes<br />
Clair Huron<br />
Superior<br />
(1975)<br />
(1974) (1975) (19<br />
24<br />
14<br />
14<br />
34<br />
1.1-115<br />
14<br />
3.6<br />
0.5-13.8<br />
11<br />
5.5<br />
0.0-18.3<br />
7<br />
1.2<br />
0.0-3.6<br />
12<br />
2.9<br />
0.0-20.0<br />
14<br />
60<br />
10-330<br />
21<br />
9<br />
8<br />
160<br />
0-1330<br />
1<br />
0.2<br />
0.0-2.0<br />
0<br />
-<br />
-<br />
0<br />
0<br />
9<br />
84<br />
20-370<br />
48<br />
5<br />
5<br />
2.9<br />
1.4-5.2<br />
4<br />
0.4<br />
0.0-1.5<br />
4<br />
0.8<br />
0-4.3<br />
2<br />
0.2<br />
0.0-0.6<br />
4<br />
0.8<br />
0.0-1.0<br />
'No organophosphorus insecticides found in any suspended solids.<br />
HCB present in 2 of 9 samples from L. Ontario - mean 1.1 ppb, range 0-5 ppb, absent in 2 samples from L. Huron.<br />
Atrazine in 3 of 34 samples in L. Ontario - mean 45 ppb, range 0-1110 ppb. No atrazine in 9 samples from L. Huron (1974).<br />
5<br />
20<br />
8-30<br />
5<br />
5<br />
2.3<br />
1.0-4.1<br />
4<br />
0.2<br />
0.0-0.4<br />
2<br />
0.4<br />
0.0-2.0<br />
1<br />
I<br />
TABLE 2.<br />
Lakes DDE TDE<br />
Lake Ontario (1974)<br />
Lake Huron (1974)<br />
Lake Huron (1975)<br />
COMPONENTS OF CDDT FOUND IN SUSPENDED SOLIDS COLLECTED<br />
AT THE MOUTHS OF RIVERS AND STREAMS ON THE CANADIAN SIDE OF THE<br />
GREAT LAKES<br />
Lakes Erie & St. Clair (1975)<br />
Lake Superior (1975)<br />
54<br />
38<br />
8.0<br />
1.4<br />
1.7<br />
76<br />
Content in suspended solids (ppb)<br />
20<br />
14<br />
6.4<br />
0.6<br />
0.5<br />
”<br />
OPDDT I P~DDT I CDDT
DISCUSSION<br />
Mr. J. CuZbertson: On the first half of the discussion, one of the tables<br />
indicated 59 and 62% of the insecticide was found on the sediment. How much<br />
sediment was this on? Did you determine the mass or surface area or Of Size<br />
these sediments?<br />
Dr. R. Miles: These are very low sediments, all less than 100 milligrams<br />
per litre. Some of them are very small, around 10 milligrams per litre. Does<br />
that answer your question?<br />
Mr. J. CuIbertson: The major point was, what was the mass of the material<br />
associated with this percent?<br />
Dr. R. Miles: I think I had that on one of the first slides, where we had<br />
parts per billion level of DDT on the suspended sediment, and I as say this<br />
suspended sediment was in the very low milligrams per litre. Now that table<br />
you asked about was the percent of the insecticide of whole the water sample<br />
that was absorbed on the sediment. We used the whole water sample in correlation<br />
with flow data that we have from Water of Survey Canada, and we<br />
actually measured the pounds of DDT. We calculated them out in weekly progression<br />
through the season and we have a paper on that with nutrients and<br />
insecticides from Big Creek that is in press. The weights are in grams per<br />
year, even some of the insecticides. It does not sound very dramatic, but<br />
the concentrations in the water in the streams are still greater than the<br />
recommendations by the EPA in the States. We have quantified them on the<br />
basis of the whole water samples or with the flow-data.<br />
Dr. E. GngZey: On the table where you showed the downstream increasing<br />
conversion of DDT to DDE and DDD this is, as I understand it, taken on the<br />
bed load or on the suspended solids fraction? I was not quite sure about<br />
that.<br />
Dr. I?. Miles: It was on the title as "Bed Material". That was bed material<br />
which we figured would take a considerable time work to down. It probably<br />
only gets moved during storm events, say, but we figured that the microorganisms<br />
in the bed material would have a longer period of work time on to<br />
the substrate as it moved downstream.<br />
Dr. E. Ongtey: Would it be correct to say that most of this would be asso-<br />
ciated with the fine fraction<br />
- the clay sized fraction?<br />
Dr. R. Miles: In Big Creek there is no real clay. Mostly gravel 1111 sand.<br />
The clay would come up in the Holland Marsh where there is a hea\ Idy
underlay under the bed material. It is a little difficult to characterize up<br />
there. The bottom mud does not get incorporated down with the clay. It<br />
forms its own bed material, the solid mass as opposed to the shifting bed<br />
load above that.<br />
Dr. E. Ongley: In your opinion we should not then perhaps restrict ourselves<br />
to looking at merely the fine sediment fraction, if we are looking at sediment<br />
quality, which is a statement that has been made earlier.<br />
Dr. R. Miles: Maybe I didn't make that point clear. The point of the bed<br />
load analyses was that the benthic fish and crustaceans are exposed to a<br />
higher concentration than you would appraise from a bed material analysis<br />
of the more permanent bed material. I think you can see, however, that there<br />
is a,considerable degradation and dilution from concentrations found in the<br />
soil. Also, with the large number of insecticides found in the soil, we are<br />
only finding a small proportion of them in the sediments.<br />
Dr. J. Weber: I have a comment. A lot of the grains of sand and coarser<br />
material are coated with fine clay particles and with fine organic substances.<br />
You can't really separate them. You may get a lot of the finer material<br />
associated with the coarser material in surface coverage.<br />
The question I have is that the analysis was on atrazine.<br />
This suggests that there is a lot of corn production. I was wondering if<br />
there was any soyabean production, if and so, had you analyzed for dinitroanilins?<br />
Dr. R. Frank: The only anilin that we have looked for is Arochlor.<br />
Dr. J. Weber: Arochlor would be an acid analid. I mean Trifluralin.<br />
Dr. R. Frank: No.<br />
Dr. J. Weber: The reason I ask is because I was also curious as to whether<br />
you had analyzed any of the sediments for some nitrosamine contaminants that<br />
have been found.<br />
Dr. R. Frank: One of the problems that we have been facing is that we only<br />
have five grams of sediment. Our first analysis is for the organochlorine<br />
group. If any sample is left we go to the triazine group. If any sample<br />
is still left we go to any other group. By then we have usually exhausted<br />
our sample.<br />
This is one of the problems we are up against. We need a<br />
system to obtain more sample. I understand that one would have to sit at the<br />
mouth of some of these streams for a month to get enough sample to do all the<br />
analyses. Perhaps Dr. Thomas could elaborate on this.<br />
70
Dr. H. Thomas: In order to investigate some of the points raised in this<br />
discussion, I should explain our procedures. We have gone up to the mouth of<br />
the Grand River with our sampling equipment. We sat there for eight hours,<br />
pumped some 4600 litres of water and recovered the solids. We have good<br />
recoveries of the solids in four size fractions. We have the associated<br />
water samples that we can supply for analysis.<br />
It is our intention to do more of this type of work. We want<br />
to fill in those samples where Dr. Frank did not have enough material. We<br />
intend to sample next spring (1977).<br />
The point that should be made is that we are not really<br />
discussing loadings in this context. We are having a look at concentrations<br />
of these materials, as they appear at the interface of the Lakes and rivers<br />
themselves.<br />
I think it is rather interesting if one looks at the organochlorines.<br />
We have done analyses on most of the residual samples from past<br />
sampling in the Great Lakes (Lake Erie, Lake St. Clair, Lake Ontario and<br />
presently Lake Huron. Lake Superior is yet to be done). This represents<br />
an enormous amount of work.<br />
Once you look at something that equates to a mass balance<br />
coming into, for example Lake Erie, one gets some shocks. Ralph Miles mentioned<br />
that in certain cases insecticides coming from these watersheds are<br />
in the grammes or kilogrammes range. Yet the amounts of these materials that<br />
are depositing in the open waters of the lakes are much higher and cannot be<br />
accounted for from these watersheds. When one looks at Lake Erie, the major<br />
impact is from the Detroit River. We know that there are not many pesticides<br />
or PCBs in Lake St. Clair. Yet once one looks at Western Lake Erie, the<br />
sediments are in terrible shape. Something is happening in that stretch of<br />
river that we have to investigate. It is a massive impact compared to a more<br />
minor impact from the watersheds themselves.<br />
Dr. R. Frank: I might just comment, that in the agricultural watersheds we<br />
are looking at a much wider range of parameters. We are not only doing that,<br />
but we are carrying field surveys to determine exactly how much material is<br />
going onto the land surface. Where we have a watershed that has got a big<br />
use of Trifluralin, we go back and look at the water in that watershed,<br />
instead of looking at it as monitoring and trying a to handle get on what is<br />
going on out there in the field and then trying to determine what is present.<br />
This is being done.<br />
Dr. J. Weber: If you took samples of the bottom deposits, has anyone sampled<br />
the concentration of the chemicals in the deposits with depth? You say DDT<br />
is decreasing with time, surely then, that means DDT the found deeper might<br />
be in higher concentrations, unless it is degraded and in that case you would<br />
find metabolites.<br />
Dr. R. Thomas: Well I think that is a function of the ability to sub-sample<br />
a sediment core with the resolution you are talking about. For example, in<br />
many lakes one centimetre would represent approximately 10 years of accumulation.<br />
You have to wait a long time to see any decline. But if you go back up<br />
through a core looking for PCBs, we have quite a nice picture in Lake Erie.<br />
It starts to appear quite strongly around 1955.<br />
79
Dr. H. Pionka: Were these sediments primarily organic? Were they very high<br />
in organic matter?<br />
Dr. I?. Thomas: We have measured total organic carbon in the suspended samples<br />
and I must confess I was very surprised indeed. I was anticipating high<br />
organic content. This is not the case. We are running up to orders of 7%<br />
organic carbon which is not high if you look in the Bay of Quinte in the Great<br />
Lakes. You are up over 20% organic carbon in samples from that area, so these<br />
are very low in organic matter.<br />
80
INTRODUCTION<br />
Anthropogenic Influences of Sediment Quality at a Source<br />
Nutrients: Carbon, Nitrogen and Phosphorus<br />
M. Miller<br />
Crop production practices have a marked influence on the amount of<br />
sediment eroding from farm fields. They may also have a marked influence on<br />
the nutrient concentration on the sediment either by altering the physical<br />
characteristics of the sediment or by altering the nutrient content of surface<br />
soil and hence of sediment.<br />
ALTERATIONS IN PHYSICAL CHARACTERISTICS OF SEDIMENT<br />
The erosion process is selective that in sediment generally contains<br />
a larger proportion of finer particles and organic matter than does the<br />
source soil. The nutrients carbon, nitrogen and phosphorus are in higher<br />
concentrations in the finer particles. This is demonstrated for phosphorus<br />
in Figure 1 in which the ratio of the phosphorus content of soil fractions to<br />
that of the total soil (P enrichment ratio) is related to the maximum particle<br />
size included in the fraction. The fact that the phosphorus enrichment<br />
in runoff sediment is related to enrichment in clay and organic matter content<br />
is also demonstrated in Table 1.<br />
Any management practice which tends to reduce the carrying capacity<br />
of the runoff water will tend to reduce the mean particle size of the sediment<br />
and hence will tend to increase the nutrient concentration on the sediment.<br />
Tillage practices can affect the runoff velocity through its effect on surface<br />
roughness, degree of incorporation of plant residues etc. Table 2 illustrates<br />
the effect of three tillage practices on the nutrient concentration in the<br />
sediment from simulated rainfall.<br />
Romkens, &. , (1973) states that "differences in the N and P<br />
concentrations in runoff sediment between tillage systems are primarily due<br />
to selective soil erosion.... Ridges across slope and/or corn residue on the<br />
soil surface act as sediment traps and natural barriers to surface runoff".<br />
Thus management practices which reduce sediment loss probably will<br />
not reduce the nutrient loss proportionately.<br />
ALTERATIONS IN NUTRIENT CONTENT OF SIRFACE SOIL AND HENCE OF SEDIMNT<br />
Carbon<br />
Crop production generally reduces the organic matter content of the<br />
surface soil. Because organic matter is critical in maintaining stable soil<br />
structure, a reduction leads to increased sediment loads in runoff from<br />
agricultural lands.<br />
81<br />
t
a2<br />
0<br />
m<br />
N<br />
Ln<br />
0<br />
N<br />
0
I<br />
Dependent Variable I<br />
TABLE 1<br />
PHOSPHORUS ENRICHMENT OF SEDIMENT FROM RUNOFF PLOTS<br />
AT GUELPH, ONTARIO AS A FUNCTION OF ENRICHMENT IN<br />
CLAY AND ORGANIC MATTER<br />
(6 EVENTS FROM 6 PLOTS - 1973-1975)<br />
Independent Variables I R2<br />
P Enrichment Clay Enrichment<br />
(Cl. Enrich. )<br />
Org. Mat. Enrichment<br />
(Org. Mat. Enrich. )<br />
0.61*<br />
0.64<br />
0.69<br />
0.70<br />
* R' - proportion of the variability in P enrichment accounted by for<br />
regression including this independent variable and all others<br />
preceding it in the table.<br />
Tillage<br />
Chisel<br />
Coulter<br />
Conventional<br />
TABLE 2<br />
EFFECT OF TILLAGE SYSTEM ON RUNOFF AND NUTRIENT<br />
CONTENT OF SEDIMENT<br />
(FROM ROMKENS. NELSON AND MANNERING, 1973)<br />
Runoff<br />
( cm)<br />
3.81<br />
4.39<br />
5.56<br />
1.68<br />
1.87<br />
24.74<br />
83<br />
I 1147<br />
2022<br />
1278<br />
616<br />
387<br />
308
Although it is difficult to increase the organic matter content of<br />
the surface soil on a sustained basis, temporary increases may occur from<br />
heavy applications of manure or sewage sludge, if they are not incorporated.<br />
Runoff from such fields would have a markedly higher carbon content, both in<br />
solution and in suspended particles during initial runoff events following<br />
application. Later runoff events would exhibit much lower differences as<br />
illustrated by the data in Table 3.<br />
Nitrogen<br />
Because nitrogen in soil is, for the most part, associated with the<br />
organic matter, the nitrogen and carbon contents of sediment will be closely<br />
related. Applications of fertilizer, manure or sewage sludge will temporarily<br />
increase the nitrogen content of surface soil. If these materials are applied<br />
to the soil surface during the late or fall winter and are not incorporated,<br />
the spring runoff will be appreciably higher in both dissolved and particulate<br />
nitrogen. If they are added at the times and rates most effective for crop<br />
production, there should be little increase in the nitrogen content of the<br />
runoff .<br />
Phosphorus<br />
The phosphorus content of the surface soil is modified by crop<br />
production practices to a much greater extent than either carbon or nitrogen.<br />
Most soils in the natural state are deficient in available phosphorus.<br />
Sustained production of high yielding crops requires that phosphorus be added<br />
to the soil to increase the available phosphorus level. This increases the<br />
total phosphorus content of the sediment and, perhaps more importantly it<br />
increases the labile phosphorus in particular. The influence of fertilizer<br />
phosphorus addition on the "extractable P" content of a soil is illustrated<br />
by the data in Table 4.<br />
Because phosphorus reacts in the soil to form only slightly soluble<br />
compounds, phosphorus applied to the surface is retained in the surface 1 to<br />
2 cm. thus increasing the available phosphorus content of this portion of the<br />
soil to a much greater extent than if the phosphorus were mixed throughout<br />
the plow layer (Table 5).<br />
The increase in "Extractable P" in the surface soil results in a<br />
similar increase in the "Extractable P" of the sediment and in the dissolved<br />
P concentration in the runoff water as illustrated by the data in 6, Tables 7<br />
and 8.<br />
The relation between dissolved P in runoff and the "Extractable P"<br />
in the sediment can be demonstrated €urther by data from runoff at plots<br />
Cuelph. For six runoff events from each of six plots during 1973-1975,<br />
"Extractable P" (0.5 N NaHC03) accounted for 55% of the variability in<br />
dissolved ortho-P in runoff. The data are plotted in Figure 2. It is<br />
apparent from Figure 2 that when manure is present on the surface, there is<br />
no relation between dissolved P and extractable P content on the sediment.<br />
However, in the absence of surface manure, the relationship is reasonably<br />
good.<br />
a4
I<br />
TABLE 3<br />
ORGANIC CARBON CONTENT OF SURFACE SOIL (0-1 CM)<br />
AND OF SEDIMENT FROM RUNOFF PLOTS AT GUELPH, ONTARIO<br />
%C<br />
Treatment Soil (0-1 cm) Sediment*<br />
No manure 1.78 2.52<br />
Manure** - on surface 1.98 2.77<br />
* Average of 9 runoff events 1973-1975<br />
** Applied each year since 1968 without incorporation<br />
TABLE 4<br />
THE INFLUENCE OF FERTILIZER P ADDITION ON "EXTRACTABLE P"<br />
CONTENT OF AN ONTARIO SOIL<br />
Fertilizer P Added<br />
(kg Plha17 yr)<br />
0<br />
75<br />
1<strong>50</strong><br />
300<br />
600<br />
* Extractable with 0.5N NaHCO 3'<br />
85<br />
"Extractable* P"<br />
(Pi? PIP)<br />
7<br />
9<br />
11<br />
20<br />
44
Depth<br />
( cm)<br />
0- 1<br />
1-2<br />
2- 3<br />
3- 4<br />
4-5<br />
5-7<br />
7-9<br />
9-11<br />
11-13<br />
13-15<br />
TABLE 5<br />
"EXTRACTABLE P" IN AN ONTARIO SOIL FOLLOWING<br />
YEARLY MANURE APPLICATIONS FOR 8 YEARS<br />
Manured<br />
Not incorporated<br />
110<br />
105<br />
95<br />
17<br />
63<br />
45<br />
31<br />
18<br />
17<br />
15<br />
* Extractable with 0.5 N NaHC03.<br />
TABLE 6<br />
Extractable P*<br />
Manured<br />
Plowed<br />
(Pg PIP)<br />
58<br />
52<br />
55<br />
47<br />
63<br />
68<br />
55<br />
65<br />
55<br />
45<br />
"EXTRACTABLE P" IN SOIL AND SEDIMENT AND DISSOLVED<br />
ORTHOPHOSPHATE CONTENT OF RUNOFF FROM PLOTS<br />
AT GUELPH, ONTARIO<br />
No<br />
Manure<br />
"Extractable P*"<br />
Soil (0-1 cm) Sediment** Dissolved**<br />
Ortho P in<br />
Treatment Range<br />
"-(~p/g) "-<br />
Manured - not incorporated 108 100-200 140<br />
Manured - plowed 57 <strong>50</strong>-95 63<br />
No manure 28 44-90 62<br />
* Extractable with 0.5 N NaHC03<br />
** Six storms 1973-1975.<br />
86<br />
(mgll)<br />
1.38<br />
0.56<br />
0.51<br />
28
I<br />
TABLE 7<br />
THE EFFECT OF PHOSPHORUS FERTILIZER APPLICATION ON<br />
"EXTRACTABLE P" IN SEDIMENT AND ON DISSOLVED ORTHOPHOSPHATE<br />
IN RUNOFF. (FROM ROMKENS AND NELSON - 1974)<br />
Extractable P* Dissolved Ortho P<br />
Fertilizer P Applied in Runoff in Sediment<br />
(kg/ha) (dg)<br />
(mg/U<br />
0<br />
56<br />
113<br />
14.6<br />
35.4<br />
57.6<br />
* Extractable by Bray P.l test (.03 N NHrF + .025 N HC1)<br />
TABLE 8<br />
0.07<br />
0.24<br />
0.44<br />
INFLUENCE OF METHOD OF FERTILIZER APPLICATION ON<br />
"EXTRACTABLE P" IN SEDIMENT (TIMMONS, BURWELL AND HOLT - 1973)<br />
Method of Application "Extractable PI'* in Sediment<br />
(VdP)<br />
No Fertilizer<br />
20<br />
Fertilized, plowed and disced<br />
20<br />
Plowed, fertilized and disced<br />
30<br />
85 Plowed, disced and fertilized<br />
* Extractable with Bray P. 1 test (.03 N NH4F + .025 N HC1)<br />
a7<br />
1
m<br />
2.0<br />
1.8<br />
1.6<br />
EL<br />
a - 1.2<br />
LC<br />
’c<br />
0<br />
c<br />
I<br />
.-<br />
c 0.8<br />
n.<br />
V<br />
al<br />
-<br />
2 0.4<br />
.-<br />
n<br />
0<br />
0. /<br />
a6b<br />
10<br />
0<br />
0<br />
0<br />
.<br />
1<br />
40 80 100 160 200<br />
Extractable P content of sediment<br />
(ppd<br />
0<br />
0 Surface Manure<br />
y = 0.029786~ - 0.000098~~ - 0.881201<br />
(r2 = 0.55)<br />
Figure 2: Relationship between NaHCO -extractable P of the sediment and dissolved P<br />
in the runoff at the exper?rnental plots in Guelph.<br />
0
From the above discussion it is apparent that application of<br />
phosphorus to soil will increase the labile P of the eroded sediment and also<br />
the dissolved orthophosphate of runoff water. However, when one attempts to<br />
relate dissolved orthophosphate in runoff to the Extractable P in the soil,<br />
the relationship is much less clear. The data presented in Figure 3 are from<br />
samples of runoff collected from farm fields in small two Ontario watersheds.<br />
The area in the field from which the runoff occurred was sampled and the<br />
"Extractable P" was determined. The data illustrates the marked effect of<br />
surface manure on the dissolved P levels. There is, however, no apparent<br />
relationship between dissolved P and extractable P in the soil, even when the<br />
samples from the manured fields are excluded. This is probably due, at least<br />
in part, to the variation from one runoff event to another in the degree of<br />
selectivity in the erosion process. The extractable P content will be greater<br />
in the finer soil particles. The degree of enrichment in clay and hence the<br />
enrichment of extractable P in the sediment will vary from one event to<br />
another as well as with management practices, it thus is not possible at this<br />
time to predict dissolved P in runoff directly from the extractable P level<br />
in surface soils.<br />
Profitable crop production requires that the available phosphorus<br />
level be increased in most soils which have not previously been fertilized.<br />
rhere is a level, however, above which no further economic increases in yield<br />
fill be obtained. This level varies from crop to crop. Generally, it is<br />
ligher for vegetable crops such as potatoes, and tomatoes, than for field<br />
:KO~S such as corn or barley. Thus soils that are used for vegetable crop<br />
xoduction are likely to have a higher available phosphorus content than are<br />
rhose used for field crop production. This is illustrated by data in Table<br />
3.<br />
There are a number of soil tests that can be used to determine the<br />
available phosphorus content of the soil and hence the fertilizer required<br />
€01- most profitable yield levels. They provide an effective tool to maintain<br />
the available phosphorus content of the soil at, but not above, the optimum<br />
level. This will assure continued production of high yielding crops while<br />
ninimizing the dissolved and total phosphorus content of runoff from agricultural<br />
fields.<br />
We must accept the fact that applications of nitrogen and phosphorus<br />
are essential to sustained production of profitable yeilds. To minimize the<br />
effects of these applications on the nitrogen and phosphorus content of<br />
runoff water we must improve our understanding of the nitrogen and phosphorus<br />
requirement of major crops and of fate the of nitrogen, particularly. when<br />
applied to the soil. This would allow us to better match the nutrient content<br />
of the soil to the needs of the crop, thus avoiding unnecessary levels<br />
in the soil and in runoff.<br />
We must also develop management systems which permit incorporation<br />
of manure and fertilizer into the soil rather than leaving them on the surface<br />
where they are much more subject to the erosional processes.<br />
a9
90<br />
w 1-<br />
I:<br />
00<br />
N U<br />
x c<br />
" C<br />
0 2 P<br />
0<br />
" C<br />
n a<br />
m a<br />
u 3<br />
L L m<br />
I<br />
al<br />
0<br />
0<br />
M O L<br />
I<br />
0<br />
0 % c<br />
r a<br />
a x<br />
U<br />
V I L<br />
c<br />
0<br />
- + .- c<br />
o m<br />
'c<br />
0<br />
0<br />
al<br />
a<br />
C<br />
m<br />
r<br />
al<br />
U<br />
m<br />
'c<br />
L<br />
3<br />
m
TABLE 9<br />
"EXTRACTABLE P"* LEVELS IN ONTARIO SOILS<br />
IN RELATION TO CROP GROWN<br />
Crop Optimum Extractable P**<br />
Average Extractable P***<br />
Mixed grain<br />
Corn<br />
Tobacco<br />
Potatoes<br />
Soybeans<br />
Tomatoes<br />
(Llg/g) (ug/g)<br />
14<br />
20<br />
115<br />
60<br />
15<br />
60<br />
1 1<br />
* Extractable with 0.5 N NaHCO 3<br />
** Test beyond which no further P required<br />
*** Samples submitted to Ontario Soil Testing Laboratory. July 1, 1974 -<br />
June 30, 1975<br />
91<br />
I<br />
12<br />
18<br />
64<br />
41<br />
18<br />
34
I, TTEM TUHE Cl TED<br />
Romkens, M. J. M., D. W. Nelson and J. V. Mannering, 1973.<br />
Nitrogen and Phosphorus Composition of Surface Runoff as<br />
Affected by Tillage Method, J. Envir. Qual., 2, 292-295.<br />
Romkens, M. J. M., and D. W. Nelson, 1974. Phosphorus Relation-<br />
ships in Runoff from Fertilized Soils, J. Envir. Qual.,<br />
- 3, 10-13.<br />
Timmons, D. R., R. E. Burwell and R. F. Holt, 1973. Nitrogen<br />
and Phosphorus Losses in Surface Runoff from Agricultural<br />
Land as Influenced by Placement of Broadcast Fertilizer,<br />
Water Res. Research, 9, 658-667.<br />
92
DISCUSSION<br />
DP. 7'. Logan: I would like to make a comment. The comment is that I think<br />
we have to be very careful about the emphasis we on put soluble phosphorus<br />
values coming off small watersheds or plots. We have to keep in mind that<br />
the adsorption reaction of phosphorus on sediment is a very rapid process.<br />
I have been doing some thinking on this regarding some of those soils that<br />
are high in available phosphorus. There are some possibilities that may<br />
be happening. One possibility is that on those soils that have appreciable<br />
available phosphorus, we might be seeing for example dicalcium phosphate<br />
that will be dissolved as we get a dilution during the runoff process.<br />
This will produce some of the soluble phosphorus that you are seeing. On<br />
looking downstream, you may see a readsorption phenomenon.<br />
Dr. 7'. Dickinson: You mentioned looking ahead to suggestions for remedial<br />
work, that one might be able to improve communication systems to get people<br />
to use better fertilizer amounts on their fields.<br />
Carrying this a step further, might it be realistic that<br />
in situations where one is applying manure or sludge, things that release<br />
nutrients more rapidly into surface runoff water, that it may be that in<br />
certain parts of watersheds there may be fields that are not very directly<br />
connected to the drainage and microdrainage system. There are fields that<br />
for only brief periods of the year might have runoff going into the drainage<br />
systems. In areas like that it might be very safe to carry on certain kinds<br />
of practices. There might be more opportunities to use a variety of management<br />
practices to be safer. On the other hand there may be parts of a<br />
drainage system near the main channel or very close to connected drainage<br />
systems where the management practices that would be allowable would be<br />
very restricted. In other words, one would have to be much more restrictive<br />
there. We mentioned that when you start differentiating between management<br />
practices on a watershed basis rather than just on a crop basis, there are<br />
going to be some problems telling the farmer over here that he can do these<br />
things and the farmer over here that he cannot. I see that as a possible<br />
way of looking to remedial measures not on just a crop basis or a soil<br />
basis, but even on a watershed basis and how that watershed may be receiving<br />
things downstream.<br />
Dr. M. MiZZer: Certainly in terms of fertilizer use we do use and apparently<br />
need to use much more phosphorus on some crops. We have to build the soil<br />
phosphate level up to much higher levels on some crops than others. If you<br />
could control the production of crops to those certain areas, it would help.<br />
I think that will be a major undertaking.<br />
93
Anthropogenic Influences of Sediment Quality at a Source.<br />
Metals<br />
G. K. Rutherford<br />
Erosion and sedimentation are natural, usually gentle, processes<br />
releasing controlled amounts of sediments to receiving waterbodies. Anthropogenic<br />
activities have burst into this process and caused far-reaching and<br />
mostly detrimental environmental changes, the extent of which has not yet<br />
been fully appreciated.<br />
Whereas the soluble ionic transport of chemicals is perhaps the<br />
most insidious and dangerous, the object of this paper is show to the myriads<br />
of ways in which sediment transported materials are damaging to our of way<br />
life. In particular I will emphasize the so-called heavy metals.<br />
BASIC ~TERIALS<br />
As most suspended particles have been soil materials in the<br />
immediate past, it would seem appropriate to consider some of the basic<br />
properties of soil materials.<br />
Soils consist of: (i) skeleton grains which form the virtually<br />
stable skeleton and are generally immobile and unreactive; (ii) plasma<br />
or the fine reactive and mobile parts; (iii) voids or the holes in the<br />
soil and (iv) water and e. Although plasma is considered to be the<br />
only solid material which moves intact through soil, the both plasma and<br />
skeleton may be elements in sediment transportation.<br />
The plasma is generally considered to consist of particles less<br />
than 2u in minimum dimension the and skeleton greater than 2U but in reality<br />
the upper size is about lop. In other words the plasma consists mainly of<br />
COLLOIDAL MATERIALS or materials possessing the properties of colloids -<br />
i.e. HAVING SURFACE CHARGES, usually negative. Not only the INORGANIC<br />
materials derived from rocks but also ORGANIC materials derived from the<br />
destructive microbial breakdown of formerly living organisms exhibit these<br />
properties. Virtually all finely comminuted materials of colloidal size<br />
possess, to some degree, this electric charge and strangely enough,<br />
amorphous materials both organic and inorganic appear to have the highest<br />
charges. The total charge, usually measured as ability to hold positively<br />
charged cations, is known as the CATION EXCHANGE CAPACITY and is this a<br />
specific property of each known compound and of major importance in the<br />
understanding of heavy metal transport in suspension.<br />
Soil materials, measured using conventional methods, exhibit a<br />
marked total positive electric charge although it has long been known that<br />
negative anions may be held or ADSORBED by soil materials. During the<br />
last 125 years many theories to explain this phenomenon have been put<br />
95
forward. Since about 1940, the theoretical background to anion holding<br />
processes has been modestly researched but since 1960 and particularly<br />
1970 the anion adsorbing powers of soils is attracting a wider circle of<br />
interested workers and a new pattern of thinking appears to be developing.<br />
Soil materials art said to have the property of IONIC EXCHANGE:<br />
in other words the adsorbed ions may be exchanged by other ions and there<br />
appear to be three major criteria determining if an ion will be held or<br />
exchanged on a colloidal body. Most heavy metals go into solution as<br />
individual cations, complex anions, or cations in a variety of forms.<br />
These parameters are generally considered to be: ionic charge, ionic radius<br />
and degree of hydration. Thus, in soils, cations tend to be bonded by van<br />
der Waals-type forces to the "parent" colloid whilst anions generally are<br />
more labile and move through and out of the soil body with drainage waters.<br />
The concentrations of POb, SOq, NOJ and C1 in lake and other water bodies,<br />
the causes of eutrophication, are a direct result of this.<br />
SOIL FORMING PFWESSES<br />
If, indeed, sediments have passed through a soil stage it is<br />
then appropriate to examine briefly the ramifications of soil forming<br />
processes for sedimentary reactions. Amongst other things, the soil<br />
forming processes tend to:<br />
(a) produce finer inorganic and organic particles - i.e. more<br />
colloids;<br />
(b) form neoformations.<br />
(i) Inorganic. These are formed by the association of ions in solution<br />
to form a new insoluble material:<br />
2M + xSi + yo -f [M Six OY]- gives new silicate minerals or<br />
z J<br />
more simply Mg + Ca + Cor + CaMgC03.<br />
If these new formations are of colloidal dimensions they will acquire a<br />
negative charge. However there is a very important exception to this and<br />
that is the sesquioxides:<br />
Fe + OH [Fe(OH) 31'<br />
or A1 + OH<br />
4<br />
(Al(0H) 31'<br />
4<br />
and there are all manner of variations over the above theme inasmuch as<br />
the final product may have a very variable composition. The same process<br />
involves such elements as Mn, Cr and V. These neoformations, particularly<br />
in the case of Fe, give soil its characteristic range of colours from the<br />
tropics to the Arctic. The sesquioxide neoformations are characteristically<br />
POSITIVELY charged and amorphous in nature when formed. With the passage<br />
of time, these become crystalline and change their charge slowly to negative<br />
and may become quite strongly negatively charged. The origin of this<br />
negative charge in soils is thoroughly explored and known. The process of<br />
positive adsorption on to silicate colloids is still largely speculative.<br />
96
(ii) Organic. The soil forming processes tend to destroy recently dead<br />
plant and animal tissue and form, amongst other things, strongly complexed<br />
compounds with metallic cations. The chelates with EDTA* are perhaps the<br />
best known and understood and occur in ionic and colloidal dimensions.<br />
These compounds commonly enhance the passage of elements such as Zn, Cu,<br />
Fe through soils and are said to SEQUESTER these elements. When these<br />
organic compounds are broken down by microbial attack the metals become<br />
available for recomplexing or precipitation. In the sequestering process<br />
the metal may simply be enmeshed or surrounded by the rest of the longchain<br />
molecule(s).<br />
(iii) Weak combination bonding. In the generation of neoformations some<br />
metal cations by virtue of size, charge or hydration may be "dragged"<br />
(seduced?) into a loose association with another compound such as iron<br />
hydroxide (oxide). Although this bonding may be loose, the heavy metal<br />
elements are strongly occluded into the newly formed compound. Our colleagues<br />
in exploration geochemistry called this SCAVENGING. Whereas the<br />
process is by no means as simple as I have implied, it is extremely<br />
important in the concentration of many elements in compounds of other<br />
elements. The examples of Cr and Co although at different ends of the<br />
scale are essentially similar. The concentration of Cr in laterites is<br />
thought to be due to this process.<br />
In order to understand the concern about heavy metals, reference<br />
must be made to the nutrition of animals and plants. N, P, K are known as<br />
MACRONUTRIENTS and must be fed to plants at the rate kg/ha; of 1 Ca, and<br />
Mg are intermediate whilst Zn, Co, Cu, I, F, B, etc., are the so called<br />
trace or MICRONUTRIENTS and are fed to plants as g/ha. The heavy metals<br />
fall in the micronutrient category and many of these are essential for<br />
plant, animal and human growth. What is one group's food is another group's<br />
poison. Some trace elements are essential for plant growth in general whilst<br />
these may not all be essential for human growth and vice versa. Na is a<br />
good example of one of these. The trace (heavy) metals are essential for<br />
one or many metabolic functions in plants and/or animals. Fe is essential<br />
for blood formation, Mg is needed for chlorophyll, is V part of cholesterol<br />
synthesis and Cr is needed for glucose synthesis. Deficiency in B causes<br />
cauliflower to malform and deficiency of Mo inhibits the nitrogen fixing<br />
bacteria, causes white muscle disease in fowls and probably plays a role<br />
in multiple sclerosis in humans.<br />
It is a common understanding that deficiency diseases are a<br />
function of a lack of a nutrient in the growth medium, however, it is<br />
equally likely to be a function of ION ANTAGONISM by which process the<br />
excess of one element may cause a deficiency of another. Zn appears to be<br />
antagonistic (and vice versa) to Ca, Cu, Se and Cd so that excess amounts<br />
of this element in industrial waste waters will cause deficiency diseases<br />
associated with the other elements. Vegetables in British Columbia in the<br />
same area showed differences of element content of <strong>50</strong>0 up to times, showing<br />
extreme differences in microenvironmental conditions.<br />
* Ethylene diamine tetra-acetate
Low pH in soils may cause AI, which is normally very stable<br />
geochemically, to go into solution. At a pH less than 3 one is measuring<br />
pAl rather than pH. A1 is extremely poisonous to plants and animals as<br />
witnessed by the failure of jute in Guyana.<br />
If heavy metals are transported by sediments and deposited by<br />
irrigation waters, as flood waters, in channel and stream bottoms, it is<br />
not unlikely that the new environment may give rise to conditions which<br />
divorce the element from its parent sediment and allow it to circulate in<br />
soil or plant nutrient solution where its ramifications and mischief may<br />
be far-reaching.<br />
ANTHROPOGENIC SCURCES OF HEAVY METALS (AND PROCESSES AFFECTING THEIR<br />
DISTRIBUTION IN SEDIMENT TRANSPORT)<br />
Some of the major sources of pollutants and their relationship<br />
to sediment transport will now be briefly discussed. The role of the<br />
transport medium and the special influence of the source will be brought<br />
out. The following man-sponsored sources of environmental pollution are<br />
perhaps the best known.<br />
Sewage, sludge and effluent. (Human metabolic processes)<br />
Animal metabolic processes.<br />
Domestic waste disposal.<br />
Industrial waste disposal.<br />
Agricultural plant protection + fertilization.<br />
Urban expansion.<br />
Industrial extraction processes; Smelting and Mining.<br />
Precipitation.<br />
Automobiles.<br />
Case study - Mercury.<br />
Human metabolic processes. The greatest uptake of heavy metals in<br />
the human intestine occurs at the end of the alimentary tract. The<br />
metabolic need for these is quite low hence human feces tend to concen-<br />
trate these elements. In the sewage disposal process the more soluble<br />
elements (geochemically) such as N, P and K tend to be in solution in '<br />
urine and the heavy metals attached as finely adsorbed organic colloids.<br />
In a secondary treatment plant much of this material and a of portion the<br />
soluble materials is precipitated out by the use of electrolytes. This<br />
SLUDGE is removed and not uncommonly used on agricultural land, primarily<br />
as a source of organic matter and P. In an untreated sewage system it is<br />
abundantly clear how effective sediment is in concentrating and carrying<br />
heavy metals.<br />
Sewage sludge has been shown to have many admirable properties<br />
for plant growth - a common limiting factor is the plugging of voids in<br />
the soil and the consequent increase in runoff which in turn results in<br />
re-entry to the waterways with sediment-borne pollutants.<br />
98
(b) Animal metabolic processes. The many stomach forms of the different<br />
domestic animals result in slight variations of the same effects as in<br />
(a) above but in general the same tendencies hold. The soil, and particularly<br />
soils with well developed A horizons have an admirable ability to<br />
adsorb and process animal manures of a carrying capacity 200 up times to<br />
that of common mixed-agriculture. However feed-lot agriculture is based<br />
on carrying capacities far higher than these. Many if not most feed lots<br />
are sited along or close to waterways. The surface soils soon become<br />
trodden and impermeable so that virtually all runoff from the lot empties<br />
into the waterway. Well fermented cattle manure from the ruminant stomach<br />
must provide about the best imaginable source of transportable sedimentborne<br />
heavy mineral pollutants. Most of the recorded fish kills are<br />
likely primarily and indirectly a function of excess dissolved nutrients.<br />
(c) Domestic waste disposal. Most domestic waste is still entered in the<br />
soil: in the case of incineration pyrolysis and other "refining" processes<br />
a treated product is still placed in earth materials. The many studies<br />
which have been done on the composition of municipal garbage show the<br />
extreme range of composition from place to place and even from day to day.<br />
However a great amount of heavy metals, in an unsuspected variety of<br />
forms, from additives to paper, tires and paint, through the agencies of<br />
microorganisms and decomposition processes may come into solution as<br />
simple or complex ions and gain access to the groundwater and become<br />
potential hazards. However, the passage and transport of these ions is<br />
affected by sediment transport under only two conditions:<br />
(i) when the entombing material is very coarse so that water may<br />
move relatively unimpeded through it, and<br />
(ii) during the process of infilling when the spoil material may<br />
be washed by rain or runoff, by sediment transport may remove<br />
certain heavy metal cations particularly in readily available<br />
form such as battery acid and metal pickling solutions.<br />
(d) Industrial waste disposal. Industrial waste disposal concerns industries<br />
which discard waste in: (i) liquid form or (ii) solid form. In<br />
general the same factors play their role in breakdown and transport as<br />
has been described above. Any industrial waste which carries finely<br />
divided materials, be it silicates or iron filings, will operate more or<br />
less as a colloidal system. The nature of the colloidal "host" will be<br />
the determining factor in how long and how effectively the heavy metal<br />
element is bound in sediment or is liberated. Host colloids which are<br />
broken down by organisms either in an oxidizing or reducing environment<br />
will liberate the heavy metal in a potentially hazardous form at an earlier<br />
stage than otherwise.<br />
Two host colloids worthy of note in this connection are: (i)<br />
tailings from ore refining processes and crushed products of coal, cement<br />
and (ii) fertilizer industries, although the same conditions will be<br />
obtained in any refining process which uses finely comminuted material.<br />
At Sudbury, for instance, The <strong>International</strong> Nickel Company<br />
produces some 35,000 tons of tailings per day which are fed into large<br />
tailings lakes. The average size of these tailings is about 3011 and thus
would have insignificant colloidal properties. Although the tailings are<br />
relatively high in some heavy metals they would not constitute a solution<br />
hazard should they accidently get into waterways. The surface mining of<br />
coal and indeed the crushing of deep-mined coal produces quantities of<br />
colloidal-sized, chemically neutral materials which are not uncommonly<br />
exposed to surface runoff waters. Not uncommonly, rock minerals in associated<br />
strata contain sulphides of Zn and other heavy metals. It is not inconceivable<br />
that the host colloidal materials may partake in the transport of heavy<br />
metals to some degree. Whereas there is an increasing volume of literature<br />
on the ionic solution of heavy metals from such sites, there is, at least<br />
to this author virtually no known literary material on the sediment transport<br />
of heavy metals from such areas.<br />
(e) Agricultural plant protection and fertilization. Most biocides are<br />
soluble and sprayed as solutions or, in fact, with colloidal suspensions<br />
such as oil or clay as the host colloids. .Those biocides which have a heavy<br />
metal as the primary killing agent or a host for a series of chlorohydrocarbons<br />
(anion), will be transported in erosional processes in the same manner<br />
as has been outlined earlier for other sources. The ease of microbial breakdown<br />
releasing the heavy metal will determine the degree of environmental<br />
hazard. Exchange relationships on inorganic colloids will be a similar<br />
deciding factor.<br />
Although N, P and K are the primary nutrients used in agriculture<br />
a series of "tailor-made" fertilizers are produced for many crops. Those<br />
fertilizers in which heavy metals are concentrated either intentionally or<br />
accidently during the manufacturing process will present potential hazards<br />
for waterways as a result of erosional activities.<br />
(f) Urban expansion. It has been convincingly shown that in many environments<br />
urban construction including highways, airports, etc., is the greatest<br />
source of sediment in waterways. As the A and B horizons are the major<br />
soil horizons exposed to erosion and as much of the land used for urban<br />
expansion has been prime agricultural land it is not unlikely that the<br />
eroded sediments carry significant amounts of adsorbed heavy metal cations<br />
bound to both organic and inorganic host colloids. As these sojl horizons<br />
are highest in potentially electropositive colloids it can be surmised<br />
that these sediments are highest in both complex and simple anions of<br />
heavy metals.<br />
As the area of urban roofs and asphalt parking lots steadily<br />
increases so must the colloid content of storm water runoff. In arid<br />
regions and in dry parts of the year in humid areas the fine dust content<br />
of cities increases strikingly. At the same time our affluent society<br />
has more careless spills of oils and solids which provide sources of heavy<br />
metals.<br />
(9) Industrial extraction processes. The smelting process if unchecked<br />
puts into the atmosphere SOz, N02, other similar gases and the stable<br />
oxides and sulphides of heavy metals as particulates. The latter fall to<br />
the soil surfaces or are swept down in the smoke plume at greater or<br />
lesser distances. For example, the concentration of these near the old<br />
smelter at Coniston is high, whilst the concentration from the 400 m new<br />
stack at neighbouring Sudbury is spread out a greater distance. Modern<br />
100
smelting methods helped by modern legislation have seemed to reduce the<br />
access of active particles to colloids for transport. The future danger<br />
will be more from dissolved ionic materials than from transported materials.<br />
However, in the past, the smoke plumes and precipitation killed vegetation<br />
and have bared the soil surface so that active erosion could take place.<br />
The less refined pollutants landing on the surface would be adsorbed first<br />
to organic colloids and transported by waterways. Cation exchange processes<br />
may then obtain and cause heavy metal cations to become available as<br />
poisons or growth inhibitors.<br />
(h) Precipitatg. Although precipitation has long been known to be far<br />
from pure near cities and industrial areas it only is recently that detailed<br />
studies of the amounts of metals being deposited have been undertaken. It<br />
is perhaps in Norway that the most prolific information is available. In<br />
recent years, pollution from rainfall has driven salmon and trout from<br />
most rivers in the south of the country. The primary effects of this<br />
precipitation in most places in North America will be found in solution in<br />
rivers and streams and in vegetation changes. This factor, although<br />
important, will have little apparent influence on sediment transportation.<br />
However in more arid regions with marked dry seasons, this precipitation<br />
factor may play an important if not critical role due to heavy metal<br />
transpcrtatior, ty sediments.<br />
(i) Automobiles. The addition of lead tetraethyl as an antiknock compound<br />
in gasoline has occurred since the early 1930's. A great deal of research<br />
has been done on soils and vegetation along highways. Most of the research<br />
has produced a self-evident result -- the soils are high in adsorbed lead.<br />
Notwithstanding the possible high lead contents of vegetables, this lead<br />
has remained rather stable. Only when road extensions and widening take<br />
place does erosion remove the lead adsorbed on colloids. Two other previously<br />
unconsidered elements are Cd and Zn in automobile tires: both elements have<br />
been found in conspicuously high amounts near highways. The extensive use<br />
of de-icing salts may cause cation exchange reactions which liberate, temporarily<br />
at least, these three elements in ionic form. Lubrication oils have<br />
also been found to have significant amounts of both Cd Zn. and<br />
FUTURE RESEARCH NEEDS<br />
Naturally my suggestions will be chauvanistic. However in<br />
addition to the present data collecting research efforts which must continue,<br />
I would submit that the following are worthy of support.<br />
1. The nature, species and charge properties of suspension<br />
transported colloids.<br />
2. Phase relationships between adsorbed and solution ions in stream<br />
channels.<br />
3. The nature of the heavy metals and all ions being transported<br />
by sediments.<br />
4. Laboratory studies on negative (anionic) adsorption.<br />
101
May I, finally, have the temerity to suggest two future events are<br />
worthy of your interest which may affect your research efforts:<br />
1. Climatic change. There is a considerable volume of evidence to<br />
suggest that we may be approaching a period where almost violent climatic<br />
fluctuations may be the norm rather than the exception. This will present<br />
a whole new range of demands on the knowledge and experience of our sedimentologists.<br />
?.. How much longer can the strained soil colloids surrounding commercial,<br />
industrial and domestic garbage dumps continue with their life saving<br />
ionic exchange without being totally clogged? Many of these dumps are<br />
perched precariously near water bodies.<br />
REFERENCE LIST<br />
- A. Some Appropriate Texts<br />
Soil Conditions and Plant Growth, E. W. Russell, 10th Edition<br />
Longmans, London, (1973).<br />
Non-Agricultural Applications of Soil Surveys, R. W. Simonson,<br />
Elsevier, (1974).<br />
Trace Elements in the Environment, E. ,L. Kothny, American Chemical<br />
Society, (1973).<br />
Cleaning Our Environment - The Chemical Basis for Action,<br />
F. A. Long, American Chemical Society, (1969).<br />
The Natural Environment: Wastes and Control, R. F. Christian<br />
4.. Goodyear Pub. Co., Pacific Palisades California,<br />
(1973).<br />
Micronutrients in Agriculture, J. J. Mortvedc &., American<br />
Society of Agronomy, (1972).<br />
Organic Substances in the Environment I & 11, M. Schnitzel,<br />
Marcel Dekker, N. Y., (1972).<br />
Cleaning Our Environment - The Chemical Basis for Action, (ed.),<br />
F. A. Long, American Chemical Society, (1969).<br />
Vaxtekologi, M. G. Stalfelt, Svenske Bokforlaget, Stockholm.<br />
- B. Symposia and Conferences<br />
Trace Substances in Environmental Health, University of Missouri<br />
I (1967)<br />
I1 (1968)<br />
111 (1969)<br />
IV (1970)<br />
Agricultural Pollution of the Great Lakes Basin, - Water EPA Quality<br />
Office, 13028-C7/71, US Govt. Printer<br />
102
'The Chemical Investigation of Recent Lake Sediments from Wisconsin<br />
and their Interpretation, US EPA Water Pollution Control Series,<br />
16010EHR03/71.<br />
Second Research and Applied Technology Symposium on Mined-land<br />
Reclamation, National Coal Board, Kentucky, (1974).<br />
Environmental Protection in Surface Mining of Coal, US EPA<br />
Environmental Protection, Technology Series, EPA -<br />
670/2/74-093.<br />
Processes, Procedures and Methods to Control Pollution from<br />
Mining Activities, US EPA, 430/9.73.011.<br />
Land For Waste Management, (ed.), B.P. Warkentin, National Research<br />
Council, Ottawa, (1974).<br />
Municipal Waste Disposal - Problem or Opportunity, Ontario Economic<br />
Council, Toronto, (1971).<br />
Solid W&tes - 11, (ed.)., S. S. Miller, American Chemical Society,<br />
(1973).<br />
Wastes - Solids, Liquids and Gases, Chemical Publishing Co.,<br />
N.Y., (1974).<br />
Canada - Ontario Agreement on Great Lakes Water Quality:<br />
Research Report No. 2 Sludge Handling and Disposal Seminar, (1974)<br />
3 High Quality Effluents Seminar, (1976).<br />
28 Removal of Phosphates and Metals from<br />
Sewage Sludge, (1973).<br />
30 Heavy Metals in Agricultural Lands Receiving<br />
Chemical Sewage Sludges, (1975).<br />
33 The Removal and Recovery of Metals from<br />
Sludge and Sludge Incinerator Ash, (1976).<br />
35 Land Disposal of Sewage Sludge, Vol. 111,<br />
(1976).<br />
38 The Harvest of Biological Production as a<br />
Means of Improving Effluents from Sewage<br />
Lagoons, (1976).<br />
Subsurface of Disposal of Waste in Canada 111, R. 0. van Everdingen,<br />
Environment Canada Technical Bulletin 82, (1974).<br />
Soils in Environmental Protection, B. C. Dept. of Agriculture, 5th<br />
B. C. Soil Science Workshop Report, (1975).<br />
First Symposium on Mine and Preparation Plant Refuse Disposal,<br />
Coal and the Environment Technical Conference, Kentucky, (1974).<br />
Sediment in a Michigan Trout Stream, USDA Forest Service<br />
NC-59, (1971).<br />
Fish Kills Caused by Pollution In 1972, US EPA 440/9-73-001,<br />
(1973).<br />
103
Analyse av organiske mikroforurensninger i nedbdr, G. Lunde og<br />
Jdrgen Gether, SNSF Project, (ed.), F Braekke, Oslo, (1974).<br />
Avsyring av sure vann, A. Henriksen og M. Johannessen, SNSF<br />
Project IR5/75, (1975). (A literary review of acid precipitation).<br />
104
INTRODUCTION<br />
Regional Overview of the Impact of Land on Use Water Quality<br />
A. 1). MeEZroy<br />
Midwest Research Institute was given the task in mid 1974 of<br />
conducting a national assessment of nonpoint source pollution for the<br />
U.S. Environmental Protection Agency. The program had two phases. In<br />
Phase I, loading functions for estimation of nonpoint pollution loadings<br />
were developed. In Phase 11, these functions were employed to calculate<br />
pollutant loadings on a national basis. The loadings were calculated<br />
and reported for minor basins, major basins, the 48 states, and the<br />
continental United States. In addition, loadings were aggregated for<br />
each of the 10 federal (EPA) regions.<br />
We were asked to develop loading functions based on "available"<br />
data. This meant generally that the more sophisticated models under<br />
development were not appropriate, since these usually require very<br />
detailed information which is not available to the user. Further, such<br />
models require a sophistication in use which usually is beyond the<br />
capabilities of the personnel involved in nonpoint planning and to whom<br />
the functions were directed.<br />
A further constraint consisted of, in most cases, steering clear<br />
of determining the impacts of nonpoint pollution on water quality. We<br />
thus estimated delivered loads, but did not estimate the resultant impacts<br />
on water quality. It goes without saying that this represents a major<br />
void which must be fiiled if we are to develop a good picture of the<br />
impacts oE nonpoint pollution.<br />
PROGRAM DESCRIPTION<br />
The program included analysis of natural background nonpoint pollution,<br />
i.e., pollution obtained in the absence of modern man's influences.<br />
One can readily appreciate that such a state is not to easy define, nor is<br />
it a simple matter to develop natural or background loadings which can be<br />
rigorously defended. Nevertheless, activities in this area have yielded<br />
results which are interesting and one of the most informative comparisons<br />
involves background and the present disturbed state.<br />
The loading functions developed and used on the program relied<br />
heavily on soil loss from erosion as a building block. Soil loss can<br />
be described and estimated with reasonable accuracy by existing equations,<br />
for which a large body of data is in existence. If one assumes that bulk<br />
loadings (or total loadings) of nitrogen, phosphorus, and organic matter<br />
can be relatcd simply to soil concentrations of these pollutants, then<br />
105
one has a fairly powerful tool for calculating loads of ROD, total<br />
nitrogen, and total phosphorus from major nonpoint sources. This approach<br />
begins to fail (or lw less accurate) when eroded sediment is relatively<br />
minor, as in well managed forests or grasslands, particularly for soluble<br />
forms of nitrogen. The soil loss equations are also a powerful tool for<br />
estimating loads of heavy metals delivered in sediments.<br />
Other approarhes are required for numerous other sources, snch as<br />
feedlot runoff, leachnte from solid waste disposal, mine drainage, irrign-<br />
t ion return flow, and urban runoff. The paper will briefly present the<br />
approaches which were taken for such cases.<br />
I'he paper will, however, deal principally with thr results of<br />
the assessment, rather than on methods for calculating loads. As indicated<br />
above, the assessment was made for minor basins, major basins, and tlle<br />
48 sL:ites. :md has been summarized by region. Data will be presented<br />
which depict land use hy state and pollutant loads by state. Similar<br />
presentations will also be made for land<br />
10 federal regions.<br />
use and pollutant loads by the<br />
DISCUSSION OF RESULTS<br />
Onr of the most usrtul comparisons involves loads from an acre of a<br />
particular source. Some comparisons follow. Acre load of phosphorus from<br />
a background condition, averaged for the 10 regions, vary from about 0.2<br />
lb/acre/year to about 6 lb/acre/year. Loads from forests are about the<br />
same as background in regions which were heavily forested in the naturcil<br />
state, and are as little as about 15% of hackground in regions which we're<br />
sparsely forested in the natural state. Acre loads of phosphorus f1om<br />
cropland range from about L to 20 times tuckground with the greatest<br />
relative increases occt~rring in regions heavily forested in Lhe nat~~ral<br />
state. In arid and semi-arid regions where cropland is chiefly irrigdtt'd,<br />
cropland represents an improvement over background. Pasture and range<br />
acre loads are 1.5 to 2 times background in Regions VI-IX, and 3 tll 20<br />
times background in Regions I-V and X. Feedlots (no control assumed)<br />
give calculated acre loads ranging from about 20 to 2,000 times back$l,round.<br />
Uncontrolled feedlots are clearly less of an insult in both relativv md<br />
absolute terms in semi-arid regions of the country. Urban runoff c
impacts occur when a natural condition in a high rainfall area is<br />
extensively disturbed (as in construction) and that the same activity can<br />
have a small relative impact in arid and semi-arid regions.<br />
CONCLUSIONS<br />
Conclusions generally supported by the results are as follows:<br />
* It is immediately apparent that loads of sediment, nutrient<br />
elements, and BOD are proportional, as one might predict, to<br />
the extent that land has been converted from its natural<br />
state to a highly disturbed state. Agriculture is the<br />
predominant "disturbed" state, and thus in gross or overall<br />
terms is the predominant influence on nonpoint source emissions.<br />
X Comparisons of background loadings with as-is loadings show<br />
clearly that the greatest relative impact of man's activities<br />
occurs in parts of the country which were relatively nonpclluting<br />
in the natural state due to the existence of an<br />
excellent ground cover of grasses and forests, but which are<br />
by reason of climate and terrain highly productive agricultural<br />
lands. The corn belt accordingly has suffered the greatest<br />
__- relative increase in nonpoint pollution compared to background.<br />
This overall effect would appear to reflect the relatively high<br />
overall complex of man's activities in such regions, i.e.,<br />
urbanization and industrialization as well as utilization for<br />
agriculture.<br />
* Total loadings tend to be high in regions which are extensively<br />
committed to agriculture even though rainfall requirements for<br />
agriculture are somewhat marginal, i.e., the Great Plains, but<br />
the relative impact of man's activities (increase over background)<br />
is less in such regions because the "natural" state is<br />
more polluting than the natural state in higher rainfall areas.<br />
* As one proceeds to the arid- semi-arid regions (the West and<br />
Southwest) man's activities tend to have a positive effect<br />
(excluding secondary effects such as salinity in irrigation<br />
return flows), since these activities involve augmentation of<br />
rainfall and generation of improved ground cover conditions.<br />
'< While silviculture is a major land use, pollutant loadings<br />
on an overall basis are substantially less than for the other<br />
major land use--agriculture--because major land disturbances<br />
in silviculture are relatively minor in extent at any one<br />
time. However, the pollutant loadings from disturbed forest<br />
lands are high, and transitory local impacts are accordingly<br />
also high.<br />
* Construction, surface mining, small feedlots, and terrestrial<br />
disposal also turn out to be relatively minor in the overall<br />
sense, and the impacts would thus appear to be significant<br />
chiefly when viewed as localized impacts, which again can be<br />
quite high.<br />
107
+ National<br />
* Particularly noteworthy are regions in which man has endeavored<br />
to "use" land which has moderate to high rainfall and poor<br />
terrain. This effect is pronounced in the states such as<br />
Kentucky and Tennessee.<br />
* An i formative set of results was obtained in a study for<br />
P<br />
NCWQ in which effectiveness of pollution control in agriculture<br />
was estimated. The results demonstrate clearly that a relatively<br />
small proportion of land in agriculture is responsible<br />
for a significant fraction of the total load, and that benefits<br />
measured in terms of pollution reduction are fairly startling<br />
if this land is converted to uses which are judged to be "adequate"<br />
from a soil conservation standpoint. These results, together<br />
with other observations noted above, indicate that marginal<br />
land use is particularly critical from the environmental<br />
nonpoint source point of view.<br />
* Heavy metal loads delivered in eroded surficial soils and<br />
sediments are calculated to be major in quantity rather than<br />
"trace" as one might intuitively expect. We are not certain<br />
what lessons can be learned or what conclusions can be drawn.<br />
It is clearly evident that large quantities of heavy metals are<br />
delivered to surface waters, either from the natural or mandisturbed<br />
states of rural lands, and that these constitute a high<br />
background against which loads,from point sources should be<br />
viewed and interpreted.<br />
* The overall results of the assessment are rather crudely<br />
interpretable in terms of the basic physical/chemical characteristics<br />
of the land and water, and of climate. Perhaps the<br />
simplest example is mine drainage, which is a function of both<br />
the capacity of a region to generate acid and its capacity to<br />
neutralize the acid--the western coal mining regions have a<br />
high neutralizing capacity, while the eastern regions do not.<br />
Similarly, relationships between delivered loads of nutrients<br />
and BOD, and observed burdens of these same pollutants indicate<br />
that surface waters differ across the country in assimilative/<br />
use capacities. The relative proportions of these pollutants<br />
further indicate that eutrophication processes can be expected<br />
to follow a fairly systematic trend one as proceeds from low<br />
rainfall to high rainfall regions.<br />
* Finally, it is usually true that nonpoint loads of pollutants<br />
are substantially in excess of in-stream burdens. One concludes<br />
that it is imperative that a better understanding be developed<br />
of the nature of assimilation of these pollutants, including<br />
adsorption on sediments or trapping out in sediments, equilibria<br />
between sediments and water, chemical and biology processes,<br />
and transport processes.<br />
Committee on Water Quality.<br />
108
U,S, EPA REGIONS<br />
I. Maine, Vermont, New Hampshire, Massachusetts, Connecticut, Rhode<br />
Island.<br />
11. New ‘fork, New Jersey<br />
111. Pennsylvania, Delaware, Virginia, West Virginia, Maryland<br />
IV. Kentucky, Tennessee, North and South Carolina, Georgia, Mississippi,<br />
Alabama, Florida<br />
V. Ohio, Illinois, Indiana, Michigan, Wisconsin, Minnesota<br />
VI. Arkansas, Louisiana, Oklahoma, Texas, New Mexico<br />
VII. Iowa, Kansas, Missouri, Nebraska<br />
VIII.Colorado, Montana, North and South Dakota, Utah, Wyoming<br />
IX. Arizona, California, Hawaii, Nevada<br />
X. Alaska, Idaho, Oregon, Washington<br />
109
DISCUSSION<br />
Dr. D. Huff: One of the things that I have been interested in, particularly<br />
in some of the earlier discussions this morning, is the direction that I heard<br />
people taking from the larger to the smaller process oriented kinds of studies.<br />
Certainly there is a lot of detail involved in those things, but in effect<br />
what you have done is to go the other way; trying to summarize on the basis of<br />
existing information what one can say about a very large area and, in particular,<br />
your last comments about things like coefficients for metabolism; say<br />
organic materials in the stream. On a large scale this may be something you<br />
might comment on. Perhaps you could be a little more specific about the<br />
kinds of things that you saw as needs at that scale.<br />
Dr. A. McEZroy: Well, I don't know that those particular needs are unique to<br />
a large scale. I think they apply to whatever scale you happen to be working<br />
at. I think we have probably seen some rather gross manifestations of what<br />
these problems are, that is differences between burdens and loads, one that<br />
does not account for very readily. We probably just illustrated or manifested<br />
these questions, and I would think for the large areas you have essentially<br />
the same need for this kind of information you as do for small areas. You<br />
need much more specific information, I suspect, for small areas because you<br />
are dealing with a much more specific situation which you want to monitor<br />
precisely, and you don't necessarily need the kind of detail that you would<br />
need if you were analyzing a larger area.<br />
Mr. J. CuZbertson: Please explain to me what an acre burden is. You have<br />
thrown in this term burden. I can't qujte understand that in the terms you<br />
have used.<br />
Dr. A. McEZroy: I should have defined it earlier. We define a burden as it<br />
relates to the actual quantity of a pollutant which is in a stream. We<br />
define that as a burden rather than a load for our purposes. In a sense it<br />
is not different from a load, but we made that distinction because, in what we<br />
call the stream-to-source methods, you start with what is a burden, or load<br />
if you wish to call it that, in the stream and you try to work backward to<br />
the source. When it got along the way we found that the loads that we<br />
calculated going from a source to a stream method were considerably larger,<br />
so we simply developed this distinction between a burden and a load. Burden<br />
refers to that quantity which actually exists in the stream as detected by<br />
sampling the water. In this case, we call it an acre burden. It is a quantity<br />
of pollutant which exists in a stream which we attribute to an acre of source,<br />
as opposed to an acre load which is that quantity which is delivered to a<br />
stream from an acre of source.<br />
111
Dr. D. Baker: For all of your loading calculations you use a delivery ratio,<br />
and I am wondering to what point you are delivering these loads when you are<br />
adding up data for counties, states or regions.<br />
Dr. A. MeElroy: We developed a delivery factor which is based on stream<br />
densities and upon soil textures and these delivery factors were developed on<br />
a land resource area. In other words, we did not develop delivery factors<br />
which were specific to a county; rather, we assigned a delivery factor for a<br />
big region to a county. We are reluctant to publish our county loads you<br />
might say, or to pass them out because they have values assigned to them in<br />
a number of cases which are typical of the region, but not necessarily<br />
specific to the county.<br />
Dr. D. Baker: As I recall your delivery ratio graphs, they are not a function<br />
of area anyway. They are more related to drainage density, which is area<br />
independent. I can't see how you can come up with a delivery ratio that isn't<br />
related to the area of the watershed.<br />
Dr. A. MeEZroy: Well, I'll see if I understand your question. If you happen<br />
to have the drainage density any in particular county, and the number of<br />
miles of stream per square mile for example, this essentially is a drainage<br />
density. If you have that figure for the county, then this would be essentially<br />
a drainage density which would in effect depict you for the average<br />
distance to the nearest permanent stream for that county. I should think<br />
in that case that it would be specific for that area.<br />
Dr. D. Huff: I think that what he is asking is "did you look at the total<br />
load delivered say at the outlet, wherever that is for the whole region,<br />
and then normalize that?" In other words, to get a per unit area you had to<br />
have an area to divide by.<br />
Dr.. A. McEZroy: Well as I said our basic unit for making the calculations<br />
was the county, however, we did use for specific sources, the acreages of the<br />
various categories of land use in that area. We would apply our delivery<br />
factor to whatever acreage we happened to come up with. I have a feeling you<br />
are not quite satisfied yet.<br />
Dr. 5'. Yaksieh: I notice you reported some of your data to 8 about significant<br />
figures. Do you really have that much confidence in it?<br />
UP. A. McElroy: It is hard to teach the computer to do otherwise. Heavens,<br />
no !<br />
Dr. S. Yaksich: What type of error estimates would you place on the numbers<br />
you are producing?<br />
112
at2. (1. hI,-!'~rY,'~t; EPA asked us to develop error analysis for our loadings.<br />
We asked some of our people to do this and they said there were not enough<br />
statistics available. We did it and if you want to consider all the possible<br />
errors, they are quite large; say <strong>50</strong>% if you want to consider enough factors.<br />
However, I think that without being able to prove it, generally we are<br />
talking about perhaps 202, and one of the reasons is that we are dealing<br />
with fairly large areas with a considerable amount of statistics which have<br />
been generated for these areas and I would rate them at about 20-25%. Now<br />
that is going to vary. For some of OUT pollutants from feed lots, for<br />
example, we used ranges of concentrations of pollutants in feed lot runoff<br />
which would range all the way from very nearly zero up to a couple of thousand,<br />
so pick a number in the middle of them. We had to For this kind of<br />
study.<br />
Dr. S. Yuksich: The reason why I asked is some of your loading functions<br />
were used by another study done by the National <strong>Commission</strong> on Water Quality<br />
in the Lake Erie drainage basin by Dalton, Dalton & Little. Some comparisons<br />
of the loadings that they got of total phosphorus coming into Lake Erie in<br />
comparison to the type of numbers that we generated from a stream sampling<br />
program at about 600 samples during the course of a year for each stream,<br />
showed their estimates were about double ours coming out of the streams.<br />
Dr. A. McBZroy: I guess I wouldn't expect the error to be that bad. You<br />
have to bear in mind that these are average numbers that we are using and any<br />
one particular year can be very much in deviation from an average year. This<br />
is one thing you need to take into consideration. I think that if you were<br />
to accept these loading functions in a sense that they are the average and if<br />
you are looking for an average condition, then probably they should for the<br />
most part be reasonably good. If you were to consider the deviation year to<br />
year and try to use an average number you might find out they would be<br />
considerably in error for a particular year.
Influence of Flow Characteristics on Sediment Transport<br />
with Emphasis on Grain Size and Mineralogy<br />
J. Culbertson<br />
The transport of sediment by water has been studied and documented<br />
since at least 1808 (Inter-Agency Committee on Water Resources, 1940).<br />
Although not documented, canal builders in India, Afghanistan, and other<br />
countries must have learned much about the subject (see Bibliography).<br />
All of those early studies were aimed at learning processes of sediment<br />
transport that related to the engineering aspects; that is, related to the<br />
building of structures and water-conveyance systems. Objectives wLre<br />
related mostly to determination and prediction of the volume of sediment<br />
being deposited in or removed from channel beds, or transported into<br />
reservoirs. Theoretical studies were related to the mass and volume of<br />
mineral sediments -- those sands and gravels that continuously to act<br />
change and modify river systems.<br />
Also, during the nineteenth century other engineers were studying<br />
the sanitary aspects of sedimentation as a result of demands for clear<br />
water piped to homes and sanitary sewer systems piped from homes. Their<br />
needs for knowledge also related more to mass and volume than anything<br />
else; however, they recognized that sediments were not all mineral.<br />
Environmental concerns over the past decade particularly, have made<br />
us more aware of the problem related to the sorption and transport of chemical<br />
constituents on sediments. With the recognition of this fact, sediment, very<br />
quickly was 1-abeled a pollutant. We now find that water chemists, aquatic<br />
biologists, sanitary engineers, geomorphologists, and sediment engineers<br />
must talk to each other - the one individual who can understand all of these<br />
different specialists calls himself or herself an environmentalist, or<br />
perhaps an ecologist. The purpose of this paper to is present a brief<br />
description of the processes that govern the transport of sediment by<br />
water.<br />
The term sediment must be defined in terms of current needs.<br />
Sediments (fluvial) are solid materials that have a specific gravity greater<br />
than 1.00 that will settle in a column of quiescent water due to the gravitational<br />
force. Sediments found in the fluvial environment can be either<br />
mineral or organic materials. Table 1 defines the kinds of materials,<br />
physical sizes of materials, and their general mode of transport in a<br />
flowing stream.<br />
11 7
TABLE 1<br />
KINDS OF MATERIALS<br />
Sediment Size Class Mode of Transport<br />
Boulders Larger than 256 m Bedload<br />
Cobbles 64-256 mm Bedload<br />
Gravel 2-64 TDUI Bedload<br />
Sand 0.062-2 UIU Bedload or Suspended<br />
Silt 0.004-0.062 UUU Suspended<br />
Clay 0.0002-0.004 TDUI Suspended<br />
Organic detritus<br />
Includes leaves, trees,<br />
biological remains, etc.<br />
Bedload or Suspended<br />
Biota Bedload or<br />
Includes bottom dwelling<br />
organisms<br />
NOTE: The size classes of mineral sediments shown are based on the<br />
Wentworth scale (Lane 5 &.. 1947).<br />
118
The mass of sediment being transported in suspension is called<br />
suspended load. The mass of sediment being transported in virtually<br />
continuous contact with the streambed is called bed load. The transport<br />
rate of suspended load is the suspended-sediment discharge, and the transport<br />
is expressed in terms of mass per unit time of (kilograms per second,<br />
megagrams per day, etc.). The range in size and specific gravity of<br />
sediments transported for both modes of transport is tremendous.<br />
The environmentalist faces a real problem in sorting out the<br />
definitions of terms that have been coined by the various disciplines over<br />
the years. Suspended sediment also is called suspended solids, nonfilterable<br />
solids, silt, turbidity, etc. Bedload materials are called bed material,<br />
bottom material, deposited sediment, benthic material. substrate, etc.,<br />
depending on the background of the individual. The definition of "solution"<br />
also enters the picture. Solution is defined by most water chemists as<br />
the liquid that passes through a 0.45 Dm (micrometre) filter. By this<br />
definition, a solution can include particles that the engineering profession<br />
classifies as fine clay and colloids.<br />
Another problem that relates to measurement and definition of<br />
fluvial sediments is that there is a continuous interchange of some<br />
sizes of sediment between the streambed and &he streamflow. Sediments in<br />
suspension may settle into the bed mve and for some period of time as<br />
bed load, or they may remain at rest until moved back into suspension<br />
for another period of time. Even the very fine silts and clays are<br />
involved in this process, particularly in reaches of sand bedded streams<br />
where there is a loss of flow from the stream to the ground water system.<br />
Zn this situation, fine materials can infitrate the sand bed to a considerable<br />
depth.<br />
In order to standardize analytical methods, sedimentologists have<br />
related particle size of sediments to quartz spheres (Figure 1). One reason<br />
is that most mineral sediments carried by streamflow consist of roughly 92-98<br />
percent of particles having an average specific gravity 2.65. of Therefore<br />
2.65 is used to represent all sediment carried when computing sediment discharge.<br />
Sedimentation diameters are used to define size classes for fine sediments<br />
because of the relative ease of analytical techniques. Size, shape, specific<br />
gravity, and viscosity of the water effect the sedimentation rate, and a<br />
small, heavy grain may have the same mass and fall velocity as a larger quartz<br />
grain.<br />
Figure 2 illustrates the basic principles of sediment transport.<br />
The velocity with depth in the general case is illustrated. The resistance<br />
at the bed is caused by the drag of the liquid over the particles, and that<br />
drag and turbulence of the flow causes the bed materials to move along the<br />
bed or to jump into suspension for unpredictable periods. The upward velocity<br />
vector must overcome the gravitational force on a particle in order for it<br />
to remain in suspension. Turbulence is greatest near the bed; therefore, the<br />
coarsest particles are carried near the bed. Finer sediments, generally finer<br />
than 62 Dm (silt and clay size by definition), require little turbulence<br />
to maintain them in suspension, and therefore, are transported throughout<br />
the depth of flow in about the same concentration. Concentration is the term<br />
used to mean the mass of sediment per unit volume of the water-sediment mixture.<br />
The discharge rate of suspended sediment varies generally with depth as<br />
illustrated by the concentration variation with depth.<br />
119
In any given fluid at any temperature<br />
VfP VfP Vf s<br />
If SGp = SGs and VIP = VIS<br />
then D = SEDIMENTATION DIAMETER of particle.<br />
If in distilled water at 24°C.<br />
9 Tq P D<br />
then D ~ STANDARD SEDIMENTATION DIAMETER.<br />
vfpl vlp2 vlp3 VIS<br />
1- . I<br />
If Vfpl, Vfp2, Vfp3 7 VfS = STANDARD FALL VELOCITY<br />
then D = STANDARD FALL DIAMETER<br />
Figure 1. Relating standard sediment dimeters<br />
to diameter of quartz spheres.<br />
120
' I .<br />
Depth Velocity<br />
of<br />
Figure 2. Schematic diagram of velocity and suspended-sediment<br />
concentration distribution in a stream vertical<br />
121
122
c1<br />
N<br />
w<br />
mg/ 1 at surface = 880 935 910 91 5 91 0 860<br />
mean in vertical = 941 930 942 920 932 907<br />
DISTANCE. IN FEET<br />
Water discharge = 1280 cubic feet per second<br />
Mean velocity = 4.41 feet per second<br />
Median diameter of bed materlal = 0 33 rnm<br />
Figure 4. Distribution of silt and clay (finer than 0.062 mm) in cross<br />
section of Rio Crande conveyance channel near Bernardo,<br />
New Mexico
I-<br />
*<br />
N<br />
c<br />
W<br />
w<br />
LL<br />
z<br />
x<br />
I-<br />
a<br />
W<br />
0<br />
C mg/ 1 at surface = 640 770 690 880 660 3<strong>50</strong><br />
mean in vertical = 1220 13<strong>50</strong> 1400 1600 1420 1190<br />
DISTANCE, IN FEET
Figure 2 also illustrates the sampling problem. Currently available<br />
suspended-sediment samplers sample only to a point from about 0.3 to 0.5 feet<br />
above the bed. It is evident that these samplers do not sample that part of<br />
the flow carrying the greatest concentration of coarse particles.<br />
Figures 3, 4, and 5 illustrate data based on actual field measurements<br />
of velocity and suspended sediment made in the Rio Grande conveyance<br />
channel near Bernardo, New Mexico (Culbertson 5 &., 1972). The conveyance<br />
channel is straight and uniform for several thousand feet. Point<br />
velocities and point suspended-sediment samples were obtained at five<br />
points in each of six verticals in order to define the isovels and zones<br />
of equal concentration of various size classes of sediment. Concentration<br />
values shown are in mg/l (milligrams per litre).<br />
Velocity distribution (Figure 3 ) was found to be quite variable<br />
because of the roughness caused by the dune bed conflguration. Note the<br />
four distinct cells of high velocity in the cross section. Also, note<br />
the slower velocity along the right bank.<br />
The finer sediments (silts and clays) were distributed almost<br />
uniformly throughout the cross section (Figure 4 ). Concentrations of<br />
samples collected at the surface were almost the same as the mean concentration<br />
in each vertical; therefore, within the limits of accuracy<br />
obtainable in most laboratories, a sample collected almost anywhere in<br />
this cross section would be considered representative for this size<br />
range.<br />
Figure 5 illustrates the distribution of the coarse suspended<br />
sediment (sand). It is obvious that the sampling problem in this case is<br />
real. If one wishes to analyze a sample of the streamflow to determine<br />
either the mass of sediment being transported or the amount of sorbed<br />
constituents on the sediments, one must have a representative sample that<br />
contains representative masses of each size class of material.<br />
The preceding discussion has related only to the general case,<br />
and the instantaneous steady uniform flow condition. Time variation of<br />
sediment transport presents another problem. Figure 6 illustrates the<br />
general relation between water discharge and suspended-sediment discharge.<br />
For a given site, it is not uncommon to find a variation in suspendedsediment<br />
discharge of about 1.5 log units for any given water discharge.<br />
This variation is caused mostly by: (1) seasonal variations in availability<br />
of sediment; (2) snowmelt runoff versus thunderstorm runoff; (3) period<br />
between floods; (4) and the difference between turbulence characteristics<br />
on rising and falling stages.<br />
Figure 7 shows the typical variation of sediment concentration<br />
with time and discharge. The differences in concentrations for a given<br />
gage height (discharge) between the rising and falling stages can be<br />
noted.<br />
In summary, the variation in transport rates of sediment in streams<br />
is dependent upon the turbulent characteristics of the flow and the avail-<br />
ability, size, and specific gravity of sediment in the system. Sediments<br />
125
1 O(<br />
la<br />
1.0<br />
0.1<br />
WATER DISCHARGE (cfs)<br />
Figure 6. Direct runoff versus sediment discharge, by day, Pigeon Roost<br />
Creek Watershed 5, .January 1957-December 1960. After Piest<br />
(in Proceedings, 1963, p. 102).
can be transported either in suspension or as bed load; however, there can<br />
be a continuous interchange between the streamflow and the channel bed.<br />
The coarser sediments require more turbulence to maintain in them suspension,<br />
and therefore, are found in greater concentrations near the<br />
stream bed. Much greater care is required when sampling streams carrying<br />
coarse sediments (sands) in order to obtain a sample that represents the<br />
streamflow.<br />
The variability of sediment concentration with water discharge is<br />
great, and many samples may be required to define the suspended-sediment<br />
discharge during floods. Variability with time also is great, and<br />
samples must be obtained throughout the year to define sediment discharge<br />
variation due to changes in land use (availability) and seasonal effects.<br />
SELECTE3 LITERATURE<br />
Abbett, R. W., (1956). American Civil Engineering Practice. V.1,<br />
sec. 2(John Wiley).<br />
An Appraisal of Water Pollution in the Lake Superior Basin. FWPCA<br />
Great Lakes Adm.. April 1969.<br />
Anderscn, A. G., (1942). Distribution of Suspended Sediment in a<br />
Natural Stream, Am. Geophys. Union Trans., 2, (Pt. 21,<br />
678-683.<br />
A.S.T.M. Designation: C136-38T, (1938). Tentative Method of Test<br />
for Sieve Analysis of Fine and Coarse Aggregates, Proc.,<br />
- 38-1, 808-810.<br />
Bagnold, R. A.. (1956). The Flow of Cohesionless Grains in Fluids,<br />
Royal So. (London) Philos. Trans., 249, 235-297.<br />
Bagnold, R. A., (1966). An Approach to the Sediment Transport Problem from<br />
General Physics, U.S. Geol. Survey Prof. Paper 422-1. 37 pp.<br />
Barthel, J. C., (1969). Pesticide Residues in Sediments of the Lower<br />
Mississippi River and its Tributaries. In: Pesticides Monitoring<br />
Jour., 3, (l), 8-66<br />
Barton, J. R., and Lin, Pin-Nam, (1955). A Study of the Sediment<br />
Transport in Alluvial Channels. Civil Eng. Dept., Colorado<br />
State Univ., Fort Collins, Colo.. 43 pp.. 21 figs., and appendix.<br />
Benedict. P. C.. Albertson, M. L., and Matejka, D. Q., (1955).<br />
Total Sediment Measurement in Turbulence Flume. Amer. SOC.<br />
Civil Engineers Trans., 120, 457-484.<br />
Bondurant, D. C., (1955). Report on Reservoir Delta Reconnaissance,<br />
Corps of Engineers, Missouri River Division Sediment Series<br />
Pub. No. 6.<br />
Borland, W. H.. and Miller, C. R.. (1958). Distribution of Sediment<br />
in Large Reservoirs, Am. SOC. Civil Engr.. Jour. Hyd. Div..<br />
84, 1587.<br />
-<br />
128
Bormann, F. H., Likens, G. E., Eaton. J. S.. (1969). Biotic Regulation<br />
of Particulate and Solution Losses from a Forest Ecosystem.<br />
Bioscience, 19. (7), 600-610.<br />
Brown, H. E., Hansen, E. A., and Champagne, N. E., Jr., (1970).<br />
A System for Measuring Total-Sediment Yield from Small Water-<br />
sheds, Water Resources Research, 5, (31, 818-826.<br />
Burrell, G. N., (1951). Constant-Factor Method Aids Computation of<br />
Reservoir Sediment, Civil Engineering, 21. (7), 51-52<br />
Chia-Shun Shih, and Gloyne, E. F., (1969). Influence of Sediments<br />
on Transport of Solutes, Jour. Hyd. Div., 95. (HY4).<br />
Colby, B. R., and Hembree, C. H.. (1955). Computation of Total<br />
Sediment Discharge, Niobrara River near Cody, Nebr., U.S.<br />
Geol. Survey Water-Supply Paper 1357.<br />
Colby, B. R., (1956). Relationship of Sediment Discharge to Stream-<br />
flow, U.S. Geol. Survey, Open-File Report.<br />
Colby, B. R., (1957). Relationship of Unmeasured Sediment Discharge to<br />
Mean Velocity, her. Geophys. Union Trans., 2, (5),<br />
708-717.<br />
Colby, B. R., (1961). Effect of Depkh of Flow on Discharge of Bed Material.<br />
U.S. Geol. Survey Water-Supply Paper 1498-D. 12 pp.<br />
Colby, B. R., (1964). Discharge of Sands and Mean-Velocity Relationships<br />
in Sand-Bed Streams, U.S. Geol. Survey Prof. Paper 462-A,<br />
47 PP.<br />
Cook, H. L., (1935). Outline of the Energetics of Stream-Transporation<br />
of Solids, Am. Geophys. Union Trans., 16, (2), 456-463.<br />
Corps of Engineers, U.S. Army, (1961). Reservoir Sedimentation Invest-<br />
igations Program, Engineering Manual 1110-2-4000.<br />
Culbertson, J. K., Scott, C. H., and Bennett, J. P., (1972). Summary<br />
of Alluvial-Channel Data from Rio Grande Conveyance Channel,<br />
N. Mex., U.S. Geol. Survey Prof. Paper 562-.I., 49 pp.<br />
David, C., (1969). Sediment Radioactivity in the Columbia River Estuary,<br />
In Selected Water Resources Abs., 2, October 1, 1969, 32 pp.<br />
Davis, Foote, and Kelly, (1966). Surveying: Theory and Practice.<br />
Fifth edition, Chap. 30 (McGraw-Hill).<br />
Davis, Foote, and Kelly, (1964). Special Surveys: Dept. of Army Tech. Manual<br />
5-235.<br />
Dendy, F . E., and Champion, W. A ., (1969). Summary of Reservoir<br />
Sediment Deposition Surveys Made in the United States 'Illrough<br />
1965, USDA Misc. Pub. 1143.
fhkin, If. M. (rev. Brown, C. B.), (1939). Silting of Reservoirs,<br />
U.S. Dept. Agr. Tech. Bull. 524, 168 pp.<br />
Einstein, H. S., and Chien, Ning, (1953). Can the Rate of Wash Load<br />
be Predicted from the Bed-Load Function?, Am. Geophys. Union<br />
Trcllls. , E, 867-882.<br />
Einstein, H. S., (19<strong>50</strong>). The Bed-Load Function for Sediment Trans-<br />
portation in Open Channel Flows, U.S. Dept. Agr. Tech.<br />
Bull. 1026, 70 pp.<br />
Einstein, H. S., (1954). Second Approximation to the Solution of the<br />
Suspended Load theory, U.S. Corps Engineers, Missouri River Div. Sediment<br />
Ser. 3, 30 pp., 12 figs.<br />
Flammer, G. H., (1962). Ultrasonic Mmeasurement of Suspended Sediment,<br />
U.S. Geol. Survey Bull. 1141-A, 48 pp.<br />
Gilbert, G. K., (1914). Transportation of Debris by Running Water,<br />
U.S. Geol. Survey Prof. Paper 86, 263 pp.<br />
Gleason, 6. R., and Ohlmacher, F. J., (1965). A Core Sampler for<br />
In-Situ Freezing of Benthic Sediments, Ocean Science and Ocean<br />
Engineering, <strong>Joint</strong> Conference Marine Tech. Soc. and Amer. SOC.<br />
Lim. and Ocean, Washington, D. C., Trans., L, 737-741.<br />
Gottschalk, L. C., (1951). Measurement of Sedimentation in Small<br />
Reservoirs, Am. SOC. Civil Engr., Proc. Hyd. Div., 77.<br />
Guy, H. P., (1968). Quality Control of Adjustment Coefficients in<br />
Geological Survey Research 1968, U.S. Geol. Survey Prof. Paper<br />
600-B, pp. B165-Bl68.<br />
Guy, H . P., (1969). Laboratory Theory and Methods for Sediment Analysis,<br />
U.S. Geol. Survey Techniques Water-Resources Inv., Book 5,<br />
Chap. C1, 58 pp.<br />
Guy, H. P., (1970). Fluvial Sediment Concepts, U.S. Geol. Survey<br />
Techniques W-R Inv., Book 3, Chap. C1, 58 pp.<br />
Guy, H. P., and Norman, V. W., (1970). Field Methods for Measurement<br />
of Fluvial Sediment, U.S. Geol. Survey Techniques W-R Inv.,<br />
Book 3, Chap. C2, 59 pp.<br />
Hubbell, D. W., (1964). Apparatus and Techniques for Measuring<br />
Bed Load, U.S. Geol. Survey Water-Supply Paper 1748, 74 pp.<br />
Hubbell, D. W., and Matejka, D. Q., (1959). Investigations of<br />
Sediment Transporation. Middle Loup River at Dunning, Nebr.,<br />
U.S. Geol. Survey Water-Supply Paper 1476, 123 pp.<br />
Inter-Agency Committee on Water Resources, (1940). A Study of Methods<br />
Used in Measurement and Analysis of Sediment Loads in Streams:<br />
Field Practice and Equipment Used in Sampling Suspended Sediment,<br />
Report No. 1.<br />
130
Inter-Agency Committee on Water Resources, (1940). Equipment for<br />
Sampling Bed Load and Bed Material, Report No. 2.<br />
Inter-Agency Committee on Water Resources, (1941). A Study of<br />
Methods Used in Measurement and Analysis of Sediment Loads in<br />
Streams: Methods of Analyzing Sediment Samples, Report No. 4<br />
Inter-Agency Committee on Water Resources, (1941). A Study of<br />
Methods Used in Measurement and Analysis of Sediment Loads in<br />
Streams: Laboratory Investigation of Suspended-Sediment<br />
Samplers, Report No. 5.<br />
Inter-Agency Committee on Water Resources, (1943). A Study of New<br />
Methods for Size Analysis of Suspended-Sediment Samples, Report<br />
No. 7.<br />
Inter-Agency Committee on Water Resources, (1952). The Design of<br />
Improved Types of Suspended Sediment Samplers, Report No. 6.<br />
Inter-Agency Committee on Water Resources, (1953). Accuracy of<br />
Sediment Size Analysis Made by the Bottom-Withdrawal-Tube<br />
Method, Report No. 10.<br />
Inter-Agency Committee on Water Resources, (1957). The Development<br />
and Calibration of the Suspended-Accumulation Tube. Report No. 11.<br />
Inter-Agency Committee on Water Resources, (1957). Some Fundamentals<br />
of Particle-Size Analysis, Report No. 12.<br />
Inter-Agency Committee on Water Resources, (1958). A Study of Methods<br />
Used in Measurement and Analysis of Sediment Loads in Streams:<br />
Operation and Maintenance of U.S. BM-54 Bed-Material Sampler,<br />
Report M.<br />
Inter-Agency Committee on Water Resources, (1959). Sedimentation<br />
Instruments and Reports, Report AA.<br />
Inter-Agency Committee on Water Resources, (1963). A Study of Methods<br />
Used in Measurement and Analysis of Sediment Loads in Streams:<br />
Determination of Fluvial Sediment Discharge, Report No. 14.<br />
Ismail, H. M., (1952). Turbulent Transfer Mechanism and Suspended<br />
Sediment in Closed Channels, Am. SOC. Civil Engineers, Trans.,<br />
- 1 I 7, 409-446.<br />
Jackson, H . W., (1970. A Controlled-Depth Volumetric Bottom Sampler,<br />
The Progressive Fish Culturist,<br />
2 (2).<br />
Kennedy, J. F., (1961). Further Laboratory Studies of the Roughrle~,~<br />
and Suspended Load of Alluvial Streams, California lnst. ‘1ecI1nology,<br />
W. M. Keck Lab. Rept. KH-R-3.<br />
Keulcgm, C. H., (1938). Laws of Turbulent Flow in Open Channels,<br />
U.S. Natl. Bur. Standards, Jour. Research, 21, ( f~),<br />
707-741.<br />
l,i\i’sev, K. H., (1970). The Role Sediments Play ill lk~trrmining the<br />
((u:llity of Water, U.S. Army Corps of Engr., ilm.tl~n [list.<br />
131
McCain, E. H., (1957). Measurement of Sedimentation in TVA Reservoirs,<br />
Am. SOC. Civil Engr., Jour. Hyd. Div., g, (HY3), Paper 1277.<br />
McCain, E. H., (1965). Operation Manual for Radioactive Sediment Density<br />
Probe, Corps of Engineers, Omaha District.<br />
Meyrr-Peter. E., and Muller, R., (1948). Formulas for Bed-Load<br />
Transport, Int. Assoc. of Hydraulic Structures Research,<br />
2nd Mtg., Stockholm, Sweden, 39-64.<br />
Proceedings of the Federal Inter-Agency Sedimentation Conference,<br />
(1948), 314 pp.<br />
Proceedings of the Federal Inter-Agency Sedimentation Conference,<br />
(1963). U.S.D.A. Misc. Pub. No. 970, 933 pp.<br />
Proceedings of the Third Federal Inter-Agency Sedimentation<br />
Conference, (1976). Sedimentation Committee of the Water<br />
Resources Council.<br />
Prych, E. A., and Hubbell. D. W., (1966). A Sampler for Collecting<br />
Sediments in Rivers and Estuaries, Geol. SOC. America Bull.,<br />
- 77, 549-556.<br />
Ritter. J. R.. and Helley, E. J.. (1969). Optical Method for<br />
Determining Particle Sizes of Coarse Sediment, U.S. Geol.<br />
Survey Techniques of Water Resources Inv., Book 5 , Chap.<br />
c3, 33 pp.<br />
Sayre, W. W., and Chang, F. M., (1968). A Laboratory Investigation<br />
of Open-Channel Dispersion Processes for Dissolved, Suspended,<br />
and Floating Dispersants, U.S. Geol. Survey Prof. Paper 433-E,<br />
71 PP.<br />
Sayre, W. W., and Hubbell, D. W., (1965). Transport and Dispersion of<br />
Labelled Bed Material, North Loup River, Nebr., U.S. Geol.<br />
Survey Prof. Paper. 433-C, 48 pp.<br />
Sellers, B., Papodopoulos, T., and Ziegler, C., (1966). Radioisotope<br />
Gage for Monitoring Suspended Sediment Concentration in Rivers<br />
and Streams, U.S. Atomic Energy Comm.<br />
Simons, D. B., Richardson, E. V., and Albertson, M. L., (1961).<br />
Flume Studies Using Medium Sand (0.45 nun), U.S. Geol. Survey<br />
Water-Supply Paper 1498-A, 76 pp.<br />
Sooky, A. A., (1969). Longitudinal Dispersion in Open Channels,<br />
Jour. Hyd. Div., 95, (HY4).<br />
Task Force on Bed Forms in Alluvial Channels of the Committee on<br />
Sedimentation, ASCE, (1966). Jour. Hyd. Div., 92, (HY3).<br />
51-64.<br />
U.S. Army, Corps of Engineers, Omaha District, (1952). Sediment<br />
Transport Characteristics Study of Missouri River at Omaha.<br />
Nebr. Unpublished Report.
U.S. Bureau of Reclamation, (1958). Interim Report, Total Sediment<br />
Transport Program, Lower Colorado River Basin: Sedimentation<br />
Sec., Hydrology Br., Div. Prof. Inv., 175 pp.<br />
U.S. Waterways Experiment Station, (1935). Studies of River Bed<br />
Materials and Their Movement, with Special Reference to the<br />
Lower Mississippi River, U.S. Waterways Expt. Sta., Paper 17,<br />
161 pp.<br />
Vanoni, V. A., (1975). Sedimentation Engineering, Am. SOC. Civil<br />
Engr., Manuals and Reports on Engineering Practice - No. 54,<br />
745 pp.<br />
Vanoni, V. A., and Brooks. H. H., (1957). Laboratory Studies of the<br />
Roughness and Suspended Load of Alluvial Streams, California<br />
Inst. Technology, Report No. E-68.<br />
Wolman, M. C., (1954). A Method of Sampling Coarse River Bed Elaterial,<br />
Am. Geophys. Union Trans., 21, (6). 951 pp.<br />
133
DISCUSSION<br />
Dr. E. ihgley: We are all aware of the fact that the relationship between<br />
sediment discharge and flow is generally non-linear when we are looking at<br />
total sediment. I am wondering, do you have any feeling for the relationship<br />
between the finer than 62 p fraction with flow? Is it because it is<br />
not depth dependent? Is it really a simple function of flow?<br />
Mr. J. 'iciiw2*tsmI; No. The fine material is that material finer than 62<br />
micron5 and I might mention that Hydro Comp has a model out where they stop<br />
at <strong>50</strong> microns. Why they selected that I don't know. This fine material<br />
has been called wash load. There is no relation between the concentration<br />
or load of wash load with the hydraulics. It is not velocity- and depth<br />
dependent. The sands do relate well with velocity-depth relations but the<br />
fine material, no. This is a real problem because vou cannot predict the<br />
fine material or washload.<br />
135
INTRODUCTION<br />
Sediment Storage and Remobilization Characteristics<br />
of Watersheds<br />
.I. Knox<br />
Accuracy in estimating long term sediment yields depends to a<br />
large degree on understanding the natural variability of the magnitude and<br />
frequency of surface runoff. Although the magnitude and frequency characteristics<br />
of surface runoff are normally presumed to display random<br />
variation over time, this presumption is often not fulfilled. Long hydrologic<br />
records spanning many decades and proxies of surface runoff that<br />
extend to century time scales indicate that natural departures from the long<br />
term average often occur in clustered sequences. Because many hydrologic<br />
records span only short periods of time, a condition which is especially<br />
true for sediment yield records in many small watersheds, it is important<br />
that observed erosion and sediment transport rates be calibrated to reflect<br />
the range of both short term and long term variations. Significant changes<br />
in erosion and sediment transport often occur when an existing balance<br />
between land cover and climate is disrupted. Disruption may occur naturallv<br />
in response to climatic change, or it may be produced in response to changes<br />
of land use. The responses of sediment yield to changes of climate or land<br />
use are mostly related to changes in the magnitude and frequency characteristics<br />
of surface runoff. It is also apparent that changes in magnitude and<br />
frequency of surface runoff, in turn, cause changes both in the functional<br />
relationship between sediment yield and water yield and in the sediment<br />
delivery ratio. Nearly all of the techniques currently in practice for<br />
estimating long term sediment yields of watersheds involve an assumption of<br />
temporal uniformity (stationarity) in the time series of variables that<br />
relate to either precipitation or runoff. Over the time span of several<br />
decades and longer, the assumption of stationarity may not be met because of<br />
changes in either climate or land use or both.<br />
THE MOVEPENT OF SEDIMENT THROU;H K~TERSHEDS<br />
Long term changes in the magnitude and frequency of surface runoff<br />
can alter the long term rate of movement of sediment through a watershed by<br />
causing changes in the rate of upland erosion and/or in the volume of sediment<br />
that is stored in the watershed as colluvium and alluvium. The volume of<br />
upland erosion is largely related to storms and surface runoff of events<br />
moderate magnitude and frequent occurrence. For example, Wischmeier (1962)<br />
found that three-fourths of the soil losses from small, cultivated, singlecover<br />
plots in the United States were caused an by average of about four<br />
storms per year. Piest (1965, p. 101) concluded that, on the average, more<br />
than one-half of the annual soil losses from 72 small watersheds from 17<br />
states was related to storms that occur more often that once per year.<br />
137
Altllougll the data of Wischmeier and Piest make it clear that most upland<br />
sediment loss is related to meteorological events that recur relatively<br />
frequently, the actual total number of events is relatively small. For<br />
rxdmplc, Knox et al. (1975, p. 42) found that, for small Iowa watersheds<br />
draining less than 10 square miles (16 km2), the suspended sediment yield of<br />
only eight to ten davs normally accounted for more than 90 percent of the<br />
annual yield. The actual number of days per year that are associated with<br />
relatively high magnitude suspended sediment yields increases slightly as<br />
drainage area increases (Figure 1). It may be concluded that the hulk of<br />
erosion and transportation of sediments from upland sites is related to a<br />
very small number of hydrologic events, whose occurrence is dependent upon<br />
the development of specific meteorological conditions. The importance of a<br />
small numher of climatic events makes climate variation critical to the<br />
explanation of long term variations of the mobility and storage of sediments<br />
in watersheds. A modest change in an expected seasonal location of a storm<br />
track or air mass boundary affects the number of occurrences of days with<br />
high mngnitude sediment yields. Climate changes of this type explain most<br />
of bottt the short and long term variations of upland sediment yields.<br />
Long term variations in the magnitude and frequency of surface<br />
runoff also affect the balance between sediment that is eroded and transported<br />
versus sediment that is stored as colluvium and alluvium (sediment<br />
delivery ratio). Millenia scale episodes of channel alluviation and channel<br />
degradation have been documented as occurring over wide regions (Starkel,<br />
1966, 1972; Haynes, 1968, 1976; Klimek and Starkel, 1974; 1NQUA Holocene<br />
Symposium, 1975; Knox, 1976; and Johnson, 1976). The regional synchroneity<br />
of many documented alluvial episodes provides evidence that the sediment<br />
delivery ratio is subject to change on long term time scales. Current<br />
estimates of long term average sediment delivery ratios have suggested that<br />
the ratio varies inversely with drainage area raised to the 0.125-0.20 power<br />
(Piest, Miller, and Vanomi, 1975, pp. 462-463; EPA, 1973, p. 58). Year to<br />
year variability in surface runoff may cause large departures from the<br />
expected average sediment delivery. For example, when land cover is held<br />
constant, the magnitude of the sediment delivery ratio increases with<br />
increasing runoff (Piest, Kramer, and Heinemann, 1975, p. 132; Elutchler and<br />
Bowie, 1976, p. 1-21). If. over the long term, vegetative cover improves to<br />
better protect the land surface from erosion and acts as a friction factor<br />
to reduce the rate of surface runoff, then sediment delivery percentages<br />
will decline (PiesL, K~dme~, and Heinemann, 1975, pp. 139-140). On the other<br />
hand, if changes in runoff are associated with highly variable flood flows<br />
with relatively frequent recurrence of large floods, then stream channels<br />
are likely to become unstable and floodplain erosion may follow (Schumm and<br />
Lichty, 1963, pp. 74-76; Stevens et 4.. 1975; and Knox, 1976, pp. 182-186).<br />
In summary, the amount of transportation and/or storage of sediment<br />
in watersheds is dominated by a relatively small number of hydrologic<br />
events that tend to occur each year. The actual frequency and the character-<br />
istics of the critical hydrologic events is strongly dependent on the<br />
prevailing climate and land use characteristics.<br />
IMPACT OF LONG TEW VPRIATICNS OF CLIMATE<br />
The importance of climate as a variable influencing short term<br />
variations of sediment yields is generally accepted, but many, if not most,<br />
investigaLors assume that climate variations are essentially random in<br />
relation to time scales than span several decades or longer. The assumption<br />
138
___<br />
FIGURE 1<br />
The relationship between days of occurrence of relatively<br />
high magnitude suspended sediment yield and drainage area.<br />
The equation explains seventy-six percent of the variance<br />
of high sediment yield days and is significant at 0.01 the<br />
probability level. High yield days, as defined here,<br />
accounted for an average of eighty-five or more percent of<br />
annual sediment yields in watersheds draining <strong>50</strong>0 square<br />
miles (1300 krn'). Only about sixty percent of the total<br />
annual suspended sediment yields were represented by the<br />
high yield days when the drainage area increased to about<br />
<strong>50</strong>00 square miles (13000 h2). The data utilized in the<br />
above equation provide quantitative documentation that a<br />
very large fraction of the total annual yield of suspended<br />
sediment from a watershed is related to hydrologic events<br />
which occur on only a small number of days during the year.<br />
The specific magnitude and frequency characteristics of<br />
the critical hydrologic events are in turn highly<br />
sensitive to variations in the dominant climatic regimes.<br />
139
nt randomness is frequently not supported by long historical records of<br />
climntc nor by proxies of long climate records (e.g., tree ring character-<br />
istics) (Lamb, 1966, pp. 56-112).<br />
Climate circulation regimes are often characterized by positive<br />
and negative feedback mechanisms that lead to persistence of anomalous<br />
conditions. Persistence of certain "preferred" circulation regimes, in<br />
turn, contribute to persistence in characteristics of hydrologic events.<br />
Persistence occurs on many time scales. For example, an analysis by this<br />
author revealed that, for the years 1941-1969, frequencies of floods in 29<br />
Upper Mississippi Valley watersheds displayed patterns of departure from<br />
,average that were strongly persistent on approximately a decadal time scale<br />
(Figures 2 and 3). The number of "runs" of consecutive occurrences of flood<br />
frequencies, expressed as five-year running averages in Figure 2, was nearly<br />
four standard deviations below the expected mean number for a statistically<br />
random arr'iv. I t was estimntrd that departures, of the above decadal scale,<br />
wtart' rt,sponsihle for four ((1 -,t~vt~n-told v.iriations in tht: average short term<br />
sediment yields.<br />
An example of how surface runoff can vary between climatically<br />
different years is prcvided in Figure 4 which represents thv Rickapoo Kiver<br />
"Strahler"<br />
St ream<br />
Order<br />
TABLE 1*<br />
Estimated Suspended Sediment Yields for<br />
Average Drainage Areas of Stream Orders<br />
in the Kickapoo Watershed Upstream of<br />
LaFarge, Wisconsin<br />
Average Sediment Yield (tonslmi')<br />
Drainage "wet"* "dry"* "average"<br />
Area (mi')<br />
1965 1963<br />
.016<br />
.07<br />
.33<br />
1.55<br />
6.65<br />
30.<strong>50</strong><br />
~<br />
24<strong>50</strong> 475 14 30<br />
2025 390 1180<br />
1640 310 945<br />
1320 2<strong>50</strong> 760<br />
1100 2 10 630<br />
88 5 170 520<br />
*The sediment yield data were generated from seasonally calibrated sediment<br />
rating curves derived from U.S. Geological Survey sediment data collected<br />
for the Kickapoo River near LaFarge, Wit;consin. For further information<br />
see Knox aJ., 1975, pp. <strong>50</strong>-57.<br />
141
Decadal scale variation in flood frequencies. A listing of the<br />
gaging stations and other descriptive characteristics of the
FIGURE 3<br />
Total Floods -Partial Duration Series<br />
29 Basins<br />
per Mississippi Valley<br />
Month<br />
- 1941 - I949<br />
"" I*Io-lv5v<br />
IVbO- I969<br />
Decadal scale variation in seasonal concentrations of<br />
flood occurrences (After Knox et al., 1975, pp. 10, "<br />
17, 30-40).<br />
FIGURE 4<br />
Hydrographs - Kickapoo River LaFarge, Wisconsin<br />
Climatic influence on runoff. Data from U.S. Geological<br />
Survey. After Knox et al., 1975, p. 51.<br />
143
FIGURE 5<br />
. , ..<br />
Annual flood series, Mississippi River at Keokuk, Iowa.<br />
Data from U.S. Geological Survey, After box - et _. a1 3<br />
1975, p. 7.<br />
1.<br />
FIGURE 6<br />
Minimum Stagor of th. Nil. Rivor at Cairo, Egypt<br />
Progressive <strong>50</strong>-year average stages of Nile River.<br />
Redrawn from Stevens' Fig. 9 closure to paper by<br />
Jarvis (1935, p. 1048).<br />
144
to reflect probable climatic trends. If long term estimates of the mobility<br />
and storage of sediments are to extend to century and longer time scales,<br />
then probabilistic interpretations of duration curves based on long records<br />
represent "best available" approximations. Planning for long term mobility<br />
and storage of sediments in watersheds should probably be viewed in relation<br />
to scenarios of possible conditions of climate influence.<br />
IWACT OF LONG TERM VARIATIONS OF [AND USE<br />
The long term mobility and storage of sediment in watersheds is<br />
influenced by land use in a way that is analogous to climate changes, because<br />
both involve disturbance of the relationships amongst precipitation, land<br />
cover, and runoff. The influence of land use on sediment yield has been<br />
investigated extensively (e.g., Federal Inter-Agency Sedimentation Conferences:<br />
1947, 1963 and 1976; U.S. Environment
Environmental Protection Agency (EPA), 1973. Methods for Identifying<br />
and Evaluating the Nature and Extent of Non-Point Sources of<br />
Pollutants: EPA - 430/9-73-014, Washington, 261 pp.<br />
HLIPP, S . C., Rittenhouse, C., and Dobson, C. C., 1940, Some<br />
principles of accelerated stream and valley sedimentation:<br />
U.S. Department of Agriculture, Tech. Bull. 695.<br />
Havrlt.s, (1, V., 1968, Geochronology of late-Quanternary alluvium:<br />
In - Means of Correlation of Quaternary Successions (R.B.<br />
Morrison .rnd II. E. Wright, Eds.) pp. 591-631, Vol. 8. Proceedings<br />
VI1 INQUA Congress, Univ. of Utah Press, Salt Lake City.<br />
<strong>International</strong> Association of Hydrological Sciences, 1974, Effects<br />
of Man on the Intrrtace of the Hydrological Cycle with the Physical<br />
".~"I_<br />
Environment: IAHS I'ublication No. 113, 157 pp.<br />
~"<br />
"_ .<br />
INQUA Holocene Symposium, 1975, Weogeographical Changes of Valley<br />
Floors in the Holocene: Biuletyn of Genlogiczny, Vol. 19,<br />
Warsaw University Press, Warszawa.<br />
.I.trvis. C. S., 1935, Flood-stage records of the river Nile: Trans.<br />
American Society of Civil Engineers, Paper No. 1944, pp.<br />
1012-1070.<br />
Johnson, W. C.. 1976, The impact OF environmental change on €luvial<br />
systems: Kivkapoo River, Wisconsin: Unpublished Pt1.D. disserat.ion,<br />
University o i Wisconhin, Department of Geography.<br />
Klimek, K., and Starkel, L., 1974. History and actual tendency<br />
of floodplain development at the border of the Polish Carpathians:<br />
In: Report OF the <strong>Commission</strong> on Present-day Processes (H. Poser,<br />
Ed.), pp. 185-196, <strong>International</strong> Geographical Union, Vandenhoeck<br />
and Kuprecht, Gottingen.<br />
box, .1. C., 1976, Concept of the graded stream: In - Theories<br />
of Landform Developms, R. Flemal and W. Melhorn, Eds., Sixth<br />
Annual Geomorphology Symposium. Binghamton, NY, pp. 169-198.<br />
Knox, J. C., Bartlein, P. J., Hirschboek, K. K., and Muckenhirn,<br />
R. J., 1975. The response of floods and sediment yields to<br />
climatic variation and land use in the Upper Mississippi<br />
Valley: University o€ Wisconsin Institute for Environmental<br />
Studies Report No. 52, 76 pp.<br />
Lamh, H. H.. 1974, The current trends of world climate--a report<br />
on the early 1970's and a perspective: University of East<br />
Anglia, Norwich, Climatic Research Unit Research Publication<br />
No. 3, 28 pp.<br />
Lamb, H. H.. 1966, On the nature of certain climatic epochs which<br />
differed from the modern (1900-1939) normal: In: The Changing<br />
Climate (H. H. Lamb). pp. 58-112, Methuen and Co., Ltd.,<br />
London.<br />
Mutchler, C. K., and Bowie. A. J., 1976, Effect of land use on<br />
sediment delivery ratios: In - Proceedings of the Third Federal<br />
Inter-Agency Sedimentation Conference, Water Resources Council,<br />
Washington, pp. 1-11. 1-21.<br />
146
Piest, R. F., 1965. The role of the large storm as a sediment contributor:<br />
Proceedings of 1963 Federal Interagency Conference on Sedimentation,<br />
Misc. Pub. 970, U.S. Department of Agriculture.<br />
Piest, R. F., Kramer, L. A., and Heinemann, H. G., 1975, Sediment<br />
movement from loessial watersheds: In - Present and Prospective<br />
Technology for Predicting Sediment Yields and Sources, U.S.<br />
Department of Agriculture, Agricultural Research Service Report<br />
ARS-S-40., pp. 130-141.<br />
Piest, R. F., Miller, C. R., and Vanomi, V., 1975, Chapter Iv -<br />
Sediment sources and sediment yields: In - Sedimentation<br />
Engineering, V. Vanomi, Ed., American Sor. Civil Engineers Task<br />
Committee on Sedimentation, NY, pp. 437-493.<br />
Renfro, G. W., 1975, Use of erosion equations and sediment-delivery<br />
ratios for predicting sediment yield: In - Present and<br />
~ _" ~<br />
Prospective Technology for Predicting Sediment Yields and<br />
__- Sources, U.S. Dept. of Agriculture, Agricultural Research<br />
Service, Publication ARS-S-40, pp. 33-45.<br />
Schumm. S. A., and Lichty, K. W., 1963, Channel widening and tlood-<br />
plain construrtion along Cimarron River in Southwestern Kansas:<br />
U.S. Geological Survey Prof. Paper 352-D, pp. 71-88.<br />
Starkel, I,., 1966, Post-glacial climate and the moulding of European<br />
relief: In - World Climate from 8000 to 0 B.C., Royal<br />
Meteorological Society, London, pp. 15-33.<br />
Starkel, L., 1972, Trends of development of valley floors of mountain<br />
areas and submontane depressions in the Holocene: Studia<br />
Geomorphologica Carpatho-Balcanica, Vol. 6, pp. 121-133.<br />
Stevens, M. A., Simons, D. B., and Richardson, E. V., 1975.<br />
Nonequilibrium river form: Proceedings American Society<br />
Civil Engineers, Journal Hyd. Division, HY5, V. 101, PP.<br />
551-566.<br />
Trimble, S. W., 1975, A volumetric estimate of man-induced soil<br />
erosion on the southern piedmont plateau: In - Present<br />
and Prospective Technology for Predicting Sediment Yields<br />
and Sources, U.S. Dept. of Agric., Agric. Research Service,<br />
Publication ARS-S-40, pp. 142-154.<br />
Trimble, S . W., 1976, Sedimentation in Coon Creek Valley, Wisconsin:<br />
In - Proceedings of the Third Federal Inter-Agency Sedimentation<br />
Conference, Washington, pp. 5-100, 5-112.<br />
U.S. Department of Agriculture. 1965, Proceedings of the Federal<br />
Inter Agency Sedimentation Conference, 1963: U.S. Government<br />
Printing Office, Washington, 933 pp.<br />
U.S. Department of Agriculture, 1975, Present and Prospective<br />
Technology for Predicting Sediment Yields and Sources:<br />
Agricultural Research Service Publication ARS-S-40, pp. 285<br />
147
DISCUSSION<br />
Dr. 0. Bouldin: One of the problems we always run into is that we have got<br />
to make estimates based on imperfect knowledge. Now I see nothing particularly<br />
wrong with that, but what really bothers me is that most of the time<br />
we have no way to estimate how sure we are about, or put confidence intervals<br />
on, those estimates, particularly sediment loads. What kinds of handles can<br />
we put on this problem? In other words how do we make an estimate of a<br />
coniidence interval<br />
years of data?<br />
for a sediment load that may be derived from two or three<br />
Dr. J. Kwxt Well, again I am sure this probably varies regionally. The<br />
situation that I am familiar with in the upper midwest is that we do see<br />
frequently decadal scale persistence in the records, and we can look at<br />
longer term records of climate or climate proxies. In this case we have<br />
records of temperature that we can take back to 1820 or so. In the case of<br />
Canada, the Hudson Bay Company has some nice records that go hack iarther.<br />
I think what we need to ds is look at these kinds of records and see to what<br />
degree the probabilities of recurrence of certain kinds of critical events<br />
that transport sediment might have varied within those records. Now, this,<br />
I think, is preferable, at least from my point of view, in estimating what is<br />
likely to happen, say over the next 10 years than just assuming pure randomness.<br />
I think you come up with a better figure, but again I think because we do not<br />
have the capability of perfect prediction, even very good prediction right<br />
now, that probably the scenario approach is still a requirement. You have to<br />
look at it in terms of what is possible and I think those probability figures<br />
are pretty good in telling you what is possible, perhaps even what is reasonably<br />
probable. We see right now, at least occuring since about 19<strong>50</strong>, a<br />
return to circulation patterns that seem to have characterized many years in<br />
the previous century, recurred with much greater frequency. Now whether<br />
that is going to continue to happen, I have no way cf knowing, but it is<br />
interesting that some of the patterns we have now apparently are similar to<br />
ones that occurred over long periods of time. Much of the first half of the<br />
20th century had recurrence frequencies of circulation patterns, preciptation<br />
characteristics and runoff that may be a little bit unusual over the long<br />
term scale of the last 200 years or so. Now, if that is true and we are<br />
working from duration curve extrapolations and we weight our data relative to<br />
the record, that is not really representative of what is going to occur in the<br />
next decade or so; then we are in trouble.<br />
149
INTRODUCTION<br />
Sediment Storage and Remobilization Characteristics<br />
of Watersheds<br />
C. Nordin<br />
The movement in a watershed of a sediment particle from its<br />
position of weathering and erosion to its point of ultimate deposition is<br />
a complex process developing in time under random influences. By definition<br />
(Cramir, 1964), this movement is a stochastic process.<br />
Most of what we know about sediment movement and sediment<br />
storage in watersheds comes from empirical observations, and despite the<br />
fact that sedimentary processes have been observed for many decades, only<br />
a few generalizations can be drawn from the extensive data accumulated.<br />
This discussion deals with the following generalizations: (1) the relation<br />
of sediment yield to drainage area and its implication in of terms sediment<br />
storage in watersheds; (2) the random nature of daily and annual sediment<br />
loads; (3) short-term in-channel storage of sediment; and finally (4) a<br />
stochastic model of sediment movement and its limitations.<br />
SEDIMENT YIELD AND DRAINAGE AREA<br />
Sediment yield usually is determined one of three ways: (1)<br />
from small experimental plots where artificial precipitation is controlled<br />
and all runoff and sediment can be measured; (2) from periodic surveys<br />
of sediment collected in reservoirs; and (3) from sampling sediment loads<br />
of streams. Auxilliary methods involving remote sensing, topographic<br />
mapping or cross sectional valley and channel surveys also are used.<br />
The relations of sediment yield to drainage area vary from arid<br />
to humid regions, but the general trend is for the unit sediment yield to<br />
decrease with increasing drainage area. The implications are clear; the<br />
movement of eroded sediment is not continuous, and the larger the drainage<br />
area, the more chances there are for storage of eroded material. The<br />
major unresolved problems with respect to sediment yield are those re-<br />
lating to storage. If all the sediment eroded from the headwater areas<br />
of watersheds is not transported out of the basin, where does this sediment<br />
go, what is its residence time while in storage, and what are the factors<br />
that control its movement and deposition? Despite the tremendous amount<br />
of empirical data collected to determine sediment yield from small watersheds,<br />
it has not been possible to draw sufficient generalizations to<br />
permit answering these sorts of questions.<br />
Most of the reliable data on sediment yield are from watersheds<br />
with drainage areas less than about 10 square kilometres, while most<br />
stream sediment discharge records are for basins with drainage areas<br />
greater than 200 square kilometres. Very little information is available<br />
for basins in the range 10 of to 200 square kilometres drainage area.<br />
151
DAILY AND ANNUAL SEDIMENT LOADS<br />
The sediment yield from large watersheds and sediment discharge<br />
to the oceans are determined from measurements of sediment discharge at<br />
gauging stations. These records are considered fairly reliable because:<br />
(1) the flow and transported sediment are confined generally a single to<br />
channel where measurements are relatively simple and standardized; and<br />
(2) many of these stations have been operated for years so that the<br />
stochastic properties of the time series of flow and sediment discharge<br />
are well-defined.<br />
A common method of displaying sediment loads is to plot them<br />
against water discharge. Generally, there is a fair correlation with an<br />
increasing trend of sediment load with water discharge, indicating that<br />
the same variables affect both runoff and sediment yield, and almost<br />
always there is considerable scatter about a mean line relating sediment<br />
discharge to water discharge. Typically, the sediment discharges from<br />
arid and semi arid regions are several Orders of magnitude higher a for<br />
given water discharge than those from more humid basins, and at low<br />
flows, the scatter in sediment load for arid regions is five or six<br />
orders of magnitude compared to about an order of magnitude for the humid<br />
regions. Part of this scatter is seasonal variation, but even after<br />
integrating over the annual cycle, there is considerable scatter in the<br />
relation of annual sediment load to annual streamflow.<br />
Land use has substantial impact on sediment yield, and most<br />
activities such as road building, urbanization, timbering, and strip<br />
mining, if not subjected to careful controls, can increase substantially<br />
the sediment yield of a specific area. The rate of rehabilitation of a<br />
disturbed area is generally rapid in urban areas and decreases with<br />
decreasing annual precipitation in rural areas.<br />
IN-CHANNEL STORAGE AND REENTRAIN"<br />
In-channel storage and reentrainment of sediment may a be longterm<br />
phenomenon associated with the building of point bars and flood<br />
plains with concurrent lateral migration of channels, or it may a be<br />
seasonal or shorter-term phenomenon related to hydrologic and hydraulic<br />
properties of the flow. There is little basis today other than projection<br />
of historical data for predicting rates of lateral migration of channels,<br />
but short-term changes related to stream hydrology OK to hydraulic sorting<br />
can be modelled with reasonable success if sufficient data are available<br />
to establish initial conditions, boundary conditions, and inputs. The<br />
two general approaches to modelling these processes are: (1) to construct<br />
stochastic models of the time series of water discharge and sediment<br />
discharge (Rodriguez and Nordin, 1968); or (2) to employ computational<br />
hydraulics as described by Bennett (1974).<br />
152
STOCHASTIC M~DELS OF PARTICLE M~VEMENT<br />
-L<br />
Mathematically, the position vector X(t) of a particle at time<br />
t is given by<br />
*<br />
where the initial position X(o) = o at time t = 0. The velocity 0,<br />
O.t<br />
is a random function of position and time so X also is a random function<br />
of position and time.<br />
ft<br />
Equation 1 says simply that a particle's position is the integral<br />
over its velocity and the description is precise whether the movement is<br />
continuous or discontinuous. From a practical point of view, though, the<br />
utility of equation 1 is restricted to some few cases where the particle<br />
velocity can be specified or where the distributions of particle step<br />
lengths and rest periods are known; so the applications are limited<br />
mostly to the hydraulic problems of predicting dispersion of fine particles<br />
or bedload transport under conditions of steady uniform flow. Considerable<br />
progress has been made in recent years on these particular stochastic<br />
models, but there still exist some disturbing departures of empirical<br />
observations from the theoretical models (Nordin, 1975), so the problems<br />
can hardly be considered solved. Nonetheless, it is encouraging that at<br />
least certain aspects of the sediment transport processes can be approached<br />
on a fairly rigorous mathematical basis.<br />
Although equation 1 is an exact description of particle movement,<br />
there are practical limitations in its applications. The first restriction<br />
requires the process be stationary; that is, the parameters describing<br />
the distributions in the process should not vary with time. A second<br />
restriction, one that is even more critical, is that the model is kinematic;<br />
so for predictive purposes, it is necessary to relate the parameters of<br />
the model to the dynamic forces of the flow. This can be done for steady<br />
uniform Flow over a flat bed. For this case, the particle steps,
Todorovic and Nordin (1975) generalize the wst important<br />
theoretical results of various stochastic models that have been proposed<br />
and derive expressions for the errors involved in the approximations in<br />
equation 2.<br />
Probably the most critical research need today is to compile<br />
and generalize existing empirical data to permit interpolation and extrapolation<br />
to areas where no field data have been collected. Such generalization<br />
would also serve as a rational basis for network design to collect<br />
additional field data where it is needed. Further development of mathematical<br />
and stochastic models should be undertaken, with particular<br />
attention to methods for treating non-steady or non-stationary processes.<br />
LITERATURE CITED<br />
Bennett, J. P., (1974) Concepts of Mathematical Modelling of Sediment<br />
Yield Water Resources Research, lo. (3). 485-492.<br />
Cram&, Harald, (1964). Model Building With the Aid of Stochastic<br />
Processes Technometrics, 5, (2), 133-159.<br />
Nordin, Carl, (1976). Simulation of Velocity Distribution and Mass<br />
Transfer Stochastic Approaches to Water Resources (ea. H. W.<br />
Shen), P. 0. Box 606, Fort Collins, CO., V. 11, chap. 22, 24 p.<br />
Rodriguez-Iturbe, I., and Nordin, C. F., (1968), Time Series Analysis<br />
of Water and Sediment Discharges Int. Assoc. for Scientific<br />
Hydrology, 13, (2), 69-86.<br />
Todorovic, Petar, and Nordin, C. F., Jr., (19751, Evaluation of Stochastic<br />
Models Describing Movement of Sediment Particles on Riverbeds<br />
U.S. Geol. Survey Journ. of Research, 3, (5), 513-517.<br />
154
DISCUSSION<br />
Dr. E. Ongley: 1 am wondering, if you have any feeling for whether there is<br />
an inverse relationship between particle size and the residence time in<br />
transport? Would you suspect that?<br />
Dr. C. Nordin: I have evidence that says that there is under steady uniform<br />
flow conditions. Experimental evidence in a laboratory flume shows that the<br />
rest period duration, or a distribution of rest periods of a particle is a<br />
function of the particle size. Once you get into a natural condition where<br />
you do not have steady, uniform flow, where you have particles depositing<br />
some place and picked up somewhere else, it is hard to say. One typical<br />
storage problem that you would run into is in most natural channels where<br />
you have material eroded from the outside of a bed being deposited on the<br />
next bar downstream. This material might be stored in that bar for centuries<br />
until the meander sweeps on downstream. So what is the rest period of particles<br />
there? They take a few hops along the channel and rest in that bar deposit<br />
for a couple of decades or a couple of centuries until the meander sweeps on<br />
downstream and picks them up. This is important in terms of nutrients and<br />
contaminants because there is a possibility for contaminants to be deposited<br />
in a flood plain and then be re-exposed a at later time on a fairly uniform<br />
basis. So you may never get these things out of the channel.<br />
Ur. J. Knor: Carl (Nordin), what time scale are you applying a randomness<br />
on? The reason I ask is that on certain time scales, things are certainly not<br />
very random; they are very episodic.<br />
Vr. C. Nordin: I do not think that the concept of persistence is in conflict<br />
with the ideas of randomness if you look at the work that Mandelbroth and<br />
Hurst have done, or even the work that G. I. Taylor did in introducing the<br />
correlation function to statisticians. There is a persistence, and until<br />
that correlation dies out this persistence determines the movement.<br />
Dr. .J. Knox: The point is really how much energy do you want to expend to<br />
make something that appears random at one scale really deterministic at<br />
another scale?<br />
155
Dr. C. Nordin: Well as an engineer I am perfectly contented to work with<br />
design lives. Most engineers are willing to work with <strong>50</strong> years or 100 years<br />
because if they design something, they are going to be gone by the time it<br />
fails. The time scales are very different. I agree with you that there<br />
are episodes of very low flow and very high flow of very intense activity<br />
and I don't know how you handle these.<br />
Dr. J. Knox: One thing that we did to improve the level of explained variance<br />
between water yield and sediment yield was to calibrate for the various kinds<br />
of events within a given physiographic area and given land use region. The<br />
explained variance rose and then you could interpret your records Over a<br />
longer term of events by looking at the stream flow from which you usually<br />
have longer records. It is a matter of calibrating over shorter periods of<br />
time, for particular times of events and then getting a long equation with<br />
dummy variable type inputs where a 1 OK a 0 is ahead of the calibration<br />
coefficient. If that event occurred in that period, it had a 1 and multiplied<br />
it in. If it did not it cancelled it out. That gives pretty reasonable<br />
results. It explains most of the variance but it takes a lot of time and much<br />
effort, but it can be a significant improvement.<br />
Dr. C. Nordin: I think I would also agree with your suggestion that when you<br />
start simulating or trying to predict these things through simulation you<br />
have to look at a large variety of scenarios or you have to run a lot of<br />
realizations of your stochastic process and get some distribution of these.<br />
156
I NTRODUCT I ON<br />
Forms and Sediment Associations of Nutrients (C.N. b p.)<br />
Pesticides and Metals<br />
Nutrients - P<br />
H. L. Golterman<br />
As most of the Dutch lakes are situated in the deltas of the Rhine<br />
and Meuse, they receive large amounts of nutrients from this source.<br />
In total they receive 6 g m-', y-l; about <strong>50</strong>% from the Rhine. The<br />
Rhine carries about 70,000 tonnes of PO+-P per year, of which 80% is<br />
transported into the North Sea. Of the remaining 15,000 tonnes about<br />
<strong>50</strong> - 60% is accumulating in the Dutch lakes, which receive an annual<br />
load of about 6 g per m2 per year.<br />
ESTIWTES OF RIVERINE PHOSPHATE<br />
Sources<br />
Three different sources of riverine phosphate - and thus of the<br />
Rhine - can be distinguished:<br />
1) natural erosion;<br />
2) human waste;<br />
3) agricultural runoff.<br />
Global Phosphate Resources<br />
A first estimate of the erosion can be made with a global mean value.<br />
Phosphate occurs in rocks as calcium apatite, in the range between 0.07 -<br />
0.13%. Van Wazer (1961) estimated the total amount of phosphate in the<br />
lithosphere to be 1.1 x loz5 g of which lo" g is present in the sea<br />
in the dissolved state, while loz1 g is buried in marine sediments. (Only<br />
3 x 10l6 g is considered to be mineable).<br />
Erosion-derived Phosphate<br />
During chemical weathering the major part of this amount is converted<br />
into a "lattice bound clay" phosphate. In unpolluted clays the phosphate<br />
phosphorus is about 0.1%.<br />
Following Meybeck's (1976) estimate for total solid riverine transport<br />
(2 x 10l6 g per year, = 5 x the dissolved transport) we arrive at a figure<br />
for erosion for PO~-P of 2 x 1013 g per year.<br />
157
For specific calculations we use Meybeck's figures for erosion of:<br />
- <strong>50</strong>0 tonnes per km2 for small alpine rivers;<br />
- 200 tonnes per km2 for middle sized basins (A= about 100,000 km2) and<br />
- 140 tonnes per km2 for large basins (A > 400,000 km2),<br />
while rivers in a low relief area have values about 10 times lower. With<br />
this kind of calculation we arrive at a value for the mine of about 4,000<br />
tonnes.<br />
Silicate - Phosphate Ratio6<br />
A second calculation makes use of the ratio Si02-Si/POs-P, which we<br />
found to be around 100/1 in many unpolluted rivers in Africa and Southern<br />
France. This calculation gives a POh-P load in the Rhine of about<br />
10,000 tonnes. Human waste can be estimated to be around <strong>50</strong>,000 tonnes<br />
per year, based on an annual POs-P production 1.5 of kg per year per<br />
person.<br />
Agricultural Phosphate<br />
Agricultural runoff can be estimated at 1,000 - 10,000 tonnes per<br />
year assuming a runoff of 0.05-0.5 kg per ha per year. The total of these<br />
three fractions is about 65,000 - 70,000 tonnes per year, not far from<br />
the measured value.<br />
Phosphate Availability - Algal Bioassays<br />
Availability of sediment phosphate was studied by algal bio-assays,<br />
using Scenedemus as a test organism. All sediment yielded a good algal<br />
growth, although not all the phosphate appeared to be available. It seems<br />
that the phosphate bound into the clay lattice (the "natural" clay phosphate)<br />
is not available for algae, although it is readily available for agricultural<br />
crops. Phosphate freshly adsorbed onto clay is easily available to algal<br />
cultures.<br />
Phosphate Availability - Chemical (NTA) Desorption<br />
It was found that with NTA (nitrilotriacetic acid) an amount of phosphate<br />
could be extracted equal to that used by algae. Iron bound phosphate is<br />
entirely available, while the availability of apatite depends on the<br />
crystal size. Whether this potential availability can indeed be used in<br />
the real lake situation depends largely of course on the hydrodynamics<br />
of the system.<br />
158
Long Term Studies<br />
Experiments in an artificial pond (enclosure type) supported the<br />
results of the bioassays. In half a year's experiment well over 1 g. per<br />
m2 of POq-P was released by the sediments. The NTA extractable phosphate<br />
fraction decreased in the same period <strong>50</strong>%. by<br />
A Sediment Phosphate Model<br />
A schematic model discussed relates to the importance of sediment<br />
phosphate to the general phosphate cycle.<br />
Colterman, H. L., 1973. Natural phosphate sources in relation to<br />
phosphate budgets. Water Research 7, 3-17.<br />
Golterman, H. L., 1977. Sediments as a source of phosphate for algal<br />
growth (In press. Proceedings of a symposium, held in Amsterdam<br />
September 1976. Interactions between sediments and freshwater)<br />
Junk/Pudoc, The Netherlands.<br />
Meybeck, M ., 1976. Total mineral dissolved transport by world major<br />
rivers. Hydrological Sciences-Bulletin-des Sciences Hydrologiques<br />
XXI, 2 611976.<br />
Van Wazer, J R., (ed), 1961. "Phosphorus and its Compounds," 2 Vols.,<br />
Vol. 2, Interscience, New York".<br />
159
DISCUSSION<br />
Dr. D. BouZdin: Does the soil in the agar disc undergo reduction during the<br />
assay or does it remain oxidized?<br />
Dr. H. Goltermun: It remains oxidized.<br />
Dr. ,J. Shapiro: What concentrations of NTA do you use for extracting?<br />
Dr. H. Golteman: 0.01 molar, pH about 7-8.<br />
L%. d. Shupiro: Do you think the concentrations that would normally be<br />
expected from use of NTA in detergents could have any effect?<br />
Dr. H. I;olteman: No, this is about a thousand times as much.<br />
161
I NTRODKT I ON<br />
Forms and Sediment Associations of Nutrients (C.N. & P.)<br />
Pesticides and Metals<br />
Nutrients - P<br />
T. CahiZZ<br />
The purpose of this discussion is to summarize current knowledge<br />
of phosphate transport, particularly as developed during the past four<br />
years. Prior to 1972, measurement of both orthophosphate and total phosphate<br />
in natural river systems was done on a grab sampling basis, usually<br />
without corresponding stream flow measurement. The inclusion of total<br />
phosphate as a regular water quality parameter was actually not widespread<br />
prior to the mid-l960's, so that the historical record is both short and<br />
largely qualitative. A typical record of this type is shown in Figure 1.<br />
The absolute variability of concentration over time can be estimated from<br />
such records, but any projection of mass flux rate or annual mass transport<br />
is fraught with error.<br />
A number of studies conducted during 1972, 1973 and 1974, (e.g.<br />
Baker and Kramer, 1973; Cahillg &. 1974) have been based on sampling<br />
procedures utilizing synchronous measurement of storm flow and chemistry,<br />
with particular emphasis upon storm events. This research has yielded<br />
several facts regarding the behaviour of phosphate loadings.<br />
The concentration of total phosphate increases with stream<br />
discharge, so that the phosphate chemograph closely parallels<br />
the hydrograph.<br />
Soluble phosphate behaves quite differently from total phosphate.<br />
Strong correlations are often found between total phosphate<br />
and suspended solids concentrations.<br />
Mass transport during storms tends to comprise very a large<br />
proportion of total annual phosphate loadings.<br />
Past methods of estimating long term phosphate loadings on the<br />
basis of average concentrations have severely underestimated<br />
annual mass transport.<br />
More recent studies have continued to build on this knowledge. I<br />
The present discussion is based on the findings of three such investigations:<br />
1) analysis of Lake Erie tributaries in the LEWMS' project (COE',<br />
1975); 2) the Maumee River Basin Study conducted for the GLBC3 (GLBC,<br />
1976); and 3 ) recent analysis of data for Honey Creek in the Sandusky River<br />
Basin of northwest Ohio. Honey Creek has served as a pilot study area in<br />
the continuation of the LEWMS project and thus has been the of focus<br />
considerable water quality analysis. (See Figure 2 )<br />
' Lake Erie Wastewater Management Study<br />
* United States Army Corps of Engineers<br />
Great Lakes Basin <strong>Commission</strong><br />
163
"""" 4<br />
"I<br />
i:<br />
4<br />
""-<br />
0 """<br />
"""- -I=I____<br />
P<br />
""<br />
n<br />
s 1<br />
""- "<br />
I
165
Increased knowledge of the mechanisms whereby phosphorus is<br />
released from insoluble forms has suggested that the proper focus of<br />
attention in lake eutrophication studies is total phosphate, rather than<br />
soluble phosphate or orthophosphate alone. Analysis of the behaviour of<br />
total phosphate in river systems has been limited primarily to examination<br />
of relationships with discharge. In the Lake Erie basin, the strongest<br />
positive relationships with discharge are found in the western tributaries<br />
such as the Maumee (Figure 3); the weakest associations occur in the eastern<br />
basins such as the Cattaraugus (Figure 4). Prior studies in the Brandywine<br />
basin of southeastern Pennsylvania have also indicated relatively poor<br />
relationships (Figure 5 ).<br />
Analysis of total phosphate is complicated by the fact that<br />
different sources and transport mechanisms are dominant under different<br />
flow conditions. Loadings at high flow consist very largely of insoluble<br />
sediment-associated phosphate derived from nonpoint sources. During lowflow<br />
conditions, soluble phosphate from point sources is likely to predominate,<br />
although nonpoint sources such as agriculture and on-site disposal<br />
of domestic waste may also be important. The behaviour of total<br />
phosphate concentrations over time is therefore likely to reflect the mix<br />
of sources present in a watershed.<br />
A convenient step in analyzing conventional water quality records<br />
is to partition total phosphate into two components: orthophosphate, and<br />
total minus ortho phosphate. The latter is treated as an estimate of<br />
particulate phosphate, although it is known to be only approximate.<br />
(Investigators must of course acknowledge that phosphate delivered to a<br />
river system in soluble form may adsorb to particulate material in while<br />
transport). Separate analysis of these phosphate components will not<br />
necessarily establish the relative importance of specific pollutant sources,<br />
but can be very helpful in clarifying the nature of loading mechanisms.<br />
Ideally, such studies should include detailed chemical/hydrologic modeling<br />
of river basins. An intermediate level of empirical analysis is possible,<br />
however, which goes beyond simplistic plotting of the data without attempting<br />
to specify explicitly all the causal linkages responsible for observed<br />
loadings. The Honey Creek findings discussed below represent the outcome<br />
of this type of intermediate analysis.<br />
ORTHOPHOSPHATE<br />
Unlike total phosphate, orthophosphate concentrations have not<br />
been found to bear a positive association with stream discharge in most<br />
river studies, and may in fact be inversely related to discharge over the<br />
lower flow range. Such an inverse relationship may be linked to dilution<br />
of point source effluents. In the Brandywine Creek studies,'orthophosphate<br />
concentrations have been found to obey relationships of the following form:<br />
166
16 7<br />
1/9W ‘d lt1101<br />
c d d 4 0 0 0 0<br />
ID 0 Lo 0 ID 0 ID 0<br />
P<br />
N 0<br />
In N 0 c: L”
1.5 -<br />
1.2 -<br />
0.9 -<br />
0.6 -I<br />
0.3 - +<br />
'<br />
.<br />
.<br />
H"H+H<br />
0 41<br />
DISCHRRGE IN CFS<br />
Figure 4. Cattaraugus Creek at Gowanda, New York<br />
Spring 1975 Sampling Period, From LEWMS, 1976
L<br />
-<br />
=<br />
2000-<br />
$i<br />
In<br />
E<br />
c!<br />
2<br />
5 1<strong>50</strong>0<br />
E<br />
W<br />
!z<br />
X<br />
v)<br />
e 1000-<br />
n<br />
d<br />
4<br />
I-<br />
O<br />
I-<br />
<strong>50</strong>0<br />
0<br />
I I I I I I<br />
4000 8000 12000 16000<br />
FLOW cubic feet per second<br />
Figure 5. Composite of Storm Samples, 1973<br />
Chadds Ford<br />
169
C Cws + ~QP (C - Cms)<br />
Q<br />
where: C = orthophosphate concentration;<br />
CWs = orthophosphate concentration from nonpoint sources;<br />
Qp = discharge from point source "P";<br />
C = orthophosphate concentration in effluent from point<br />
P source "P";<br />
Q = stream discharge.<br />
The quantity Cms represents a base-level orthophosphate concen-<br />
tration which is related to the land use characteristics and natural features<br />
of the watershed. In the Lake Erie basin, values of Cms appear to range from<br />
100-200 pg/l in the heavily-impacted western tributaries such as the Portage,<br />
to 10-20 pg/l in the eastern watersheds. Loss of orthophosphate due to adsorption<br />
during transport may affect these relationships, although the influence<br />
of this factor is undetermined.<br />
Somewhat different findings have been obtained for Honey Creek,<br />
a basin which was chosen as a test area partly because of its agricultural<br />
nature and absence of major point sources. The orthophosphate concentration<br />
in Honey Creek appears to be positively related to discharge--but only during<br />
certain periods of the year. Analysis of Honey Creek data has been conducted<br />
by partitioning streamflow into two components; direct runoff, and groundwater<br />
outflow. The former consists of surface runoff from rainfall and snowmelt<br />
events, plus a portion of infiltrated water which passes rapidly to the tile<br />
drains underlying agricultural land. Flow separation has been accomplished<br />
solely on the basis of discharge records at the watershed mouth; experimentation<br />
indicates that the methodology utilized is not critical. The analysis of<br />
orthophosphate has then been based on the assumption that each observed<br />
concentration represents a weighted average of orthophosphate concentrations<br />
in the two flow components. That is,<br />
where: CD =<br />
CG =<br />
Q,, Q, =<br />
orthophosphate concentration in direct runoff;<br />
orthophosphate concentration in groundwater outflow;<br />
streamflow due to direct runoff and groundwater<br />
outflow, respectively;<br />
The imputed orthophosphate concentrations CD and CG have been<br />
estimated by regression analysis, utilizing the 1976 record for Honey Creek<br />
which spanned 7 months and contained more than 300 observations. Values of<br />
C and C were allowed to vary among different portions of the sampling<br />
D G<br />
period (through the use of dumy variables yielding the interesting results<br />
shown in Figure 6). Both of the imputed concentrations followed a similar<br />
pattern, falling from moderate levels in mid-winter to low values in April,<br />
then rising to high levels in June and July. The seasonal variation was<br />
more pronounced for the imputed orthophosphate Concentration in direct runoff;<br />
and this quantity was generally higher than the imputed groundwater concentration.<br />
170
0.200 0.200- -<br />
"1 "<br />
' DIRECT RUN OFF<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
0.160 0.160- -<br />
I<br />
I<br />
-I<br />
3<br />
z_<br />
z 0.120 0~120-"~"""""g -<br />
1""""""g<br />
P<br />
t a<br />
+<br />
z<br />
w<br />
y 0.08 -<br />
y 0.08-<br />
0<br />
0<br />
0.04 -<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
r""-d<br />
r""-d<br />
I<br />
p m " l<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
I<br />
GROUNDWATER<br />
OUTFLOW<br />
Figure 6. Imputed Orthophosphate Concentrations in Direct Runoff and Groundwater<br />
Outflow<br />
Honey Creek Watershed, 1976
The rise in concentrations from April to June is believed to be<br />
related to fertilizer application on agricultural land. This finding, if<br />
verified in other watersheds, could provide an important index of the<br />
agricultural contribution to phosphate loadings. The decline in concentrations<br />
from January to April could represent exhaustion of the soluble<br />
phosphate available from agricultural activities in the previous year. The<br />
mid-February decline in the direct runoff concentration could also be<br />
related to another factor. Discharge during the previous period was caused<br />
primarily by snowmelt, and contained unusually low suspended solids concentrations<br />
(see discussion below). The rate of phosphate adsorption during<br />
transport could therefore have been relatively low, due to lack of available<br />
sites. The decline in orthophosphate concentrations which followed the<br />
major rainstorm in February 16 could thus represent, in part, simply a<br />
return to more normal circumstances with regard to phosphate transformation.<br />
In any case, linkage of orthophosphate concentration to the crop production<br />
cycle appears to be the only plausible explanation for the overall patterns<br />
shown in Figure 6.<br />
The two-compartment approach utilized here for analysis of orthophosphate<br />
could easily be expanded to a three-compartment approach by<br />
incorporation of the point source equation given earlier. This of mode<br />
analysis is considered promising in cases where full-scale chemical/<br />
hydrologic modelling is not feasible.<br />
PARTICUATE PHDSPHATE<br />
There is little question that most of the total phosphate in<br />
transport during storm flow conditions is occurring in association with<br />
particulate matter, as measured by suspended solids. The overall correlation<br />
between total phosphate and suspended solids is good for most, but<br />
not all of the Lake Erie basins, (Figure 7). Linear regressions can be fitted<br />
to these data and take the form of y = mx+b, as in the Maumee;<br />
TP (ug/l)=l. 32(SSmg/1)+153 (Maumee at Waterville, 1975).<br />
The Brandywine Basin Data also found a good relationship between<br />
total phosphate and solids, but used "Residue on Evaporation" (ROE) rather<br />
than suspended solids. bThose data showed the best curve (r' fit = 0.86) to<br />
be the power curve y=ax<br />
, yielding;<br />
Total Phosphate (pg/l) = 1.48 (ROE) 0.975<br />
Of course, the ROE measurement includes both suspended and<br />
dissolved solids, and would not be suitable for basins with relatively high<br />
dissolved solids, such as the Maumee.<br />
Recognizing that the bulk of phosphate transport occurs with<br />
particulates, it is of interest to take that portion of the total phosphate<br />
which is non-soluble, defined as TP (TP - OP = TP ), and compare its ratio<br />
P P<br />
to suspended solids as the concentration changes over a hydrograph. The<br />
linkage of total minus ortho phosphate to suspended solids concentration is<br />
extremely strong. In the statistical analysis of Honey Creek data, the<br />
only additional factor needed to explain total minus ortho P concentrations<br />
172
173<br />
N N 4<br />
LD 0 v)<br />
9 Y 9<br />
4 0 0<br />
1/9W 'd ltJ101
was stream discharge (which had a very low estimated exponent). By combining<br />
and rearranging the predictive relationships for total minus ortho<br />
and suspended solids, an expression has been obtained for the of ratio the<br />
former to the latter, as a function of discharge, seasonality, and time<br />
since start of storm. This predictive equation for the phosphorus-tosediment<br />
ratio (excluding orthophosphate) is depicted graphically in Figure<br />
8. The curves in Figure 8 relate to storm periods in the spring; summer<br />
conditions would differ slightly. The phosphorus-to-sediment ratio is<br />
found to be linked positively to time since start of storm, which is plotted<br />
on the horizontal axis, and negatively to discharge.<br />
Both of these associations are probably related to systematic<br />
differences in the composition of the suspended load carried by the stream<br />
during different flow conditions. In the early stages of storm events, and<br />
during periods of high discharge generally, a large proportion of the<br />
suspended load may consist of coarse grained materials which are relatively<br />
low in phosphorus content. Since these are the materials most likely to be<br />
lost from storm water during storage and transport, the sediment load at<br />
other times tends to be limited primarily to fine colloidal particles,<br />
which are relatively high in phosphorus. Although additional studies are<br />
needed to provide fuller understanding of these relationships, it is clear<br />
that the phosphorus-to-sediment ratio is fairly stable across storm events.<br />
During non-storm periods, the ratio tends to be somewhat higher than the<br />
values shown in Figure 8 (due in part to the more prominent role played by<br />
soluble orthophosphate).<br />
CHANNEL SCOUR AND I~POSITICN<br />
An issue which has been the subject of some discussion is the<br />
role of the stream channel in sediment transport, i.e., the extent to which<br />
observed suspended solids concentrations reflect SCOUK and deposition<br />
processes in the channel system. The Honey Creek data for the February<br />
snowmelt/thaw period provide an interesting perspective on this issue. In<br />
the four-day period from February 10 to 14, high discharges occurred entirely<br />
as a result of snowmelt and thawing of the soil surface. Suspended solids<br />
concentrations at Melmore would thus reflect channel scour, and land erosion<br />
due to surface flow, rather than raindrop impact. The low concentrations<br />
observed suggest that channel scour tends to be of relatively minor importance.<br />
The following table compares the February snowmelt period with a<br />
four-day storm period in March, which involved a somewhat higher peak<br />
discharge but similar total runoff. Both of these periods occurred approx-<br />
imately two weeks after a major storm; initial discharge in each case was<br />
90 cfs.<br />
Suspended Solids<br />
Peak<br />
Discharge in cfs Load in concentration<br />
Period Duration Peak Average metric tons (md1)<br />
Feb. 10-14 4.0 days 947 820<br />
March 3-7 4.0 days 1279 805<br />
174<br />
522 104.1<br />
19<strong>50</strong> <strong>50</strong>6.5
.0030<br />
.Ei .0020<br />
c,<br />
a<br />
a:<br />
w<br />
0<br />
a,<br />
2 .0015<br />
m<br />
><br />
.OOlO<br />
-<br />
Q= <strong>50</strong><br />
.0025 - Q=i<strong>50</strong><br />
Q=1100<br />
-<br />
-<br />
I I I I<br />
0 1 2 3 4<br />
Time Since Storm Start, in Days<br />
Figure 8.<br />
Values of the Phosphorus-to-Sediment Ratio (Excluding<br />
Orthophosphate) by Time Since Storm Start and Discharge<br />
(Q) Honey Creek Watershed, 1976.<br />
175
The total suspended solids loading was nearly four times as great<br />
in the March storm as in the February snowmelt period; and the peak concentration<br />
was five times as great. There appears to be no reason, other than<br />
the somewhat lower peak discharge, why net channel scour should be less in<br />
the February period than in the March period. Scour should perhaps be greater,<br />
due to the low utilization of transport capacity by sediment from the land<br />
surface. Thus, if these figures can be regarded as representative, one<br />
might conclude that channel scour is responsible for no more than 25% about<br />
of the total suspended solids load in a moderate storm (and possibly much<br />
less, in view of the other potential sources of sediment during snowmelt/thaw<br />
periods). The implication would be that the bulk of suspended solids and<br />
phosphorus loadings from small basins such as Honey Creek tend to originate<br />
from the land during the same storm period which in they are observed.<br />
Hydrologic Response<br />
A great deal of analysis has been done (LEWMS, 1975) which considers<br />
the variation in concentration of total phosphate with hydrologic response<br />
in different watersheds, showing the seasonality, storm duration mag- and<br />
nitude effects. Several multipeaked hydrographs have also been analyzed,<br />
and demonstrated the "hysteresis" effect. The conclusion drawn from these<br />
data is that a rigorous analysis of individual events in a watershed is<br />
necessary to understand the generation of diffuse pollutants, and any<br />
application of "annual loading factors" is misleading at best. Of particular<br />
interest in the data analyzed was the large variability in mass<br />
transport produced with different storms. It was seen that any estimate of<br />
load contributed per unit area of watershed (kg/ha) would depend entirely<br />
upon the period sampled, and thus we can begin to understand why the literature<br />
shows such a great variability when different sets of data are compared<br />
in this way. Expressed quite simply, there is no such thing as "average"<br />
mass flux produced in a watershed, but rather the diffuse pollutant transport<br />
which occurs is entirely a function of the hydrologic response of the<br />
basin, and the mechanisms which generate the response determine the pathways<br />
by which pollutants are brought into suspension or solution and pass<br />
through the system. The fact that greater storms produce greater transport<br />
per "unit area" could be interpreted to mean that an increased percentage<br />
of the basin land area was having pollutants removed form its surface, as<br />
"partial area" hydrology would suggest (Dunne, 1973). Dunne and others<br />
have suggested that the hydrologic response a of basin does not occur a on<br />
uniform basis, but that incident precipitation on certain areas produces<br />
the immediate hydrograph rise. These partial areas are flat, impervious<br />
soils with high water table, usually contiguous to the drainage channels.<br />
Since diffuse pollutant transport occurs in association with this runoff,<br />
the management implication is that only a small portion of a total watershed<br />
need be regulated to reduce the production of these pollutants.<br />
TOTAL PHOSPHATE - (~GANIC (COD) TRANSPORT<br />
One might question the possibility that much of the total phos-<br />
phate measured with storm transport is occurring in organic matter, or<br />
detritus, rather than in association with inorganic matter or soil particles.<br />
176
Everything from decaying vegetation to macroinvertebrates are flushed from<br />
a river bottom or land surface and of course contain phosphorus. The only<br />
parameter measured over hydrographs which might express the organic transport<br />
has been Chemical Oxygen Demand (COD). It has been observed that<br />
there is a very great increase in concentration of COD during runoff,<br />
reaching values averaging 40 mg/l and peaks of over 100 mg/l. This represents<br />
an organic transport which, in many basins, far exceeds the input<br />
from point sources.<br />
As far as the relationship between total phosphorus and COD goes<br />
however, it is very poor; that is, while both materials are in transport<br />
during a runoff event, they do not seem to occur in association with each<br />
other. While these data is far from conclusive, they do not support the<br />
"organic phosphorus" source possibility.<br />
PHOSP~DRUS TRANSPORT THROUGH ESTUARIES<br />
A key question pertaining to the storm transport of phosphorus<br />
out of river basins into a lake such as Lake Erie, is just how much phosphorus<br />
actually gets flushed through the estuary and reaches the lake. Knowing<br />
the nature of these estuaries, the tremendous dredging required to maintain<br />
the shipping channels and the sediment-associated nature of most of the<br />
total phosphorus, one might speculate that we have exaggerated the loading<br />
problem, and in actuality only a fraction of a tributary basin's nutrients<br />
actually reach the lake. However, synchronous sampling of riverine and<br />
estuarine stations in both the Maumee and Cuyahoga basins during several<br />
spring storms in 1975 strongly suggest, although not conclusively, that<br />
most if not all of the sediment-associated phosphate is flushed through the<br />
estuary during a large storm. A great deal more work, however, is necessary<br />
to answer this question satisfactorily.<br />
BIOLOGICAL UPTAKE DURING NON-STORM CONDITIONS<br />
One final question pertains to the phosphate which is discharged<br />
from treatment plants and either taken up by aquatic vegetation or precipitated<br />
by calcium ions to settle on the stream bottom. These mechanisms<br />
are significant in many basins, especially during summer, (TMACOG', 1976).<br />
This "point source residual" can accumulate in a stream, and subsequently<br />
may be scoured during storm runoff. However, a continuous mass balance<br />
analysis for several watersheds with large point source inputs, such as the<br />
Portage basin, demonstrated that only a minor portion of the total mass<br />
transport of phosphate during storms can be accounted for by this residual.<br />
SEARCH NEEDS<br />
In an effort to better guide the management strategies which are<br />
evolving with respect to the reduction and control of diffuse or non-point<br />
sources in the Great Lakes, some general knowledge gaps can be identified<br />
' Toledo Metropolitan Area Council of Governments<br />
177
egarding phosphate which have broad applicability to other pollutants.<br />
Because of the immediate need for this information, these deficiencies receive<br />
a higher priority than some of the long term research needs. These knowledge<br />
gaps can be filled by four general programs carried out jointly by the various<br />
agencies operating in the Great Lakes. They as are follows:<br />
(1) Evaluate the effectiveness of land management techniques,<br />
especially agricultural, for nutrient load reduction;<br />
(2) Analyze storm transported phosphate to determine long<br />
term bioavailability;<br />
(3) Develop hydrologic response models which more accurately<br />
simulate pollutant production and transport mechanisms, and<br />
(4) Evaluate both the positive and negative aspects of widespread<br />
acceptance of tillage modification techniques, such as no-till.<br />
While a great wealth of water quality data has been developed<br />
throughout the Great Lakes Basin, is it likely that additional sampling<br />
will be required for selected watersheds to accomplish the first three<br />
programs. Other important research questions should not be neglected,<br />
including a reevaluation of erosion loss analysis techniques (USLE) as<br />
applied to watersheds, modelling particulate transport during storms, lake<br />
bottom release rates and tile drainage effects, to mention only a few.<br />
However, the primary objectives of research during the next to two five<br />
years should centre on land management techniques which will actually<br />
reduce diffuse pollutants and the evaluation of present erosion control<br />
techniques to determine if they will accomplish the necessary reduction.<br />
LITERATURE CITED<br />
Baker, D. B. and Kramer, J. W., (1973). Phosphorus Sources and<br />
Transport in an Agricultural River Basin of Lake Erie,<br />
Proc. 16th Conf. Great Lakes Res.<br />
Bowen, D. H. M., (1970). The Great Phosphorus Controversy, Environmental<br />
Science and Technology, 6, 725-726.<br />
Cahill, T. H., P. Imperato and F. H. Verhoff, (1974). Evaluation of<br />
Phosphorus Dynamics in a Watershed, Journal of the<br />
Environmental Engineering Division, ASCE, April<br />
100, (EEZ), Proc. Paper 10445.<br />
-<br />
Cahill, T. H., J. Adam and D. Baker, (1976). The Importance<br />
of Diffuse Pollutants in River Chemistry, Presented at the 19th<br />
Conference on Great Lakes Research, May 1976.<br />
Cahill, T. H., P. Imperato, P. K. Nebel and F. H. Verhoff, (1975).<br />
Phosphorus Dynamics In A Natural River System, in WATER-<br />
1974, 145, 71, A.I.Ch.E., New York.<br />
Cleaves, E. T., Godfrey, A. E. and 0. P. Brinker, (1970). Geochemical Balance<br />
of a Small Watershed and its Geomorphic Implications, Geological<br />
Society of America Bulletin, e, 3015.<br />
178
Connell, C. H., (1965). Phosphates in Texas Rivers and Reservoirs, Water<br />
Research News No. 73, Southwest Water Research Council,<br />
Fort Worth Tex.<br />
Deevy, E. S., Jr., (1970). Mineral Cycles, Scientific American, 223,<br />
(3), 149-158.<br />
Dunne, T., and Black R. D., (1970). Partial Area Contributions to Storm<br />
Runoff in a Small New England Watershed, Water Resources<br />
Research, 6, 1269.<br />
Hammer, Thomas, H., (1976). Interim Report; Urban Land/Water<br />
Relationships, U.S.E.P.A., Cont. No. 68-01-3551, June 1976.<br />
Hutchinson, G. E., (1969). Eutrophication, Past and Present, Eutrophication:<br />
Causes, Consequences, Correctives, National Academy of Sciences,<br />
Washington, D. C., pp. 17-26.<br />
Johnson, N. M., g., (1969). A Working Model for the Variation in<br />
Stream Water Chemistry at the Hubbard Brook Experimental Forest, New<br />
Hampshire, Water Resources Research, 1, 1353.<br />
Keup, L. E., (1968). Phosphorus in Flowing Streams, Water Research,<br />
-<br />
2, 373.<br />
Lee, 6. F., (1969). Analytical Chemistry of Plant Nutrients, Water and<br />
Sewage Works, 116, R-38.<br />
Logan, T. J. and G. 0. Schwab, (1976). Nutrient and Sediment Character-<br />
istics of Tile Effluent in Ohio, Journal of Soil and Water<br />
Conservation, 2, (1).<br />
Mackenthun, K. M., (1965). Nitrogen and Phosphorus in Water, An Annotated<br />
United States Selected Bibliography of Their Biological Effects,<br />
Publication 1305. United States Department of Health, Education<br />
and Welfare, Division of Water Supply and Polution Control,<br />
pp. 1-112.<br />
Mackenthun, K. M., (1968). The Phosphorus Problem, Journal of the Water<br />
Works Association, 60, 1047-1053.<br />
McKee, 6. D., &, (1970). Sediment-Water Nutrient Relationships, Part 2,<br />
Water and Sewage Works, 177, 246.<br />
National Assessment of Trends in Water Quality, Environmental Control,<br />
Inc., National Technical Information Service, Springfield,<br />
Va., June, 1972.<br />
Rendon-Herrero, 0. J., (1974). Estimation of Washload Produced on Certain<br />
Small Watersheds, Journal of Hydraulics Division, ASCE, 100, 835.<br />
Rigler, R. H., (1956). A Tracer Study of the<br />
Water, Ecology, x, 5<strong>50</strong>-562.<br />
179<br />
Phosphorus Cycle in Lake
Sandoval, M. T., T. H. Cahill, and F. H. Verhoff, (1975). Mathematical<br />
Modellings of Nutrient Cycling in Rivers, Div. of Env. Chem.<br />
ACS., Phila., Pa., April 6, 1975.<br />
Wang, W. C. and Evans, R. L., (1970). Dynamics of Nutrient Concentrations<br />
in the Illinois River, Journal Water Pollution Control Federation,<br />
- 42, 2117.<br />
Wolman, M. G., (1971). The Nation's Rivers, Science, 174, 905.<br />
180
I NTRODKT I ON<br />
Forms and Sediment Associations of Nutrients (C.N. & P.)<br />
Pesticides and Metals<br />
Nutrients - N<br />
T. Logan<br />
Nitrogen along with phosphorus are nutrients that are required by<br />
most organisms in large quantities. These organisms range from the<br />
smallest bacteria to algae and also higher plants used by man for food.<br />
Many authors have cited nitrogen and phosphorus as the nutrient<br />
elements limiting productivity in waters (Mackenthun &., 1967;<br />
Hasler, 1970). Keeney indicated that N may be more important than P<br />
in limiting growth in eutrophic lakes, especially those high P in<br />
(Keeney, 1972).<br />
This review will consider:<br />
1. The forms of nitrogen involved in fluvial transport with emphasis<br />
on sediment N.<br />
2. The relative sources of nitrogen entering fluvial systems.<br />
3. The microbiological and chemical transformations of nitrogen<br />
involved in sediment-aquatic environments.<br />
4. The role of the C/N ratio in nitrogen transformations.<br />
5. Identification of research problems and knowledge gaps.<br />
1, FORMS OF NITROGEN<br />
Nitrate-N<br />
Ions<br />
- NO-3<br />
Ammonium-N NH+u<br />
Nitrite-N NO-2<br />
181<br />
soluble anion; product of<br />
nitrification; used by most<br />
organisms; not retained in<br />
soil, leaches readily.<br />
soluble cation; product of<br />
decomposition of organic<br />
materials; utilized by most<br />
organisms; retained on neg-<br />
atively charged clay particle.<br />
soluble anion; product of<br />
biological oxidation; toxic<br />
to higher plants; does not<br />
usually occur in high concentrations;<br />
will accumulate in<br />
soil or sediment at high pH<br />
and under anaerobic conditions.
Ammonia-N<br />
Nitrous oxide<br />
Nitrogen<br />
PARTICULATE FORMS<br />
Gases<br />
product of biological<br />
decomposition; toxic;<br />
evolved when pH is high<br />
or under dry soil conditions.<br />
product of denitrification;<br />
constituent of atmosphere;<br />
product of denitrification.<br />
Many attempts have been made to identify the mostly organic forms<br />
of nitrogen In biological systems. In addition to the biologically viable<br />
compounds in fauna and flora tissue including protein, amino acids, and<br />
nucleic acids. there are the complex N forms found in decomposed organic<br />
materials. This organic matter, also called humus is the product of<br />
primarily microbiological decomposition. Brenmer (1967) summarized the<br />
extensive work in soil organic nitrogen characterization. Based on this<br />
work, soil organic nitrogen may be divided into several categories (Table<br />
1). Much of the soil organic N remains unidentified and may include amino<br />
acids other than those previously identified and heterocyclic nitrogen<br />
compounds (Bremner, 1967).<br />
+<br />
The small amount of fixed MI, is primarily a consequence of the<br />
+<br />
physiochemical entrapment of NHI, ions between the internal surfaces of<br />
expandable clays such as illite (Figure 1). The degree of fixation will<br />
vary with expandable clay content and the extent of interlayer weathering.<br />
The bioavailability of particulate N has concerned agronomists<br />
for years and is also of interest to ecologists studying the impact of<br />
tributary loadings on lake water quality. Attempts to correlate N<br />
bioavailability with a particular fraction or fractions has been unsuccessful.<br />
In recent years, more attention has been given to direct bioavailability<br />
and mineralization tests (Stanford, 1968) and correlations<br />
between chemical extraction and N released by incubation (Stanford and<br />
Smith, 1976). These tests show some promise in estimating potential N<br />
mineralization in soil but have not been extended to aquatic systems.<br />
2, SOURCES OF NITFKIGEN ENTERING FLUVIAL SYSTEMS<br />
a) Runoff and Erosion - the main product of runoff and erosion is<br />
surface soil and some crop residue, leaves and other debris. Some<br />
soluble Nos-N and N1k-N is transported during runoff; however,<br />
if erosion is significant the organic soil N contribution<br />
will be much greater (Armstrong et&., 1974). Runoff sediment<br />
is enriched with nitrogen, i.e. the total N content of the<br />
sediment is higher than the soil from which it originated.<br />
Hensler and Attoe (1970) found that total N enrichment was<br />
2.7 to 5 fold. This is due to selective clay transport and<br />
also the lower density of some organic matter. The total<br />
particulate N content of sediment can be estimated<br />
from a knowledge of the soils from which it was derived.<br />
182
TABLE 1.<br />
FORMS OF PARTICULATE N IN SOILS<br />
Form X of total N<br />
Amino - N 20-40<br />
Hexosamine 5-10<br />
Purines and pyrimidines -1<br />
Unidentified forms 30-<strong>50</strong><br />
Fixed NH4 3-8<br />
TABLE 2.<br />
CHARACTERISTICS OF WASTEWATER EFFLUENTS<br />
Primary Trickling Secondary Ponds<br />
Activated<br />
Sludge<br />
”----% 1-<br />
Total N 31 16 23 23<br />
NO 3-N 0.3 6.3 3.9 0.7<br />
NH4-N 23 5.9 17.0 a. o<br />
183
CI<br />
0<br />
0<br />
0<br />
0<br />
0<br />
0<br />
0<br />
0 0
Jenny (1941) in his classic thesis on soil formation indicated<br />
that soil N increased with increasing rainfall and showed that<br />
soils developed under grasses contained higher N levels than<br />
under forest vegetation (Figure 2). For most of the Great<br />
Lakes basins, the total N of virgin soils would be about 0.1<br />
- 0.25%. Cultivation has resulted in substantial mineralization<br />
of soil N (Figure 3) and present levels are about 60-70% of the<br />
virgin soil N. Further net soil N loss is quite low and crop<br />
residue management and adequate N fertlization can maintain<br />
soil N levels.<br />
b) Tile Drainage and Seepage - this source will contain mostly<br />
nitrate; low concentrations of NHb-N and dissolved organic -N<br />
may be found in tile drainage (Armstrong et&., 1974).<br />
c) Precipitation - varying amounts of N (5-<strong>50</strong> kg/ha) can be added<br />
in rainfall; made up of both nitrate and ammonia.<br />
d) Sewage - the major source of N from domestic sewage is that<br />
discharged as secondary treated effluent. Pound and Crites<br />
(1973) indicated that depending on the method of treatment (Table<br />
Z), effluent will contain varying amounts of N forms with<br />
organic N as the major constituent. The organic N is quite<br />
stable and resistant to further mineralization. Septic tank<br />
leakage can be a major component N of loading in some watersheds,<br />
but this source is difficult to quantify. Most input<br />
would be as nitrate.<br />
e) Livestock Wastes - Improper disposal of livestock wastes due<br />
to inadequate storage facilities or because of excessive<br />
application to soil can result in significant N additions to<br />
streams. Livestock wastes are highly variable in their<br />
characteristics depending on livestock type and the degree of<br />
decomposition of the manure. Runoff of fresh manure from<br />
barnlots or feedlots can result in high BOD and NH3 loadings<br />
which can have disastrous effects on downstream aquatic<br />
habitat. Leaching of NO,-N from excessively manured land will<br />
mean increased N loading. Livestock wastes vary in total N<br />
content (Table 3) and the percentage of the total N that is<br />
subject to mineralization. In general, about <strong>50</strong>% of the total<br />
N is mineral ( NHJ + NO3) and about 25-<strong>50</strong>% of the organic N is<br />
mineralizable in the first year.<br />
f) Processing Wastes - may be high in both NHk-N and organic N.<br />
BOD levels are usually high. A localized problem.<br />
g) Overall Sources of N to Great Lakes - Various estimates of<br />
point and nonpoint sources of nitrogen would indicate that about<br />
30-<strong>50</strong>% of the total N loadings are from point sources, particularly<br />
wastewater effluents. Loadings of N to Lake Erie<br />
from various tributaries (Table 4) indicate that most of the N<br />
is in the form N03-N of with smaller amounts of organic and<br />
NH4-N. If we assume an annual mineralization of 3% for the<br />
organic N (based on rates for soil N), then it would appear that<br />
sediment N is relatively insignificant as a source N of loading<br />
to Lake Erie.<br />
185
c<br />
C<br />
0,<br />
0<br />
C<br />
0<br />
C<br />
al<br />
0)<br />
2 4- .-<br />
0.300<br />
- 0 Grassland soils<br />
-<br />
8 Grassland soils (averages)<br />
- t Forest soils<br />
- + Forest soils (averages)<br />
-<br />
-<br />
-<br />
$J 0.200 -<br />
a<br />
- -<br />
.-<br />
5:<br />
-<br />
LC -<br />
0 - -<br />
C<br />
0,<br />
c -<br />
-<br />
-<br />
-<br />
- Q<br />
E 0.100 -<br />
Humidity factor (N.S.Q.)<br />
+<br />
New Jersey<br />
Figure 2. Effect of rainfall on soil N content (Jenny, 1941)<br />
+
18 7<br />
I<br />
0<br />
0<br />
0..<br />
0<br />
0
Stream<br />
Cuyahoga<br />
Sandusky<br />
Black<br />
Huron<br />
Portage<br />
TABLE 3.<br />
CHARACTERISTICS OF LIVESTOCK WASTES<br />
Dairy Cow 3.9<br />
Beef Feeder 4.9<br />
Swine Feeder 7.5<br />
Total N (Percent of total Solids)<br />
Sheep Feeder 4.5<br />
Poultry Layer 5.4<br />
Poultry Broiler 6.8<br />
Horse 2.9<br />
TABLE 4.<br />
TRANSPORT OF NITROGEN FROM STREAMS DRAINING INTO LAKE ERIE<br />
(1974-1975) LAKE ERIE WASTEWATER MANAGEMENT STUDY<br />
Sediment<br />
mt/yr x lo3<br />
1222<br />
483<br />
270<br />
136<br />
71<br />
48<br />
* Percent of total Kjeldahl-N<br />
~_______<br />
N03-N + N02-N<br />
mtlyr % *<br />
34700 594<br />
2020 120<br />
5170 85 2<br />
3520 297<br />
1180 317<br />
2100 808<br />
18 8<br />
Ammonia-N<br />
mt/yK %<br />
966 16.5<br />
476 20.3<br />
213 35.1<br />
338 28.5<br />
46 12.4<br />
57 21.9<br />
Organic-N<br />
mt/yK %<br />
5174 88.6<br />
1200 71.4<br />
394 64.9<br />
847 71.5<br />
326 87.6<br />
203 78.0<br />
Kjeldahlmtlyr<br />
5840<br />
1680<br />
607<br />
1185<br />
372<br />
260
3. BIOLOGICAL NITROGEN TRANSFONTIONS IN SEDIENT/WATER SYSTEMS<br />
The important sediment-nitrogen reactions are visualized<br />
in Figure 4 (Keeney, 1972).<br />
a) Nitrogen Fixation - primarily by blue-green algae (Cyanophyceae).<br />
Some fixation by anaerobic heterotrophs has been reported but<br />
N fixation in sediments is probably of only minor importance<br />
(Brezonik, 1971).<br />
b) Immobilization - also termed assimilation. In aquatic systems,<br />
+<br />
NHs-N assimilation is probably more significant than N03-N.<br />
Whereas, in terrestial systems (soils) bacteria and fungi are<br />
dominant in N assimilation, in aquatic systems the phytoplankton<br />
and herbivorous zooplankton are more important because of their<br />
numbers and capacity to assimilate organic materials. Bacteria<br />
play an important role in the decay of these organisms. Bacteria<br />
may aLso be very important in utilizing organic N secreted by<br />
zooplankton and converting it to mineral N (Keeney, 1972).<br />
c) Mineralization - Ammonification - Decomposition of organic<br />
materials by heterotrophic bacteria results in the release of<br />
mineral N as NH;. N regeneration, i.e. net N release, is<br />
determined by the stability of organic forms and the ability<br />
of the bacteria to convert organic C to cellular C (Foree<br />
" et al., 1971). Net mineralization appears to be less efficient<br />
under anoxic conditions than when the system is aerated. The<br />
degree of mineralization of organic N in sediments will depend<br />
on the extent to which the organic material is humified (Bremner,<br />
1967). Recent organic deposits will be mineralized to a greater<br />
extent than organic N from soils. In addition, there is some<br />
degree of stability imparted by the interaction of organic compounds<br />
with clay minerals. Zooplankton are involved in ammonification by<br />
excreting NH3 and amino acids which they gain by feeding on<br />
phytoplankton + detritus. Johannes (1968) believes this<br />
to be the dominant mechanism of ammonification in surface<br />
waters. Direct autolysis after cell death may account for<br />
30-<strong>50</strong>% of N released from plant and animal material. Bacterial<br />
decomposition is considered to be of minor importance in N<br />
mineralization in shallow lakes except where sewage contributes<br />
large amounts of organic matter. In sediment systems, however,<br />
bacterial and fungal mineralization is the dominant process.<br />
d) Nitrification - Biological oxidation of nitrogen to nitrite and<br />
nitrate is performed by heterotrophs including bacteria, fungi<br />
and algae; however, these are of limited importance compared with<br />
the autotrophic bacteria. The net nitrification is given as:<br />
N H + ~ 20; -f NO; + H ~ + O ZH+ + energy<br />
Nitrite, NO;, is an intermediate oxidation product, but is rzrely<br />
present in significant concentrations because the rate NOz of +<br />
oxfdation is much faster than the initial oxidation NHr of to<br />
N02.<br />
189
ATMOSPHERE<br />
WATER NO,-, NH,', Organic N<br />
SEDIMENT ~,y<br />
{ Rainwater 3<br />
External Sources<br />
Particulate organic N-NH,'<br />
[-I - NH,' (Soluble<br />
(Nitrification)<br />
Exchangeable)<br />
9<br />
NO,- in Groundwater<br />
Figure 4. Generalized nitrogen cycle in waterlsediment system (Keeney, 1972)
The conditions for nitrification are favourable in fluvial Systems<br />
above 1 O C and there is evidence to indicate that nitrification<br />
will also proceed in bottom sediments if they are stirred and pH<br />
buffered (Keeney, 1972).<br />
e) Denitrification - The biological reduction of nitrate to gaseous<br />
N (NO, NzO, N2). This is a significant reaction in streams and<br />
lakes, leading to loss of nitrogen from the system. Bacteria<br />
responsible for denitrification include species of the genera<br />
Pseudomonas, Alcaligenes, Achromobacter, Bacillus and Micrococcus.<br />
Denitrification occurs in the absence 0,, of induced by 0,<br />
consumption during decomposition of organic material (BOD) and<br />
by the limitation of O2 diffusion in quiescent water. Denitrification<br />
is limited by temperature (optimum at 60-65°C<br />
and considerable activity above 20°C) and pH (optimum at pH's<br />
near 7). Denitrifiers require a readily available source of<br />
organic carbon. Denitrification will be high in high BOD stream<br />
sections, especially where the organic carbon is easily decomposable.<br />
Denitrification - nitrification can occur simultaneously (Keeney,<br />
1972) and this process is responsible for much of the disappearance<br />
of NOB-N from lakes. The role of denitrification in fluvial N03-N<br />
balance has not been studied extensively; however, it would appear<br />
that conditions for denitrification would be optimum during low<br />
flow summer periods if sufficient carbo? is present. Reddy<br />
al. (1976) have recently shown that NHs in the anoxic zone<br />
of sediment can be denitrified NH,, + as diffuses into the oxic<br />
zone and is nitrified. The nitrate then diffuses down into the<br />
anoxic zone where it is decitrified.<br />
4, THE F~LE OF THE CARBON/NITROGEN RATIO IN NITROGEN TRANSFORNITIONS<br />
All organisms have a characteristic C/N ratio, depending on<br />
the relative amounts of protein in their biomass. Higher plants<br />
may range from as high as 1OO:l for some straw residues to as low<br />
as 2O:l for some of the legumes (Brady, 1974). Bacteria and other<br />
microorganisms have a ratio of about 9:l. During the heterotrophic<br />
decomposition of organic materials, mineral N (N03-N) may be assimilated<br />
or released depending on the C/N ratio of the mineral being decomposed<br />
(Figure 5). Much of the N assimilated by the heterotrophs will be<br />
mineralized as these organisms themselves die and are decomposed. The<br />
N not mineralized becomes part of the solid phase (soil or sediment)<br />
as organic matter with a C/N ratio of about 10-12:l. This nitrogen<br />
is quite resistant to further decay. The C/N ratio of decomposable<br />
material in transport will determine if there is a net gain or loss<br />
of N from the system due to microbiological transformations.<br />
5. SEDIPENT-NITROGEN TRANSFORMATIONS DURING FLUVIAL TRANSPORT<br />
a) During Storm Runoff - during periods of maximum transport in the<br />
early spring, biological nitrogen transformations will be minimal<br />
due to the short transport intervals and cooler temperatures.<br />
191
192
Sediment nitrogen will closely reflect source materials (eroded<br />
soil, resuspended bottom sediments). These sediments are likely<br />
to have C/N ratios of about 10-12:l to and be quite stable.<br />
Low Flow Winter Runoff - this is a period of minimum sediment<br />
transport. Most of the nitrogen transported will be NOS-N from<br />
seepage and tile flow with some NHq-N from point discharges.<br />
There will be a minimum of biological activity.<br />
Low Flow Summer Runoff - during this period, sediment transported<br />
will be low; nitrogen transport will be minimal and confined to<br />
point sources. Biological activity will be high. Sediment-water<br />
phase reactions involving biological nitrogen transformations<br />
will be operating under optimum conditions. Nitrate will be<br />
removed from the water phase by assimilation and denitritifation.<br />
The net addition or removal of N frpm the system will depend<br />
on the environmental conditions determined by stream characteristics.<br />
Sediment acts to buffer the transport of nitrogen as it<br />
does for phosphate. The multitude and complexity of biological<br />
N transformations make N transport a very dynamic process and<br />
makes estimation of delivery ratios to streams difficult (McElroy<br />
" et al., 1976).<br />
6, GAPS IN CURRENT WWLEDGE<br />
Most of the available literature pertains to terrestial or lake<br />
systems. In-stream nitrogen transformations involving sediment<br />
nitrogen have not been studied extensively.<br />
The extent of microbial and algal N fixation in streams not is<br />
known.<br />
A satisfactory model for N transformation during fluvial transport<br />
is needed; this is especially true of in-stream assimilation and<br />
denitrification.<br />
The extent of transport of N in organic materials (crop residues,<br />
leaf litter, detached aquatic vegetation and zooplankton) needs<br />
to be further elucidated.<br />
Analytical methods to determine bioavailability of suspended<br />
sediment nitrogen should be developed.<br />
The effect of estuaries on nitrogen loadings to the lake is not<br />
known.<br />
In summary, the processes of nitrogen cycling and interchange<br />
in sediments are complicated and poorly understood. The mechanisms whereby<br />
nitrogen is exchanged between water and sediments are probably known,<br />
and in some cases qualitative rankings can be given to their importance.<br />
However, almost no information is available to establish the in situ<br />
rates and controlling factors for these processes (Brezonik, 1971).<br />
193
LITERATURE CITED<br />
Armstrong, D. E., K. W. Lee, P. D. Uttomark, D. R. Keeney, and R. F.<br />
Harris, (1974). Land Use/Water Quality Relationships in the<br />
U.S. Great Lakes Basin. Task A6: Nutrients. PLUARG, IJC,<br />
Windsor. Ontario.<br />
Brady. N. C., (1974). The Nature and Properties of Soils, 8th Edition,<br />
Macmillan, New York.<br />
Brewer, J. M., (1967). Nitrogenous Compounds. In Soil Biochemistry,<br />
A. D. McLaren and G. H. Petersen, Eds., Marcel Dekker, N. Y., pp.<br />
19-66.<br />
Brezonik. P. L., (1971). Nitrogen: Sources and Transformations in<br />
Natural Waters. Presented at American Chemical Society, Los<br />
Angeles, California.<br />
Foree, E. G., W. J. Jewel1 and P. L. McCarty, (1971). The Extent of<br />
Nitrogen and Phosphorus Regeneration from Decomposing Algae.<br />
Water Research, I (27).1-15.<br />
Hasler, A. D., (1970). Man-Induced Eutrophication of Lakes. Global<br />
Effects of Environmental Pollution, S. F. Singer, Ed. Springer-<br />
Verlag, N. Y., pp. 110-125.<br />
Hensler, R. F. and 0. J. Attoe. (1970). Rural Sources of Nitrates<br />
in Water. Proc. 12th Sanit. Eng. Conf., Nitrate and Water Supply,<br />
Source and Control, Univ. Illinois. Bull., %,(2),86-98.<br />
Jenny, H., (1941). Factors of Soil Formation. McGraw-Hill, New York.<br />
Johannes, R. E., (1968). Nutrient Regeneration in Lakes and Oceans.<br />
Adv. Microbiol. Sea., 1,203-212.<br />
Keeney, D. R.. (1972). The Fate of Nitrogen in Aquatic Ecosystems.<br />
Eutrophication Information Program, University of Wisconsin,<br />
Madison, Wisc.<br />
McElroy, A. D., S. Y. Chiu, J. W. Nebgen, A. Aleti and F. W. Bennett,<br />
(1976). Loading Functions for Assessment of Water Pollution from<br />
Nonpoint Sources. Midwest Research Institute, EPA-600/2-76 151.<br />
Mackenthun, K. M. and W. I. Ingram, (1967). Biological Associated<br />
Problems in Freshwater Environments - U.S. Dept. Interior, FWPCA,<br />
Washington, D. C.<br />
Nomnik, H.. (1965). Ammonium Fixation and Other Reactions Involving a<br />
Nonenzymatic Immobilization of Mineral Nitrogen in Soil. In<br />
Soil Nitrogen, Amer. SOC. Agron. Monograph No. 10.<br />
Pound, C. E. and R. W. Crites, (1973). Characteristics of Municipal<br />
Effluents. Proc. <strong>Joint</strong> Conf. Recycling Municipal Sludges and<br />
Effluents on Land, Champaign, Ill.<br />
Reddy, K. R., W. H. Patrick, Jr. and R. E. Phillips, (1976).<br />
Ammonium Diffusion as a Factor in Nitrogen Loss from Flooded<br />
Soils. Soil Sci. SOC. her. Journ., 3,528-533.<br />
194
Stanford, G., (1968). Extractable Organic Nitrogen and Nitrogen<br />
Mineralization in Soil. Soil Sci., 106,345-351.<br />
Stanford, 6. and S. 3. Smith, (1976). Estimating Potentially<br />
Mineralizable Soil Nitrogen from a Chemical Index of Soil<br />
Nitrogen Availability. Soil Sci., =,71-76.<br />
ADDITIONAL LITERATURE<br />
Likens. G. E., (1972). Nutrients and Eutrophication: The Limiting-<br />
Nutrient Controversy, Symposium. American Society of Limnology<br />
and Oceanography, Allen Press, Lawrence, Kansas.<br />
Allen, H. E. and J. R. Kramer, (1972). Nutrients in Natural Waters.<br />
Environmental Science and Technology Series, Wiley-Interscience,<br />
John Wiley and Sons, New York.<br />
195
DISCUSSION<br />
Dr. R. Swank: Relative to your problem on nitrogen balances in marshes or<br />
the ability of marshlands and wetlands to attenuate the nitrogen loads,<br />
there is a large study going on now, sponsored by the Smithsonian Institute<br />
in the Chesapeake Bay area in Maryland, in which I understand, they are<br />
focusing primarly on nitrogen, phosphorus and carbon in wetlands. This is<br />
in both salt water marshes and freshwater marshland. We hope to get some<br />
useful data out of that. The laboratory where I work (U.S. EPA, Athens,<br />
Georgia) is also sponsoring some work at the Westpoint Military Reservation<br />
where there are rather extensive small freshwater marshes driven almost<br />
entirely by sub-surface flow, intermittent flows and runoff from primarily<br />
forested and pasture type land use. There are no pasturing animals on it,<br />
just grassland. That might also be very useful for this purpose. We are<br />
focusing on that area with regard to some modelling studies. It is a major<br />
plus because the data so far suggest that marshes are particularly effective<br />
in absorbing nitrogen - i.e. denitrification. They appear to be great sinks<br />
for nitrogen of all forms, less so for phosphorus and the carbon situation<br />
is unclear. Frankly, there apparently seems to be an equilibrium in the<br />
marshes, but there are a lot of data being generated there lot and of a<br />
research interest in that domain as well.<br />
With regard to the nitrogen sources there is some work that<br />
was done by EPA in the agricultural runoff model. We are taking a very<br />
close look at the runoff forms of nitrogen by continuous simulation, but<br />
it is a process model. It is essentially a marriage of the lump parameter<br />
hydrology model with a process nitrogen model. We are doing a lot of calibration<br />
of that model or testing it, as well as calibration of parameters in<br />
various locations around the U.S. We have four years of data at Watkinsville<br />
which is in Piedmont, Georgia, near the location of my laboratory. Those<br />
data are about to be published and should be of great interest both from<br />
pesticides, sediment, water yield and nitrogen yield for agronomic crop growth<br />
operations. There are about four different watersheds, a variety of practices,<br />
a variety of crops and a variety of management practices.<br />
We have extended that study rather comprehensively to Iowa<br />
State University on Four Mile Creek. Iowa State, Purdue and EPA are involved<br />
in that one as well. We are looking at in-stream processes there, trying to<br />
make some kind of rational judgements about the kinds of nitrogen work you<br />
are talking about as a data gap. We recognize it also as a severe data gap.<br />
We hope to have some data out of that, in of terms sediment movements in streams,<br />
loading and dynamic loading. Continuous simulation is the approach we are<br />
taking. We have extended that further to do some regionalization based on<br />
197
the Smithsonian Institution's work primarily in the Chesapeake Bay area. We<br />
are participating in that study again with the of idea calibrating on a<br />
regional basis the R model with this kind of concept. The U.S. Department<br />
of Agriculture is involved in that with some of their continuous simulation<br />
approaches. I think they are going to look at ACMO and some of the other new<br />
models they have developed recently. So we again are hoping to "piggy-back"<br />
onto many researchers for this kind of work. I just wont to indicate that<br />
there is a lot of work underway in some of these data gap areas that may<br />
directly relate to the problems of the Great Lakes, even though they are not<br />
in fact being done in assocation with any of the Great Lakes programs.<br />
198
I NTRODKT I ON<br />
Forms and Sediment Associations of Nutrients (C.N. & P.)<br />
Pesticides and Metals<br />
Pesticides<br />
H. 8. Pionke<br />
It is my intention to assist in the PLUARG endeavour by identifying<br />
significant gaps in current knowledge and to define associated research<br />
needs relative to specific problems of the Great Lakes Basin. The emphasis<br />
is on: (1) determining the impact of land use activities on boundary waters<br />
rather than upstreams areas; (2) defining pesticide research needs to answer<br />
specific problems or to meet needs of the Great Lakes Basin rather than to<br />
answer general issues in pesticide research; and (3) developing practical<br />
remedial measures for correcting the identified problems. The last two needs<br />
imply a sufficiently good forecasting and inventory capability to establish<br />
present pesticide use and near-future trends.<br />
Because the impact of a pesticide in boundary or upstream waters<br />
is going to be related to pesticidal bioactivity in some phase such as the<br />
sediment, aqueous, or vegetative phases, the planner's objective is to<br />
estimate or determine this bioactive concentration. The bioactive concentration<br />
depends on the pesticidal concentration and its toxicity. In turn,<br />
the pesticide concentration observed in a phase at a downstream point<br />
depends on upstream use, persistence, the characteristic pesticide distribution<br />
between phases, and the resultant hydrologic dilution. Relatedly,<br />
the expected toxicity may be abnormally reduced by adsorption or incorporation<br />
of the pesticide or abnormally increased to some target species by<br />
the presence of other pesticides or chemicals (i.e. synergism).<br />
The title of this paper "Forms and Sediment Associations of<br />
Pesticides" defines an important part of the environmental system that<br />
exerts controls on pesticidal toxicity and concentration in selected phases.<br />
For example, pesticidal persistence, toxicity and concentration of the<br />
pesticide distributed between phases can be substantially altered by sediment<br />
during loss, transport and in the impact area. However, it is the author's<br />
opinion that environmentally-oriented, future pesticide research must first<br />
be considered in the context of the total system before one component of<br />
this system, i.e., the adsorbed and sediment-associated phase can be isolated<br />
and considered separately. The current gaps in present knowledge relative<br />
to this workshop's objectives are still at a system rather than a component<br />
scale. Thus, the pesticide-sediment interaction will not be discussed<br />
separately but within the context of the dominant environmental and pesticide<br />
properties controlling the pesticide bioactivity in a phase.<br />
199
In order to usefully answer the three questions posed by IJC the<br />
in term of this assignment and the workshop objectives. a more appropriate<br />
set of questions needs to be asked. Basically, these are: "Which pesticides<br />
have potentially the greatest impact?" and "For those pesticides selected<br />
as having potentially the greatest impact, what are the dominant environmental<br />
conditions reasonably occurring in the Great Lakes that substantially<br />
alter the expected downstream environmental hazard?" Specific answers to<br />
these two questions would likely place research emphasis on a select few<br />
pesticides and the dominant environmental conditions.<br />
My paper will refine and expand these two questions into a philosophy<br />
to select pesticides and their key environmental interrelationships<br />
for special research emphasis. This is followed by several specific recommendations<br />
most of which are aimed at improving the selection process.<br />
The selection process is described by answering the questions which are<br />
subdivided and mare clearly defined for purposes of better organizing the<br />
folloving discussion.<br />
They are: (1) Which pesticides are and will be applied in large<br />
quantities to major watersheds within the Great Lakes Basin? (2) Of these,<br />
which pesticides have the basic properties that alone or in combination<br />
create a potential environmental hazard under a reasonably selected standard<br />
set of environmental conditions? and (3) What known or expected environmental<br />
changes in subunits of the watershed could substantially alter the potential<br />
environmental hazard of pesticides earlier identified by answering the<br />
previous two questions? Generally, the text will follow the flow chart<br />
(Figure 1).<br />
Which pesticides are and will be applied in large quantities to<br />
major watersheds in the Great Lakes Basin? This is the most important<br />
information need for selecting pesticide research. To do this adequately,<br />
annual surveys by specific pesticide according to subwatersheds are needed.<br />
This will tie time and area distributions of application together. To<br />
date, this information is not available for the Great Lakes Basin. Best<br />
current sources of information are the periodic surveys (1964. 1966, 1971)<br />
by the Economic Research Service, USDA, which are presented according to<br />
combined State rather than watershed boundaries and by the Ontario Ministry<br />
of Agriculture and Food publication, which more closely coincides with<br />
watershed boundaries. However, the latter is still not current, the latest<br />
year of publication being 1973. The Great Lakes states (Michigan, Minnesota<br />
and Wisconsin) were chosen for comparison because this grouping had the<br />
highest percentage of land area draining into the Great Lakes of Basin any<br />
state grouping. This was approximately one-half the total land area of<br />
these three states.<br />
Note that by using these data to designate present use and to<br />
establish use trends in selected Great Lakes states and Ontario (Table l),<br />
it is apparent that the organochlorine insecticide use has decreased<br />
dramatically. Increased use of methoxychlor, chlordane and the relatively<br />
stable usage of toxaphene in the U.S. contradict this overall trend. The<br />
200
FIGURE 1 A FLOW DIAGRAP: FOR IDENTIFYING PESTICIDE RESEARCH PRIORITIES IN<br />
THE GREAT LAKES BASIN<br />
\<br />
(Toxic, persistent<br />
and adsorbed)<br />
(intermediate<br />
icology and/or persistence)<br />
I<br />
tox- t<br />
RESEARCH AUTOPIA-<br />
TICALLY CONSIDERED<br />
FOR ENVIR- COMMON<br />
ONMENTL CONDITIOMS<br />
PEST IC IDES 7 PERT I NENT ENV I RONMENTAL<br />
EXCLUDED (Toxicity PROPERTIES THAT SUBSTAN-<br />
FRY; STUDY ~ ~ ~ { ~ ~ c TIALLY ~ e rALTER - TOXICITY,<br />
greatly<br />
reduced)<br />
PERSISTENCE OR ADSORP-<br />
TIVITY (NON STANDARD BUT<br />
EXPECTED ENVIRONMENTAL<br />
CONDITIONS)<br />
I<br />
(Toxicity andfor<br />
persistence. adsorptivity<br />
greatly increased)<br />
4<br />
INDICATE WHICH PESTI-<br />
CIDES SHOULD BE STUD- t<br />
IED AND IN WHAT CONTEXT<br />
201<br />
I
N<br />
0,<br />
Table 1. Quantities of Organochlorine Insecticides (1000 lb units active ingredients) used in Lake States<br />
(Michigan, Wisconsin and Minnesota) for 1964, 1966 and 1971 and in Ontario, Canada for 1968-1973.<br />
Lakes State-<br />
11<br />
Ontario, Canada- 21<br />
Insecticide 1964 1966 1971 Trend 1968 1970 1969 1973'<br />
- -<br />
Aldrin 761 717 93 Greatly decreased 3263 27<br />
Chlordane - 154 225 Increase (8)- 41<br />
(74) 82 (105)<br />
DDD 104 108 1 Greatly decreased 296 85 463 -<br />
DDT 511 430 2<br />
I,<br />
Dieldrin 69 97 - 0.3 0.8 -<br />
Endosulfan - 32 2 Stable U U U<br />
Endrin 12 - - Decrease (3) (3) (0.2)<br />
Heptachlor 105 48 10 Greatly decreased U U U -<br />
Lindane 24 58 1 Decrease (2) (2) 6 (3)<br />
Methoxychlor - 5 76 Increase U U U 3<br />
Toxaphene 53 111 40 Stable U U U -<br />
" - none reported U = unknown<br />
'/ Farmers Use of Pesticides, 1964, 1966, 1971 - Quantities, Economic Research Service, USDA, Agricultural<br />
Economic Reports Nos. 131, 179, 252.<br />
2/ Agricultural Pollution of the Great Lakes Basin, (1971). Environmental Protection Agency, Water Quality Office<br />
Publication 13020---07/71.<br />
Roller, Nick F. Survey of Pesticide Use in Ontario, 1973. Economics Branch, Ontario Ministry of Agriculture<br />
and Food Parliament Buildings, Toronto, Ontario. M7A 1B6, October 1975. Total amount applied determined by<br />
summing specific use categories in Appendix IV.<br />
Parenthetical statements are average annual quantities calculated from the total sale records over the following<br />
two year periods: 1968-1969, 1970-1971 and 1972-1973. Frank, R.; Smith, E. H.; Braun, H. E.; Holdrinet,<br />
M.; and Wade, J. W. (1975), Organochlorine Insecticides and Industrial Pollutants in the Milk Supply of the<br />
CA..+.L"" n- , ~ I 79<br />
C<br />
-<br />
15
use of carbamates has increased substantially whereas total organophosphate<br />
insecticide usage has remained essentially stable. However, the trends in<br />
these data must also be interpreted relative to regulatory actions which<br />
have severely prohibited the use of organochlorine pesticides (Table 2, in<br />
particular note chlordane and toxaphene). The present applications of<br />
such pesticides have either declined to near insignificance or are in<br />
imminent danger of being severely restricted for agricultural use. Thus,<br />
in the context of research leading to future remedial programs that are<br />
directed toward land use effects on pollution in the Great Lakes, research<br />
into the organochlorine insecticides should be severely downgraded if not<br />
dropped from consideration. The most recently available pesticide usage<br />
and use trends are summarized in Tables 3 and 4.<br />
For trend analysis, results of any survey need to be supplemented<br />
with a knowledge of imminent or recently instituted Provincial, State or<br />
Federal regulatory actions. This should include the projected effect of<br />
new pesticide introductions. In the context of applying remedial measures,<br />
it may take 5-10 or more years to develop a research idea to the level of<br />
an effective watershed-scale remedial program. Thus, if successful implementation<br />
of remedial measures depends on prior research, long-term pesticide<br />
use projections will have to become part of the research planning process.<br />
The pesticides listed on Tables 3 and 4 in addition to methoxychlor<br />
and toxaphene were used to construct Table 5. The fungicides were not<br />
included because they were applied in much smaller quantitites were than<br />
the insecticides and herbicides. Table 5 incorporates some basic pesticidal<br />
properties, determined under select conditions, which serve as the base<br />
information for the next step.<br />
Of these, which pesticides have the basic properties that or alone<br />
in combination create a potential environmental hazard under a reasonably<br />
selected standard set of environmental conditions? The three basic pesticidal<br />
properties considered here to be of primary importance in the Great Lakes<br />
are: persistence; toxicity; and the basic pesticide distribution between<br />
phases, e.g., water, sediment and vegetative phases. Considerable information<br />
exists and much of it is already available for guidance on making<br />
some of the most basic decisions regarding research emphasis.<br />
On the basis of trend data presented earlier, the shift in pesticide<br />
consumption has been from the persistent insecticides of relatively low<br />
mammalian toxicity to those of much less persistence but often greater<br />
toxicity.<br />
The persistence times of the most commonly used pesticides are<br />
presented in Table 5. The persistence categories of low (C6.0 months),<br />
intermediate (6-18 months) and high (218 months) were chosen according to<br />
time required to dissipate an accumulated 10 year residue once application<br />
had stopped according to a first order disappearance rate (Tables 6 7). and<br />
Unless the pesticide degrades to substantially longer-lived phytotoxic<br />
daughter-products, the impact of the short-lived pesticides ($6 months onehalf<br />
life) is expected to be upstream or on watersheds rather than in<br />
boundary waters. Thus, the impact of the short-lived compound is probabilistic,<br />
i.e., based on the probability of a selected rainfall event or<br />
series occurring within a certain period after application, which will<br />
cause the pesticide concentration in some phase downstream to exceed a<br />
203
Table 2. Major regulatory action limiting agricultural use of Organochlorine<br />
Insecticides in Ontario. Canada and the U. S.<br />
Aldrin<br />
BHC<br />
Chlordane<br />
Dieldrin<br />
DDD<br />
DDT<br />
Endrin<br />
Heptachlor<br />
Hept. Epox.<br />
Lindane<br />
Methoxychlor<br />
Mirex<br />
Toxaphene<br />
~~~ ~~<br />
Ontario, Canade<br />
11<br />
United States-<br />
21<br />
Action-<br />
31<br />
Date Act ion-<br />
31<br />
Date<br />
Restricted 1969<br />
Cancelled<br />
Never registered for<br />
agricultural use<br />
-<br />
To be reviewed<br />
Being reviewed at 1976<br />
Federal level<br />
Suspended<br />
Restricted 1969 Cancelled<br />
Restricted 1970 Cancelled<br />
Restricted 1970 Major restrictions<br />
Cancelled<br />
Restricted 1972 To be reviewed<br />
Restricted 1969<br />
Suspended<br />
Never registered -<br />
Suspended<br />
No restriction -<br />
To be reviewed<br />
No restriction -<br />
No restriction<br />
Never registered -<br />
Restricted to non<br />
food crops (intent<br />
to ban)<br />
Restricted at 1972 No restriction<br />
Federal level<br />
1972<br />
1976<br />
1975<br />
1972<br />
1971<br />
1969<br />
1972<br />
1976<br />
1975<br />
1975<br />
"Agricultural Pollution of the Great Lakes Basin, Combined Report of Canada and<br />
the United States, 1971. Environmental Protection Agency, Water Quality<br />
Office Publication 13020-"07171. Also private communication from Dr. R. Frank<br />
Ontario Ministry of Agriculture and Food, University of Guelph, Guelph, Ontario<br />
1976<br />
-<br />
?/The Pesticide Review by D. L. Fowler and J. N. Mahan. Agricultural Stabilization<br />
and Conservation Service. USDA, 1968 to 1975 (annual publication). Also<br />
private communication from J. Ellenberger, Regulatory Entomologist, EPA,<br />
Washington, D. C.<br />
"Statement may not be legally correct but essentially so based on impact on<br />
agricultural use.<br />
Cancel. = No application allowed.<br />
Suspension = No manufacture allowed.<br />
Review = Initiate process that can lead to suspension or cancellation.<br />
Restrict = Restricted to non agricultural uses.<br />
204<br />
1971<br />
-
Table 3. Top six ranking insecticides according to amount applied with use<br />
trends in parentheses.<br />
I<br />
Lakes States (1971) Ontario (1973)<br />
Carbaryl (stable)<br />
Bux (stable)<br />
Carbofuran (greatly increasing)<br />
Diazinon (stable)<br />
Phorate (greatly increasing)<br />
Azinphosmethyl (stable)<br />
Carbaryl<br />
(unknown)<br />
Chlordane (unknown)<br />
Dursban (unknown)<br />
Carbofuran (unknown)<br />
Phorate (unknown)<br />
Phosvel (unknown)<br />
Table 4. Top six ranking herbicides according to amount applied with use<br />
4,<br />
trends in parentheses.<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
Lakes States (1971)<br />
Atrazine (greatly increasing)<br />
Propachlor (greatly increasing)<br />
2,4-D (stable)<br />
EPTC-vernolate (greatly increasing)<br />
Amiben (greatly increasing)<br />
Alachlor (greatly increasing)<br />
Used mostly in combination with Atrazine.<br />
See Table 1 for references.<br />
205<br />
Ontario (1973)<br />
Atrazine (unknown)<br />
*<br />
Butylate (unknown)<br />
Alachlor (unknown)<br />
2.4-D (unknown)<br />
Amiben (unknown)<br />
*<br />
Cyanazine (unknown)
206
207
0<br />
N<br />
m<br />
li = llllknow~: L = Laboratory Study; F - Field Study; S - Sediment predominant node of transport; W - Water.<br />
*~’er~l~~cci~~ is the experimentally dvtermined ~ lme required for the original pesticide concentration in soil to he reduced to<br />
one-ll,?tf. is the concentretlcln lethal to <strong>50</strong>% of the population exposed for a given number of hours. Hours are in<br />
pazenLllrtlcr1 statement. Kd is ~lle equilibrium concentration adsorbed on the soil or organic carbon (OC) divided by that in<br />
<strong>50</strong>10210n.<br />
i/il.maher, .I. W. 1972. Decomposition: quantitative Aspects, Chapter 4 In Organic Chemicals in the Soil Environment: Vol. 1<br />
Harcal Lkkker. New Kork.<br />
?/Edwards, C. A. 1972. Insecticides. Chapter 8 Organic Chemicals in the Soil Environment: Vol. 1. Uarcel Dekker.<br />
E(eu Yurk.<br />
J’l~lmentcl, I). 1971. Ecological Effects of Pesticides on Non-Target Species. Executive Office of the President. Office of<br />
Srlcncc and Technnlogy. GPO Stock No. lalob-0029.<br />
d’Becaose no experimentally determined values available, approximate values based on relative rnohilities are provided (see<br />
C2irlr 8).<br />
~~ll.~m.aker, .I. W. and J. M. Tllompson. 1972. Adsorption, Chapter 2 Organic Chemicals in the Soil Environment: Vol. 1.<br />
H~r~el Dekker. New Yurk.<br />
fi’St
critical concentration established according to some criteria. Pesticides<br />
originally selected according to use and use trends that disappeared from<br />
soil at approximately first order rates with half-lives in excess 18 of<br />
months, were considered persistent and automatically worthy of further<br />
research consideration. The remaining pesticides or resulting phytotoxic<br />
daughter-products, with half-lives intermediate between the short-lived and<br />
persistent compounds, would require further examination; particularly for<br />
c.onditions in which the half-life may be extended under reasonably changed<br />
environmental conditions to more than an 18 month half-life. These were<br />
defined as following approximate first order kinetics with half-lives<br />
between 6 and 18 months under normal soil conditions.<br />
The second property, toxicity, should be operationally defined as<br />
a critical concentration exceeding some tolerance level for chosen indicator<br />
organisms. In this case (Table 5), the Rainbow Trout was used as the indicator<br />
organism to group pesticides into low, intermediate, and high acute toxicity<br />
levels because of the general availability of the data for the pesticides<br />
of interest. There is no intent on the author's part in suggesting Rainbow<br />
Trout should be the only test organism considered. Chronic toxicity levels,<br />
i.e., 96 or more hours exposure, which should be considered were not included<br />
for purposes of this discussion. Testing for increased toxicity due to<br />
possible synergistic effects among pesticides should be considered provided<br />
that the pesticides are applied both in quantity in and proximity to each<br />
other as determined from survey data.<br />
The third pesticidal property following persistence and toxicology<br />
is the adsorptivity. Numerous references are available on how adsorptivity<br />
will increase persistence and reduce bioactivity of the compound. Sometimes<br />
a portion of the pesticide is irreversibly sorbed and may be essentially<br />
removed from the system. Furthermore, adsorption may provide the mechanism<br />
for transport and accumulation in selected phases of the stream and lake<br />
environment. Determined under standard conditions, the K or distribution<br />
d<br />
coefficient of the pesticide between phases may be a useful indicator.<br />
These values are expressed on both the soil and organic matter basis (Table<br />
5). Because these are not available for many pesticides, a rough correlation<br />
was observed between Kd and the relative mobility of pesticides in soils<br />
(Table 8) which was used to approximate missing data in Table 5. This<br />
relationship was observed by Gerber and Guth (1973)'. Also, the phases may<br />
include vegetation and fish in addition to sediment and water. These biotic<br />
phases often concentrate pesticides by an order or several orders of magnitude<br />
above that found in sediments. The K is expected to be useful for: a)<br />
estimating zones of accumulation or pisticide distribution among phases; b)<br />
identifying primary transport mechanisms for modelling purposes; or c)<br />
indicating which pesticides are most susceptible to irreversible sorption<br />
or most likely to exhibit increased persistence and reduced toxicity upon<br />
adsorption.<br />
The K between water and sediment may be especially useful for<br />
d<br />
making some rough cut decisions regarding erosion and fluvial transport of<br />
selected pesticides (Figure 2). This figure shows how Kd influences the %<br />
total pesticide load carried by the sediment fraction relative to the suspended<br />
sediment concentration.<br />
' Short Theory, Techniques and Practical Importance of Leaching and Adsorption<br />
Studies, Proc. Eur. Weed Res. Coun. Symp., Herbicides-Soil, pp. 51-69, 1973.<br />
209
N<br />
0<br />
SEDIMENT DILUTION AND EFFECT ON THE X PESTICIDE LOAD TRANSPORTED IN THE SEDIMENT RUSE<br />
100<br />
80<br />
60<br />
40<br />
20<br />
0<br />
DILUTION FACTOR<br />
loox lox 4x 2x 0<br />
SEDIMENT CONCENTRATION k /L)
Table 6. Accumulated pesticide residue (kg/ha) resulting from annual applica-<br />
tions of 2 kg/ha active ingredients (calculated according to C = Coe-kt).<br />
Years of Persistence in Half Lives<br />
Application 3 mo 6 mo 1 yr 2 yr 4 yr 8 yr 12 yr<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
7<br />
8<br />
9<br />
10<br />
.125 0.<strong>50</strong> 1.00<br />
.133 0.63 1.<strong>50</strong><br />
.134 0.66 1.75<br />
.134 0.67 1.88<br />
.134 0.67 1.94<br />
.134 0.67 1.97<br />
.134 0.67 1.99<br />
.134 0.67 2.00<br />
.134 0.67 2.01<br />
.134 0.67 2.01<br />
1.41 1.70<br />
2.41 3.13<br />
3.12 4.35<br />
3.62 5.38<br />
3.98 6.26<br />
4.23 7.00<br />
4.40 7.63<br />
4.49 8.17<br />
4.55 8.62<br />
4.60 9.00<br />
1.83<br />
3.51<br />
5.05<br />
6.46<br />
7.76<br />
8.95<br />
10.05<br />
11.05<br />
11.97<br />
12.80<br />
Table 7. Remaining pesticide residue (kg/ha) after application-free interim<br />
periods of 1-5, 10, 15 and 20 years following 10 years of sequential annual<br />
application of 2 kg/ha active ingredients (calculated according to<br />
-kt<br />
C=Ce ).<br />
1.89<br />
3.67<br />
5.35<br />
6.94<br />
8.44<br />
9.86<br />
11.19<br />
12.45<br />
13.64<br />
14.80<br />
Lapsed Time<br />
Following Final<br />
Persistence in Half Lives<br />
Application (yrs) 3 mo 6 mo 1 yr 2 yr 4 yr 8 yr 12 yr<br />
0<br />
1<br />
2<br />
3<br />
4<br />
5<br />
10<br />
15<br />
20<br />
0.134<br />
0.01<br />
9.0<br />
0.0<br />
0.0<br />
0.0<br />
0.0<br />
0.0<br />
0.0<br />
0.67<br />
0.17<br />
0.04<br />
0.0<br />
0.3<br />
0.0<br />
0.0<br />
0.0<br />
0.0<br />
211<br />
2.01 4.60 9.0 12.8 14.8<br />
1.00 3.25 7.6 11.7 14.0<br />
0.<strong>50</strong> 2.30 - - -<br />
0.25 1.63 - - -<br />
0.12 1.15 - - -<br />
0.06 0.81 3.8 8.3 11.0<br />
0.00 0.14 1.6 5.4 8.3<br />
0.00 0.03 0.67 3.5 6.2<br />
0.00 0.00 0.28 2.3 4.7
*<br />
Table 8. Relative mobility of pesticides in soils<br />
594<br />
TCA+<br />
Dalapon<br />
2,3,6-TBA<br />
Tricamba<br />
Dicamba (0.0)<br />
Chloramben (0.3)<br />
Picloram (0.45)<br />
Fenac<br />
Pyrichlor<br />
MCPA<br />
Amitrole<br />
2,4-D (1.6)<br />
Dinoseb<br />
Bromacil<br />
Propachlor<br />
Fenuron (1.0)<br />
Prometone (13)<br />
Naptalam<br />
2,4,5-T (1.0)<br />
Terbacil<br />
Propham<br />
F luome t uron<br />
Norea<br />
Diphenamid<br />
Thionazin<br />
**<br />
Mobility Class (decreasing mobility<br />
3<br />
Endothall<br />
Monuron (33)<br />
Atratone<br />
WL 19805<br />
Atrazine (25)<br />
Simazine (15)<br />
Ipazine<br />
Alachlor<br />
Ametryne<br />
Propazine (25)<br />
Trietazine<br />
2<br />
Siduron<br />
Bensulide<br />
Prometryne (55)<br />
Terbutryn<br />
Propanil<br />
Diuron (196)<br />
Linuron (147)<br />
Pyrazon<br />
Molinate<br />
EPTC<br />
Chlorthiamid<br />
Dichlobenil<br />
Vernolate<br />
Pebulate<br />
Chlorprouham<br />
Azinphosmethyl<br />
Diazinon<br />
1<br />
Neburon<br />
Cloroxuron<br />
DCPA<br />
Lindane (321)<br />
Phorate<br />
Parathion<br />
Disulfoton<br />
Diquat<br />
Chlorphenamidine<br />
Dichlormate<br />
Ethion<br />
Zineb<br />
Nitralin<br />
C-6989<br />
ACNQ<br />
Morestan<br />
Isodrin<br />
Benomyl<br />
Dieldrin<br />
Chloroneb<br />
Paraquat<br />
Trifluralin<br />
Benef in<br />
Heptachlor<br />
Endrin<br />
Aldrin<br />
Chlordane<br />
Toxaphene<br />
DDT (10,000)<br />
Helling, C. S., Kearney, P. C., and Alexander, M., 1971. Belwviour of Pesticides in Soils. Adv. Agron.<br />
- 23,147-240.<br />
**<br />
Class 5 compounds (very mobile) to Class 1 compounds (immobile); in each class pesticides are ranked in<br />
estimated decreasing order of mobility. Underlines designate the most commonly used pesticides by survey.<br />
Parentheses enclose average Kd's from Hamaker, J. W. and J. M. Thompson, (1972),Adsorption. Chapter 2 &<br />
Organic Chemicals in the Environment, Vol. I. Marcel Dekker, N. Y., 440 pp.
Washoff losses of pesticides are likely to be predominantly<br />
associated with the eroded rather than the aqueous phase at or above relatively<br />
low Kds, e.g., 100. However, once eroded, the initial transport<br />
will be heavily weighted toward the aqueous phase unless the 1000 Kd or is<br />
more with reasonably high sediment concentration, i.e., <strong>50</strong>0 over mg/l for<br />
the larger Great Lakes tributaries. Further transport downstream increases<br />
the likelihood of dilution by other water exhibiting lower sediment concentration.<br />
From Figure 2, hydrologic dilution of the pesticide by 10 times<br />
substantially shifts the pesticide load to the aqueous phase unless the Kd<br />
equals or exceeds 10,000. The dilution factor has to be very large, e.g.,<br />
100 times or greater to significantly shift the load from the adsorbed to<br />
aqueous phase if the Kd is this high. Furthermore, if a dynamic equil-<br />
ibrium exists between fluvial sediment and the aqueous phase, and the Kd<br />
is large, substantial and perhaps rapid pesticide losses may occur because<br />
of pesticide adsorption on exposed stream banks and bottoms and associated<br />
vegetation within relatively short travel distances. Usually the Kd for<br />
most pesticides used today is 1000 or less, thus weighting the load transported<br />
to the aqueous rather than adsorbed phase (Figure 2). However,<br />
adsorptivity or oganic matter may be 10 to 1000 times greater than that for<br />
soil (Table 5). Thus, in situations where organic soils, organic sediments<br />
or transported organic materials predominate, transport may be heavily<br />
weighted toward a sediment fraction of low density. Such highly adsorptive<br />
systems are usually primary candidates for investigation related to irreversible<br />
adsorption, increased persistence and decreased bioactivity due to<br />
adsorption. The same consideration would apply to highly polluted stream<br />
sediment to which the pesticide distribution could be greatly altered.<br />
Table 9 summarizes these three properties for the pesticides originally<br />
chosen according to the survey.<br />
From this table, both chlordane and toxaphene potentially provide<br />
both upstream impact (toxicity) and downstream impact (persistence), being<br />
primarily lost by erosion and transported in both the aqueous and sediment<br />
phase. Azinphosmethyl, phorate and diazinon potentially provide upstream<br />
impact only, because although they are toxic they lack persistence. They<br />
are lost primarily by erosion but transported primarily in the aqueous<br />
phase. Thus, hydrologic dilution likely dominates the concentration of<br />
this material downstream and this concentration dissipates rapidly. Dursban<br />
does potentially provide upstream impact and is transported primarily in<br />
the aqueous phase. The persistence was not known. The impact of phosvel<br />
and carbofuran is limited to upstream areas because of low persistence.<br />
The persistence for bux is unknown. The remaining compounds are not likely<br />
to be of concern.<br />
Thus, this whole approach provides some guidelines for research:<br />
Firstly, these compounds which are important on the basis of use; secondly,<br />
the identification of the important pesticidal properties and how they might<br />
indicate particular research needs and provide research emphasis assuming<br />
normal environmental conditions. This kind of approach not only attempts<br />
to direct attention to the most environmentally hazardous properties of<br />
a compound but also points out weak or missing data needed to make of some<br />
these basic assessments and perhaps provide some insight. However, this<br />
213
Table 9. Selected pesticides grouped according to combinations of properties.<br />
Pesticide<br />
Chlordane<br />
Toxaphene<br />
Azinphosmethyl<br />
Phorate<br />
Phosvel<br />
Dursban<br />
Methoxychlor<br />
Diazinon<br />
Bux<br />
Carbofuran<br />
Atrazine<br />
Cyanazine<br />
EPTC<br />
Vernolate<br />
Carbaryl<br />
Butylate<br />
Amiben<br />
2,4-D<br />
Alachlor<br />
Propachlor<br />
Group Ranking for each Property<br />
Toxicity Persistence Adsorptivity<br />
I I I<br />
I I I<br />
I I11 I1<br />
I I11 I1<br />
I 111 U<br />
I U I11<br />
I U U<br />
I, I1 I11 I1<br />
I1 U U<br />
I1 I11 U<br />
I11 I1 I11<br />
I11 I1 I11<br />
I11 I11 I1<br />
I11 I11 I1<br />
I11 I11 I11<br />
I11 I11 U<br />
I11 I11 I11<br />
I11 111 I11<br />
I11 I11 I11<br />
I11 I11 111<br />
I = Toxicity (0.003 - .05 mg/l, LC<strong>50</strong> over 24 48 to hour for Rainbow Trout),<br />
OK persistence 5 18 months (observed time for one-half the original<br />
concentration to disappear from soil) OK adsorptivity 2 1000 Kd (Kd =<br />
concentration on sediment (x/m) versus that in solution (C at equilibrium<br />
from Freundlich relationship n=l). at<br />
ed<br />
I1 = Toxicity (0.052 - 0.3 mg/l ditto) or 6 months < persistence < 18 months<br />
OK 100 5 Kd < 1000.<br />
I11 = Toxicity (1.0 - 20, mg/l ditto) or persistence 5 6 months or Kd < 100.<br />
U = Unknown.<br />
214
has as yet only treated properties under standard or a selected set of<br />
environmental conditions. This is often not the case.<br />
What known or expected environmental changes in subunits of the<br />
watershed could substantially alter the potential environmental hazard of<br />
those pesticides earlier identified by answering the previous two questions?<br />
Answering this third question assumes that the dominant local stream and<br />
lake environments on a seasonal or annual basis are known. Then those<br />
dominant, yet nonstandard environmental conditions which substantially<br />
extend pesticidal persistence beyond the middle-range persistence OK bias<br />
the distribution of pesticide among phases causing a potentially hazardous<br />
environmental level, can be identified and their impact researched. This<br />
investigation would apply to those pesticides of intermediate persistence<br />
or toxicity as determined from answering questions 1 and 2. These could<br />
include the effect of: pH changes from field to stream to lake (persistence<br />
and redistribution between phases of pH-dependent charged pesticides);<br />
chemical reducing conditions on persistence; temperature on persistence;<br />
exposure to sediment associated with industrial type pollutants, e.g.,<br />
petroleum products mixed with sediment, on persistence and selective distribution<br />
of pesticides in this phase; exposure to organic soils and<br />
sediments on toxicity, persistence and adsorptivity, etc. Obviously, the<br />
criterion of reasonability should be followed regarding the likelihood of<br />
these conditions existing and their severity. The test conditions chosen<br />
should not be extreme or unreasonable relative to the expected in range<br />
pertinent environmental conditions.<br />
CONCLUSION<br />
In summary, if a pesticide is used extensively or in increasingly<br />
larger amounts, is known to be toxic to selected aquatic organisms, and is<br />
either persistent or has an intermediate half-life that may be extended by<br />
adsorption and/or abnormal environmental conditions that are known to occur<br />
within the area, then these should be the primary subject of research<br />
emphasis.<br />
RECOWMIATIONS<br />
1.<br />
2.<br />
' 3.<br />
Better surveys of individual pesticide usage on an annual basis are<br />
needed in order to establish present usage and trends in usage. This<br />
should be assembled by the IJC according to subwatershed divisions<br />
within the Great Lakes Basin.<br />
The organochlorine insecticides should not be the subject of new<br />
research unless justified on the basis of present and projected<br />
use in the Great Lakes Basin.<br />
The pesticides selected on a usage or use trend basis should be<br />
further evaluated according to persistence, toxicity, and adsorptivity<br />
under some set of standard conditions. Synergistic effects on selected<br />
key aquatic organisms should be evaluated where justified on pesticide<br />
use basis. Then the "nonstandard" but reasonably expected local<br />
environmental conditions that substantially alter persistence and<br />
toxicity occurring downstream from application areas should be<br />
identified and researched. An exhaustive literature review could<br />
supply much of this information.<br />
215
This overall approach provides a means of selecting those pesticides<br />
that should be the primary candidates for subsequent laboratory and field<br />
research and monitoring programs. Furthermore, this approach should<br />
specifically delineate or further identify needed research.<br />
4. We need standardization OK development of methodology to experimentally<br />
and routinely determine: a) standard persistence, toxicology and<br />
adsorptivity; b) the altered toxicity upon adsorption or in<br />
association with other pesticides (synergistic effects); and c)<br />
the altered persistence upon adsorption, changed 02 pH, status,<br />
temperature, etc. Perhaps the study of the selected pesticides<br />
in multiphase microcosms may be useful for isolating potential<br />
problems. Obviously, the pesticides studied should not be chosen<br />
at random, but should be of at least intermediate persistence, toxicity<br />
and used in considerable amount according to survey.<br />
5. Certain environmental exceptions exist that should be researched even<br />
if not defensible by pesticide use. These include: a) dredge spoil<br />
as a renewed source of adsorbed pesticides, particulary the organochlorine<br />
insecticides; and b) altered K and persistence of selected<br />
pesticides partitioned into or exposed t,d highly polluted sediments<br />
(lipophilic wastes, industrial wastes, municipal sewerage, etc.).<br />
216
DISCUSSION<br />
/A,. L. HetZing: The way you are going at the pesticide issue is usage. Is<br />
it possible or thinkable to go at it the other way? We are interested in<br />
those pesticides or those types that are bio-accumulating in the environment.<br />
Could one go and take certain test organisms or test fish or something else<br />
at a lake and measure them for the range of these things, and if they are<br />
not accumulating, not worry about them?<br />
Dr. H. Pionkc: I wasn't really suggesting that we start on a full-blown<br />
programme, collecting all these numbers. I am saying that in certain cases<br />
there are probably missing data that could be supplied. I think that some of<br />
the EPA people would like to speak to this, but I think there is already a<br />
movement afoot to try and get some of this basic information. As I mentioned<br />
earlier, this is in the context again of the IJC. We are talking about the<br />
Great Lakes watershed. There are many compounds in use across the country.<br />
They are not used in any amount on the Great Lakes watershed. I think if<br />
you turn it around and get the cart before the horse and start talking about<br />
what compounds could create a potential problem without asking whether or not<br />
they are being applied, is the wrong way to go.<br />
217
Forms and Sediment Associations of Nutrients (C.N. 6 P.)<br />
Pesticides and Metals<br />
Trace Metals<br />
Ulrich F6rstner<br />
The availability of trace metals for metabolic processes is<br />
closely related to their chemical species both in solution and in particulate<br />
matter. For this reason a thorough investigation into the<br />
behaviour of trace metals in the dissolved state was undertaken. In comparison,<br />
very little is known about the chemical association of metals in<br />
suspended materials and sediments, although the knowledge of characteristic<br />
bonding types may be the key to the detection of specific pollution sources<br />
in the aquatic environment.<br />
TRANSPORT OF TRACE MALS IN RIVERS<br />
The source of trace metals in rivers significantly determines<br />
their distribution ratio between the aqueous and solid phases. For example,<br />
the bulk of the detrital trace element particulates never leaves the<br />
solid phase from initial weathering to ultimate deposition. Similarly,<br />
metal dust particles (e.g. from smelters) and effluents containing heavy<br />
metals associated with inorganic and organic matter, undergo little or no<br />
change after being discharged into a river. This is attributable to the<br />
average residence times (of the order of days or weeks), too short €or the<br />
establishment of stable, dynamic equilibria between water and suspended<br />
material. '<br />
There are characteristic differences in the metal distribution<br />
between the aqueous and solid phases. Examples are given in Table 1,<br />
indicating the fractions of metals in particulate form as percentages of the<br />
total metal discharges from U.S. rivers', rivers in West Germany3 and from<br />
the Rhine River in the Netherlands' respectively.<br />
The order of sequence of the mobility for a few selected metals<br />
is as follows. Alkali and alkali-earth metals are predominantly present in<br />
a dissolved form; for trace metals like boron, zinc and cadmium, the ratio<br />
of dissolved species to particulate species is between 2:l and 1:l; copper,<br />
mercury, chromium and lead exhibit ratios of the solid phases to the aqueous<br />
phases of between 2:l and 4:l; whereas iron, aluminum (and manganese under<br />
normal Eh conditions) are almost totally transported as solid particles.<br />
The preferential bonding of heavy metals in effluents to the solid phase is<br />
of particular significance for tracing sources of pollution. During<br />
reduced rates of river flow, suspended material settles to the river bed,<br />
becoming partially incorporated into the bottom sediment. By virtue of their<br />
composition, sediments conserve heavy metal contamination and "express the<br />
state of a water body"'. Vertical sediment profiles (cores) often uniquely<br />
preserve the historical sequence of pollution intensities6; lateral distributions<br />
(quality profiles) serve to determine and evaluate local sources<br />
of pollution .<br />
219
Metal<br />
(example)<br />
Sodium<br />
Calcium<br />
Strontium<br />
Boron<br />
Cadmium<br />
Zinc<br />
Copper<br />
Mercury<br />
Chromium<br />
Lead<br />
Aluminum<br />
Iron<br />
*Federal Republic of Germany<br />
TABLE 1<br />
Percentage particulate-associated metals<br />
of total metal discharge (solid + aqueous)<br />
U.S. Rivers' F.R.G.* Rivers3 Rhine (Neth.)'<br />
-<br />
0.5<br />
2.5<br />
21 %<br />
30 %<br />
-<br />
-<br />
30<br />
40 % 45<br />
63 % 55<br />
-<br />
59<br />
76 % 72<br />
84 % 79<br />
98 % 98<br />
98 % 98<br />
220<br />
%<br />
%<br />
-<br />
-<br />
% 45 %<br />
% 37 %<br />
% 64 %<br />
% 56 %<br />
% 70 %<br />
% 73 %<br />
% -<br />
% -
Two effects, however, must be considered when sediment analyses<br />
are used for the identification of sources of pollution. Firstly, under<br />
conditions of high water discharge, erosion of the river bed takes place,<br />
and generally leads to a lower degree of local contamination. LSzl6’, for<br />
example, obtained data from the Sajo River, Hungary, which indicated that<br />
three months after a flood, the mercury concentration in bottom sediments<br />
had been reduced to approximately one-quarter of the values found immediately<br />
before the flood. Secondly, it is often overlooked that it is imperative<br />
to do base metal analyses from river sediments on a standardized procedure<br />
with regard to particle size’, since there is a marked decrease in the<br />
content of metals as sediment particle size increases. An example is given<br />
in Figure 1, indicating the concentrations of cadmium in different grain size<br />
fractions of sediments from the highly polluted Rhine and Main Rivers in<br />
the Federal Republic of Germany”. Maximum concentrations of Cd occur in<br />
the fractions of 0.2 pm - 0.6 pm; from there the metal contents decrease<br />
both toward finer and coarser grain diameters (relatively high levels of Cd<br />
in the sand fractions are probably due to the input of coarse waste particles).<br />
Several methods have been introduced in order to include most of<br />
the substances active in metal enrichment, i.e., hydrated oxides, sulphides,<br />
amorphous and organic materials, but to exclude coarser-grained sediment<br />
fractions which are largely chemically inert: (i) reduction of grain size<br />
effects, e.g. the pelitic fraction of < 2 pm diameter”, by extrapolation<br />
from the grain size distribution” or by correction for the specific surface<br />
area”; (ii) selective chemical treatment for the extraction of acidsoluble<br />
fractions by including 0.1 M hydrochloric acid’l*, 0.1 M nitric<br />
acid15 and aqua regia/ignition lossIb procedures; (iii) using mineral<br />
separation techniques as exemplified by the quartz correction method”;<br />
(iv) taking conservative elements, e.g. aluminum, silicon, potassium, and<br />
sodium as a reference base for cultural metal inputs’’”’. The question as<br />
to which trace metals are characteristic of nonpoint or diffuse sources,<br />
which is of particular interest to the present Workshop, cannot be answered<br />
to any degree of satisfaction. In areas of heavy environmental pollution<br />
such as highly industrialized areas, one generally finds high levels of<br />
lead, zinc, mercury and cadmium in the river deposits whereas at the same<br />
time other metals such as iron, manganese, nickel, and cobalt have only<br />
increased insignificantly in comparison. High levels of chromium, cadmium<br />
and copper can usually be attributed to industrial waste”, particularly<br />
from the tanning and electroplating industries; enrichment of lead and zinc<br />
can usually be connected with diffuse sources such as atmospheric emissions<br />
in the case of lead, and urban sewage effluents for zinc”. Apart from<br />
this, the temporally changing influence in the various catchment areas as<br />
well as seasonal effects must be taken into consideration as was done by<br />
Schleichert” in his study on the metal transport of suspended matter in<br />
the Rhine.<br />
CHEMICAL FORMS OF TRACE BTALS IN FLWIATILE SEI)IE:MS<br />
The ratio of heavy metal distribution between the aqueous and solid<br />
phases is subject to change due to a general shift of equilibria toward<br />
the solid phase. Thus, the total amount of heavy metals in solution generally<br />
diminishes along the course of a river during normal flow conditions.<br />
This is of importance for the elimination of dissolved wastes, e.g. from<br />
metal processing (electroplating, galvanizing), the removal of trace metals<br />
from the aqueous phase being effected by mechanisms as such sorption,<br />
precipitation, co-precipitation and complexing.<br />
221
In spite of their different origins, environmentally related<br />
types of metal bonding are comparable to the natural forms. The most<br />
common associations of heavy metals in particulate substances are compiled<br />
in Table 2:<br />
(1) Heavy metals are transported and deposited as major, minor<br />
OK trace constituents in the natural rock detritus, mostly<br />
in inert positions.<br />
(2) Slight alkalinity prevailing in normal surface waters results<br />
in the formation and precipitation of hydroxides, carbonates,<br />
sulphides and phosphates of heavy metals.<br />
(3) Sorption and cation exchange particularly takes place on<br />
fine-grained substances with large surface areas. A generalized<br />
sequence of the capacity of the solids to sorb heavy was metals<br />
established by Guy 6 ChakrabartiZ4 in the order<br />
MnOz > humic acid > hydrous iron oxides > clay.<br />
Experiments with different sorbents and seawater indicated an<br />
extensive extraction of zinc, copper and lead by hydrous iron-and manganeseoxides,<br />
clay minerals and peat substances; nickel, cobalt and chromium are<br />
closely associated with Mn-oxidesZ5. In experiments on competing exchange<br />
of metal cations performed with equal concentrations of humic substancesz6,<br />
copper was preferentially sorbed (53%). followed by zinc (21%), nickel<br />
(14%). cobalt (8%) and manganese (4%).<br />
(4) Dissolved organic polymer is increasingly being regarded as a<br />
carrier material in the transfer of metals from the mineral<br />
phase into the organic material. The younger, less condensed<br />
humic acids (fulvic acids) play an especially important role<br />
in the bonding of heavy metals in aquatic systems because of<br />
their large number of functional groups”. In a summary of<br />
organic matter-metal interactions in recent sediments, Nissen -<br />
baum L Swaine” mention concentrations of up to 0.4% copper and<br />
0.45% zinc in humic substances from marine reducing environment^^^.<br />
A high degree of positive correlation between the content of<br />
organic substances, and metal concentrations may, however, not<br />
always be interpreted as being the result of direct bonding of<br />
heavy metals to organic substances. In highly polluted areas,<br />
for instance, contamination by characteristic heavy metals simply<br />
coincides with the accumulation of organic substances, both derived<br />
from urban, industrial or agricultural sewage effluents.<br />
Extraction of metals from fulvic and humic acids in sediments<br />
from Lake Malawi indicated that only zinc, copper and vanadium<br />
were accumulated in organic material, whereas iron, manganese,<br />
chromium, cobalt and nickel are more or less diluted by the organic<br />
acids”.<br />
(5) Co-precipitation of heavy metals with hydroxides and carbonates<br />
appears to be an important mechanism controlling the metal<br />
concentrations in the aquatic environment”’ 32. Experiments on<br />
calcium carbonate precipitation in the presence of heavy metal<br />
ions33 indicated that heavy metal carbonates having a low solubility<br />
(e.g. cadmium and lead) will be completely eliminated<br />
222
TABLE 2<br />
CARRIER SUBSTANCES AND MECHANISMS OF HEAVY METAL BONDING<br />
Minerals of natural rock debris<br />
(e. g. heavy minerals)<br />
Heavy metal<br />
- hydroxides<br />
- carbonates<br />
- sulphides<br />
Clay minerals<br />
(Sorption)<br />
PH<br />
~ Bitumen, Lipids PH<br />
Humic substances<br />
, Residual organics<br />
Hydroxides and oxides<br />
of Fe/Mn<br />
Calcium carbonate<br />
PH<br />
PH<br />
223<br />
metal bonding predominently<br />
in inert positions<br />
Precipitation as a result of<br />
exceeding the solubility product<br />
in the area of the water course<br />
Physico-sorption<br />
(electrostatic attraction)<br />
Chemical sorption<br />
(exchange of H+ in SiOH, AlOH and<br />
A1 (0H)z)<br />
Physico-sorption<br />
Chemical sorption<br />
(exchange of H+ in COOH-, OH-groups)<br />
complexes<br />
Physico-sorption<br />
Chemical sorption<br />
(exchange of H+ in fixed positions)<br />
Co-precipitation as a result of<br />
exceeding the solubility product<br />
Physico-sorption<br />
Pseudomorphosis<br />
(dependent on supply and time)<br />
Co-precipltation<br />
(Incorporation by exceeding the<br />
solubility product)
from the solution as a result of co-precipitation with calcium<br />
carbonate (Table 3). Examples from the hcavily polluted Elsenz<br />
River (a tributary to the Neckar River) and the Elbe River in<br />
West Germany have demonstrated the actual importance of coprecipitation<br />
phenomena with hydroxides of iron and calcium carbonate<br />
in the natural environment 34 ' . The presence of large amounts<br />
of iron hydroxide in the Elsenz River was caused by the mixing<br />
of normal Elsenz water with alkaline waste water from a local<br />
galvanizing industry. The measured concentration of heavy<br />
metals found in iron hydroxide concretions are far higher than<br />
the concentrations found in the clay-sized fraction of the<br />
surrounding Elsenz sediments. Compared to this surrounding<br />
clay-sized sediment fraction, heavy metal enrichment by factors<br />
of up to 30 times for Cd, 17 for Zn and 1.4 for Cu were measured<br />
in the iron hydroxide concretions". Groth's3' investigations<br />
on the co-precipitation of trace metals with hydrous Fe/Mn<br />
oxides (also taking the competition of organic complexing agents<br />
into account), indicated an almost complete co-precipitation of<br />
copper occurring under all conditions, whereas zinc and cobalt<br />
are precipitated to a much lesser extent in the presence of strong<br />
EDTA complexing agents than of natural organic chelating substances<br />
(Table 4 ).<br />
In contrast to iron hydroxide, calcium carbonate was found to have<br />
concentrated heavy metals far less than those found in the<br />
surrounding clay size ELbe sediment fraction". Calcium carbonate<br />
coatings had been formed locally in the Elbe River as the result<br />
of mixing effects upon the contact of normal Elbe River water<br />
with alkaline waste waters from a local Dow Chemicals Plant.<br />
Compared to the geochemical background values of heavy metals<br />
in carbonate rocks37, enrichment factors of approximately 10<br />
times for Cd, 6 for Cu, 4.5 for Zn and 4 for Co were measured<br />
in calcium carbonate coatings.<br />
SELECTIVE EXTRACTION PROCEDURES<br />
Many attempts have been undertaken for a selective extraction of<br />
the different forms of metal association in both natural and polluted<br />
sediments: Hirst & Nicholls3* differentiated the metal contents in the<br />
detrital and non-detrital fractions of carbonate rocks by acid treatment.<br />
Goldberg & Arrhenid6 used EDTA to establish the partition of elements<br />
between detrital igneous minerals and authigenic phases in pelagic sediments.<br />
Arrhenius & Korkish4' tried to separate the iron fraction of the<br />
ferro-manganese nodules with 1 M hydrochloric and dilute acetic acid.<br />
Chester & Hughes" distinguished the lithogenous and non-lithogenous components<br />
in recent aquatic sediment by acid-reducing 1 M hydroxylamine<br />
hydrochloride + 25% acetic acid; this method reached particularly wide<br />
application in marine ge~chemistry~'-~'. A combination of different<br />
extraction procedures was first used Ni~senbaum~~<br />
by<br />
for sediments from the<br />
Okhotsk Sea (Table 5a), then by G i b b ~ for ~ ~ analyses of trace metals<br />
associated with different carrier substances in suspended materials of the<br />
Amazon and Yukon Rivers (Table 5b). Engler and co-workers4' developed a<br />
separation technique, particularly for dredged materials, precluding<br />
atmospheric oxidation at sensitive steps (Table 5c); sediments from Mobile<br />
224
TABLE 3<br />
CO-PRECIPITATION OF TRACE METALS WITH CALCIUM CARBONATE<br />
-LOG L = NEGATIVE EXPONENTS OF THE SOLUBILITY PRODUCTS OF METAL CARBONATES<br />
Original Metal contents in the precipitate<br />
concentration in % of the original concentration<br />
(Pdl) Cd Pb Co Zn Cu Ni<br />
25 100 100 100 100 96 96<br />
2<strong>50</strong> 100 100 100 100 - 43<br />
2000 100 100 96 96 75 -<br />
-log L 13.2 12.8 12.0 10.2 9.85 6.85<br />
TABLE 4<br />
CO-PRECIPITATION OF TRACE METALS WITH Fe/Mn-HYDROXIDES<br />
Solution Percent of metal additive in the precipitate<br />
Fe cu Zn Mn co<br />
distilled water 98% 95% 14% 47% 80%<br />
lake water<br />
no admixture (a) 99% 98% 86% 67% 25%<br />
EDTA (b) 99% 88% 18X 25% 25%<br />
Peat extract (c) 99% 97% 88% 75% 25%<br />
225
TABLE 5<br />
SELECTIVE EXTRACTION PROCEDURES FOR METAL ASSOCIATIONS IN SEDIMENTS<br />
(PRE- AND INTER-TREATMENT STEPS - WASHING, CENTRIFUGATION, FILTRATION<br />
ETC. - ARE NOT INCLUDED IN THE TABLE; - DEFINITATION A<br />
OF THE CHEMICAL<br />
PHASE ACCORDING TO THE AUTHORS)<br />
- a. NISSENBAIIM, 1972<br />
Treatment of sediment Ref. Chemical phasea<br />
1) 30% Hz02<br />
2) 2 N acetic acid (pH - 3)<br />
3) 6 N HC1<br />
4) Fuse with Na~C03<br />
- b. GIBBS. 1973<br />
Perox. fraction<br />
Acetic ac.-fraction<br />
HC1-fraction<br />
Residue fraction<br />
Treatment of sediment Ref. Chemical phasea -<br />
1) 1 N MgClz-solution. pH=7 Cation exchange + adsorption<br />
2) Reduction with sodium dithionite/<br />
complexing with sodium citrate<br />
Metallic coatings<br />
3) Sodium hypochlorite, pH=8,5; see (2) Solid organic material<br />
4) lithium metaborate lOOO"C, HN03 Detrital crystalline material<br />
- c. ENGLER, BRANNON, ROSE & BRIGHAM, 1974 (modified by GUPTA & CHEN, 1975)<br />
Treatment of sediment Ref. Chemical phasea -<br />
1) 1N ammonium acetate, sediment-pH<br />
2) 1M acetic acid (G & CH)<br />
3) 0.1M hydroxylamine hydrochloride<br />
solution in 0.01M nitric acid<br />
4) 30% hydrogen peroxide at 95OC,<br />
extract with 1N NHbOAc, pH=2,5 or<br />
extract with 0.01M HN03 (G 6 CH)<br />
5) Sodium dithionite/citrate or<br />
0.04M NH20H.HC1 in 25% acetic<br />
acid at 100°C for 3 hrs (G 6 CH)<br />
6) Digestion with HN03, HF, HClOs<br />
- d. PATCHINEEJAM, 1975<br />
Exchangeable ions<br />
Carbonate, some Fe/Mn-oxides<br />
Easily reducible phase<br />
(manganese oxide + amorphous<br />
iron oxide)<br />
Organic phase<br />
(organics + sulphide)<br />
Moderately reducible phase<br />
(iron oxide)<br />
Lithogenous fraction<br />
Treatment of sediment Ref. Chemical piesea<br />
1) 0.2 N BaClz-tri.ethanolamine Adsorption + cation exchange<br />
2) 0.1 N NaOH Humates + Fulvates<br />
3) COZ-treatment Carbonate co-precipitates<br />
4) 1 M NHzOH.HC1/25% acetic acid Hydrous Fe/Mn-oxides co-prec.<br />
5) Digestion with HF/HClO,, HN03 Detrital silicates<br />
226
Bay (Alabama), Ashtabula, Ohio (Lake Erie) and Bridgeport, Connecticut (Long<br />
Island Sound) were investigated4'. Further modification of the Engler scheme<br />
was performed by Gupta & C~~II'~ and applied on sediments from Los Angeles<br />
Harbor. Patchineelam differentiated the metal contents associated with cation<br />
exchan e positions, humic acids, carbonate minerals and hydrous Fe/Mn<br />
oxides". The first three examples' 5' ' compiled in Figure 2, represent<br />
relatively unpolluted systems (Dead Sea: 14 ppm Cu, 13 ppm Pb, 36 ppm Zn),<br />
while both the latter area^'^'^^ are strongly contaminanted by heavy metals<br />
(Los Angeles Harbor: 568 ppm Cu, 342 ppm Pb, 632 ppm Zn). In the example<br />
of the Lower Rhine sediment3', two groups of metals can be distinguished<br />
from a comparison of metal data of recent Rhine deposits with analyses of<br />
ancient Rhine sediments obtained from drilling cores in the Cologne area".<br />
High percentages of detrital fractions are found for those elements which<br />
are less affected by man's activities, e.g. iron (3.0% in fossil Rhine<br />
deposits - 3.4% in recent pelitic sediments of the Rhine), nickel (85 ppm -<br />
165 ppm), and cobalt (14 ppm -35 pprn); on the other hand, lattice-held<br />
fractions are very low for metals such as (30 lead ppm - 333 pprn), zinc<br />
(115 ppm - 1558 ppm) and cadmium (0.3 ppm - 28.4 ppm). In the Rhine sediments,<br />
lead, copper and chromium are particularly associated with the<br />
hydroxide phases. These findings can be explained by the specific sorption<br />
of lead to iron-hydroxide5', by the effective co-precipitation of copper<br />
together with hydrous Fe/Mn oxides36, and by the absence of carbonate<br />
phases of chromium in natural aquatic systems. The preferential carbonate<br />
bonding of zinc and cadmium, on the other hand, be attributed can to the<br />
relatively high stability of zinc-carbonate and cadmium-carbonate under the<br />
pH-Eh conditions of common island as well as to the characteristic<br />
co-precipitation of these components with calcium-carbonate. Copper and<br />
cadmium contents are found to be closely associated to organic substances.<br />
The metal fractions in cation exchange positions represent less ten than<br />
percent of the total metal content associated to sediment. Similar results<br />
were obtained by Gupta & Chen" particularly with respect to the low metal<br />
concentrations in exchangeable form and to the sequence of metals associated<br />
with detrital (silicate) minerals. The only exception is copper (98% in<br />
detrital phase) of which 0nJ.S; 10 to 14% is bonded to silicates in Nissenbaum's<br />
study on the Sea of Okhotsk .<br />
THE RLEASE OF TRACE F~ALS FROM RIVER SEDIMENTS<br />
Heavy metals which are "immobili~ed"~~ in the bottom sediments of<br />
rivers and dams constitute a potential hazard to water quality since they<br />
may be released as a result of chemical changes in the aquatic milieu:<br />
(1) Increased salinity of the water body leads to competition<br />
between dissolved cations and adsorbed heavy metal ions and<br />
results in partial replacement of the latter. Such effects<br />
should particularly be expected in the estuarine environment.<br />
Apart from the highly complicated situation there5', however,<br />
it was demonstrated, that the decrease in heavy metal concentrations<br />
at the river/sea interface is caused by mixing processes<br />
of polluted river particulates with relatively "clean" marine<br />
sediments5' rather than by solubilization and/or desorption.<br />
(2) A lowering of pH leads to the dissolution of carbonate and<br />
hydroxide minerals and also - as a result of hydrogen ion<br />
competition - to an increased desorption of metal cations.<br />
227
N<br />
N<br />
m<br />
FIGURE 1: Cadmium concentrations in different grain size fractions of<br />
sediments from the rivers Maine (*) and Rhine".
SEA OF OKHOTSK<br />
(NISSENBAlJM.1972)<br />
"Pwox ~ Froctlon"<br />
"Acotlc acid "<br />
YUKON RIVER<br />
( OIBES, 1973 1<br />
Mn<br />
Fe Ni Co Cr Cu Pb Zn Cd<br />
FIGURE 2:<br />
Extraction of different forms of metal associations in sediments<br />
(examples).<br />
229
Long-term changes of the pH-conditions have been observed from<br />
highly industrialized areas by atmospheric SO2 emissions 59 ' 6o mainly in waters poor in bicarbonate ions. Acid mine drainage<br />
effects a 1,000 - 10,000- fold increase of iron, manganese,<br />
nickel, cobalt, copper and zinc61'62.<br />
A change in the redox conditions is usually caused by the increased<br />
input of nutrients. Oxygen deficiency in the sediments leads to<br />
an initial dissolution of hydrated manganese oxide, followed<br />
by that of the analogous iron compound63. Since these metals<br />
are readily soluble in their divalent states, any co-precipitates<br />
with metallic coatings become partially remobilized. There are<br />
indications, that, for example, copper, zinc and cadmium are<br />
released from anoxic sediments into surface water64. These<br />
processes, however, seem to be controlled rather more by bacterial<br />
activity and subsequent complex formation than by ion migration65;<br />
since the calculated stability for the sulphide compounds is<br />
strongly exceeded by the concentrations of Cu, Cd, Zn, Pb and<br />
other metals in the pore<br />
The growing use of synthetic complexing agents (e.g. nitrilotriacetic<br />
acid) in detergents to replace polyphosphates6' increases<br />
the solubilization of heavy metals from aquatic sediment69'70.<br />
A considerable number of heavy metal chelates are not destroyed<br />
during water pr~cessing~~, for example by bank filtration7',<br />
and may lead to a potential danger for the drinking-water supply.<br />
Microbial activities enhance the release of metals in three<br />
main ways: (i) by formation of inorganic compounds capable<br />
of complexing metal ions; (ii) by influencing the physical<br />
properties and the pH-Eh-conditions of the environment;<br />
(iii) by means of oxidative and reductive processes catalyzed<br />
by enzymes, microorganisms can convert inorganic metal compounds<br />
to organic molecules; organo-mercurial transformations show that<br />
such mechanisms may strongly increase metal toxicity' 3'74.<br />
The latter two mechanisms may be further aided by physical<br />
effects such as erosion, dredging and bioturbation, which<br />
also affect the release of pore solutions rich in metals.<br />
The senior author (U.F.) wishes to express his thanks to the<br />
organizers of the Workshop for their kind invitation to participate;<br />
additional support by the Centre Europben d'Etudes des Polyphosphates is<br />
gratefully appreciated. We are indebted to Dr. G. T. W. Wittmann (Pretoria)<br />
for providing data and valuable suggestions.<br />
LITERATURE CITED<br />
1. Wittmann, G. T. W. & Forstner, U. Water S.A., (1976) 2, 67-72.<br />
2. Kopp, J. F. & Kroner, R. C.. (1968). Trace Metals in Waters of the<br />
United States. U.S. Dept. Interior, FWPCA, Cinncinnati.<br />
230
3. Heinrichs, H., (1975). Diss. Gottingen, 97 pp.<br />
4.<br />
5.<br />
6.<br />
7.<br />
8.<br />
9.<br />
10.<br />
11.<br />
12.<br />
13.<br />
14.<br />
15.<br />
16.<br />
17.<br />
18.<br />
19.<br />
20.<br />
21.<br />
22.<br />
23.<br />
24.<br />
25.<br />
DeGroot, A. J., &., (1973) Sware Metalen in Fluviatiele en<br />
Mariene Ecosystemen. Symp. May 24-25.<br />
Zullig, H., (1956). Schweiz. Zeitschr. Hydrologie, 18. 7-143<br />
Forstner, U., (1976) Naturwissenschaften, 63, 465-470<br />
Forstner, U. & Muller, G., (1974). Schwermetalle in Flussen und Seen<br />
als Ausdruck der Umweltverschmutzung. Spring-Verlag Berlin<br />
Laszlo, F., (1975) Abstr. Int. Conf. Heavy Metals Environm., Toronto,<br />
C-130.<br />
Wittmann, G. T. W. 6 FoKstneK, U.S. (1976) Afr. Jour. Sci. I?_,<br />
242-264<br />
Working Group Metals, Water Research Program of the German<br />
Research Society (Deutsche Forschungsgemeinschaft), unpubl.<br />
data.<br />
Banat, K., et&., (1972) Naturwissenschaften. 2, 525-528<br />
DeGroot. A. J., e. (1971) Geol. en Mijnb., <strong>50</strong> 393-398<br />
Oliver. B. G., (1973) Environmn. Sci. Technol., 2, 135-137<br />
Piper, D. 2.. (1971) Geochmin. Cosmochin. Acta, 25, 531-5<strong>50</strong>.<br />
Jones, A. S. G., (1973) Mar. Geol., g, Ml-M9.<br />
Literathy, P. 6 Laszlo, F., (1975). Int. Symp. Geochim. Natural<br />
Water, Burlington.<br />
Thomas, R. L.. (1972) Can. J. Earth Sci., 2. 636-651 E.,<br />
(1973) lo. 194-204.<br />
Bruland, K. W., d., (1976). Environmn. Sci. Technol. 5, 425-432.<br />
Kemp, A. L. W., etg., (1976) Fish. Res. Board Can., 22, 440-462.<br />
Forstner, U. & Muller, G. Geoforum, (1973a). g 53-61 Rupert0<br />
Carola, (1973b), 51, 67-71 Fortschr. Miner., (1976) 12, 271-288.<br />
Schleichert. U. & Hellmann, H. (1973) Lit. Rept. Bundesanstalt<br />
fur Gewasserkunde Koblenz, 32 pp.<br />
Schleichert, U. (1975) Deutsche Gewasserkundl. Mitt., 19, 1<strong>50</strong>-157.<br />
Forstner, U. 6 Patchineelam, S. R., (1976) Chemikerzeitung, 100,<br />
49-57.<br />
Guy, R. D. 6 Chakrabarti, C. L. (1975) Abst. Inst. Conf. Heavy<br />
Metals Environnm., Toronto, D-29.<br />
Krauskopf, K. (1954) Geochmin. Cosmoschim. Acta, 2, 1-34.
26.<br />
27.<br />
28.<br />
29.<br />
30.<br />
31.<br />
32.<br />
33.<br />
34.<br />
35.<br />
36.<br />
37.<br />
38.<br />
39.<br />
40.<br />
41.<br />
42.<br />
43.<br />
44.<br />
45.<br />
46.<br />
47.<br />
48.<br />
Rashid, M. A., (1974) Chem. Geol., 13, 115-123<br />
Rashid, M. A., (1971) Soil Sci., 111, 298-306<br />
Nissenbaum, A. & Swaine, D. J. (1976) Geochim. Cosmochim. Acta<br />
809-816<br />
Presley, B. J. et g., (1972) Geochim. Cosmochim. Acta, 2, 1073-1090<br />
Forstner, U. (1976) Proc. SIL/UNESCO-Symposium on Interactions Between<br />
Sediments and Freshwater, Amsterdam, 6-13 Sept., (in press)<br />
Lee, G. F., (1975) In: Heavy Metals in the Aquatic Environment (P.A.<br />
Krenkel, ed.), 137-147<br />
Patchineelam, S . R., (1975) Unpublished Diss. Heidelberg, 137 p.<br />
Popova, T. P., (1961) Geochemistry 12. 1256-1260<br />
Patchineelam, S. R. Paper Submitted for Publication<br />
Patchineelam, S. R. Paper Submitted for Publication<br />
Groth, P., (1971) Arch. Hydrobiol. 68, 305-374<br />
Wedepohl, K. H. (1970) Verh. Geol. Bundesanstalt Wien, 6, 692-705<br />
Hirst, D. M. 6 Nicholls, G. D. J. (1958) Sediment Petrol., E. 461-468<br />
Goldberg, E. D. & Arrhenius, G.O.S. (1958) Geochim. Cosmoschim. Acta,<br />
- 13, 153-212<br />
Arrhenius, G.O.S. & Korkish, J., (1958) Am. Assoc. Adv. Sci.,<br />
Chester, R. & Hughes, M. J., (1967) Chem. Geol., 2, 249-262<br />
Chester, R. & Hughes, X. J., (1969) Deep-sea Res., 16, 639-654<br />
Chester, R. & Hessiha-Hanna, R. G., (1970) Geochim. Cosmochim. Acta,<br />
- 34. 1121-1128<br />
Sayles, F. L. etg., (1975) Bull. Geol. SOC. her. 36, 1423-1431<br />
Nissenbaum, A. (1972) Israel J. Earth Sci., 11, 143-154 w.,<br />
(1976) 3, 111-116<br />
Gibbs, R. J. (1973) Science 180, 71-73<br />
Engler, R. M. a. (1974) 168th ACS Natl. Meeting, Atlantic City,<br />
N.J.<br />
Brannon, J. M. et 4.. (1976) U.S. Waterways Experim. Station Misc.<br />
Paper D-76-18.<br />
232
49. Cupta, S . K. & Chen, K. Y. (1975) Environm. Lett., 10, 129-158<br />
<strong>50</strong>. Jackson, M. L. (1958) Soil Chemical Analysis, Prentice-Hall Inc.,<br />
Englewood Cliffs, N. J., 498 pp<br />
51. Holmgren, 6 . S. (1967) Soil Sci. SOC. Amer. Proc., 3, 210-211<br />
52. Chao, L. L. 1974) Soil Sci. SOC. Amer. Proc., z, 764-768<br />
53. Volkov, I. I & Fomina, L. S. (1974) APPG Mem., 2, 456-476<br />
54. .Jenne, E. A.<br />
55. Hem, .J. D. ( 1 972) Water Resour. Res. S, 661-679<br />
56.<br />
57.<br />
58.<br />
59.<br />
60.<br />
61.<br />
62.<br />
63.<br />
64.<br />
65.<br />
66.<br />
67.<br />
68.<br />
69.<br />
70.<br />
71.<br />
72.<br />
73.<br />
74.<br />
(1968) Am. Chem. SOC. Adv. Ser. G, 337-387<br />
Koppe, P. (1973) Gas-Wasserfach, 114, 170-175<br />
Ouinker, .J. C. 6 Nolting, R. F. (1976) Neth. J. Sea. Res. LO, 71-<br />
102<br />
Muller, C. & Forstner, U., (1975) Environm. Geol., 1, 33-39<br />
Oden, S. & Ah., T. Sartryck Ur Ymer, 103-122 (Arsbok 1970)<br />
Whitby, L. X. &. (1976) Can. Mineralogist, 14, 47-57<br />
Forstner, U. & Wittmann, G. T. W. (1976) Geoforum 1. 41-49<br />
Hiller, K. D. (1973) in: Cycling and Control of Metals, EPA Cincinnatti,<br />
91-94<br />
Tessenow, U. & Baynes, Y. (1975) Naturwissenschaften, 62, 342-343<br />
Reinhard, D. & Forstner, U.<br />
301-320<br />
(1975) N. Jb. Geol. Palaont. Mh.,<br />
Cline, J. T. & Upchurch, G. B. (1975) Proc. 16th Conf. Great Lakes<br />
Res., 349-356<br />
Brooks, R. R. &. (1968) Geochim. Cosmochim. Acta, 2, 397-414<br />
Elderfield, H. & Hepworth, A. (1975) Mar. Poll. Bull., 6. 85-87<br />
Epstein, S. S. (1972) Int. J. Environm. Stud., 2, 291-200<br />
Banat, K. 9 &. (1974) Chem. Geol. 14, 199-207<br />
Chau, Y. K. & Shiomi, M. T. (1972) Water, Air, Soil Poll., 1_, 149-155<br />
Schottler, U. (1975) Ztschr. Dtsch. Geol. Ges. 126, 373-384<br />
Lagerwerff, J. V. (1967) Am. Assoc. Adv. Aci. Publ. 95, 343-364<br />
Fagerstrom, T. & Jernelov, A. (1972) Water Res., 6, 1193-1202<br />
Wood, J. M. (1974) Science, 183, 1049-1052<br />
233
I NTRODUCT I ON<br />
Geochemistry of Sediment/Water Interactions of Nutrients,<br />
Pesticides and Metals, Including Observations on Availability.<br />
Nutrients<br />
D. R. Bouldin<br />
During the period September 1972 through September 1975, phosphorus<br />
fractions and flow were monitored in a 300 km2 (126 mi2) watershed in central<br />
New York. In addition several studies were made of composition and flow in<br />
selected subwatersheds for portions of this experimental period.<br />
The water samples were collected manually. Sampling frequency was<br />
concentrated during periods of high discharge rate. The total phosphorus in<br />
the water was separated into three fractions according to the following<br />
scheme. Within 2 to 3 hours after removal from the stream, the sample was<br />
centrifuged at high speed to remove particulate matter. The untreated supernatant<br />
was analysed immediately for inorganic phosphorus and this fraction<br />
was labelled molybdate reactive phosphorus (MRF'). Another portion of the<br />
supernatant was oxidized with persulfate and the results of this analysis<br />
were labelled TSP. Finally, samples of the particulate matter were analysed<br />
for total phosphorus. Hence we derive 3 fractions: a) a molybdate reactive<br />
fraction which is probably mainly dissolved inorganic phosphorus, b) a<br />
fraction which includes the molybdate reactive plus organic phosphorus and<br />
c) the particulate fraction which is primarily the phosphorus associated with<br />
the suspended solids.<br />
The total quantity of these various fractions carried out of the<br />
watershed during a specified time interval (loading) was calculated as follows.<br />
The concentration of MRP increased as discharge rate increased and the concentration<br />
of MRP was higher on the ascending limb of the hydrograph relative to<br />
the descending limb; hence, regressions of MRP on discharge rate and rate of<br />
change of discharge rate were calculated and these regressions were used for<br />
interpolation purposes. The loading of MRP was then calculated as the sum of<br />
the product of discharge per 2 hours, times concentration calculated from the<br />
regression. In general the difference in concentration between MRP and TSP<br />
was relatively constant and seemed to vary in a random fashion about the mean<br />
of 12 microgrammes P/1. The phosphorus content of the particulate matter<br />
varied randomly about a mean 0.11% of P.<br />
There are 3 aspects of the data which will be discussed in detail.<br />
These are: 1) The removal from solution of soluble phosphorus inputs from<br />
point sources during movement downstream, presumably by reaction with the<br />
streambed. 2) A procedure for allocating the loading of soluble phosphorus<br />
to a) biogeochemical ("natural" or "background") sources, b) diffuse sources<br />
associated with human activity and c) point sources associated with human<br />
activity. 3) A rationale for the working hypothesis that the soluble phosphorus<br />
will have a major impact on the biology of lakes while the phosphorus<br />
associated with the particulate matter will have only a minor impact.<br />
235
Two independent subwatersheds which together comprised about 3/4 of<br />
the total area of the whole watershed were selected for detailed study of<br />
behaviour of soluble phosphorus. One of these subwatersheds (location 16)<br />
contained a secondary sewage treatment plant serving 1200 people who effluent<br />
was emptied into the stream. This served as a major point source of soluble<br />
phosphorus. The other subwatershed (location 15) was similar in other aspects<br />
but did not contain such well a defined point source. One set of data which<br />
illustrates that the total soluble phosphorus (TSP) was not conserved in<br />
the stream is presented in Table 1. Particularly noteworthy are the losses<br />
during low flow periods, which were primarily the summer months. Presumably<br />
this loss is associated with reaction between phosphorus in the water and<br />
material in the streambed. During the periods of higher flow, there appeared<br />
to be some redissolution of this phosphorus, but recovery was not complete.<br />
Two small subwatersheds which were almost devoid of human activity<br />
were selected for study of total soluble phosphorus loading in the absence of<br />
human activity, and in addition the water from 4 wells sunk in an active<br />
agricultural area located on well-drained outwash was monitored. All of the<br />
samples from these sites contained about same the amount of MRP and TSP<br />
regardless of source. The averages were 62 0.3 microgrammes MRP/1 (123 samples)<br />
and 15% 0.9 microgrames TSP/1 (107 samples). Thus the loading of these phosphorus<br />
fractions in the absence of human activity was set equal to these concentrations<br />
times total discharge, which averages about 0.5 meter/ year in the<br />
area we studied.<br />
The remainder of the MRP and TSP was allocated to the sum of the<br />
diffuse and point sources. The landscape is a mosaic of point and diffuse<br />
sources. There are numerous single family dwellings (%3000), a sizeable<br />
fraction of which are served by septic tank-disposal fields which are not<br />
functioning properly and many simply overflow into drainageways. Milk house<br />
wastes are often emptied into drainageways. There are about 125 dairies in<br />
the watershed most of which have barnlots and the manure from these is spread<br />
on the associated cropland. Since the strategy for control of this multitude<br />
of sources is very different and since control of all is probably impractical,<br />
some means of estimating the relative importance would provide useful insights.<br />
The rationale for the separation of these sources is as follows.<br />
The diffuse sources have a major impact on water composition only when water<br />
runs over the soil surface and into drainage ways. Thus there is a soil<br />
factor and a precipitation intensity-distribution factor. Some soils are so<br />
permeable to water that only on very rare occasions will water run over the<br />
surface and into flowing drainageways. These are usually the most intensively<br />
farmed soils, and also receive the most manure. On the other hand, overland<br />
flow of water is common on less permeable soils and this is particularly true<br />
for barnlots where the compaction from the animal traffic reduces permeability.<br />
In contrast, milk house wastes and sewage inputs are mostly the same each day<br />
regardless of precipitation intensity.<br />
The foregoing discussion suggests that the major impact of diffuse<br />
source loading will be on the high discharge associated with precipitation<br />
events which produce large amounts of surface runoff. A complicating factor<br />
(during high discharge) is the dissolution of phosphorus from bed sediment<br />
which has reacted with point sources during the preceding low discharge<br />
intervals.<br />
236
TABLE 1<br />
Comparison of loading of total soluble phosphorus during selected<br />
periods for 2 major subwatersheds (locations 15 and 16) and the<br />
total watershed (location 1). The areas of subwatersheds 15 and 16<br />
are 147 and 104 km2 respectively and the area of watershed 1 is<br />
330 km’. Note that during low discharge intervals, the MRP loading<br />
from 15 and 16 was considerably higher than that at location 1,<br />
even though location 1 included 15, 16 and an additional 78 km2<br />
of area. Location 16 includes the secondary sewage treatment plant.<br />
r<br />
Discharge at<br />
location 1<br />
Period<br />
(m3 x<br />
15<br />
May 1974<br />
through<br />
October 1974<br />
November 1974<br />
through<br />
May 1975<br />
May 1974<br />
through<br />
August 1975<br />
40<br />
153<br />
210<br />
237<br />
340<br />
822<br />
1253
We proceeded to analyse high discharge events as follows utilizing<br />
the hydrograph analysis of Chow. This separates flow into 3 components:<br />
a) that associated with a base flow reservoir<br />
b) that associated with an interflow reservoir and<br />
c) that associated with surface runoff.<br />
Then a plot of the concentration MRF' of against surface runoff as a<br />
fraction of total flow was made and the results were extrapolated to 100%<br />
surface runoff. which served to estimate the concentration of MRP in the<br />
surface runoff.<br />
The results for the whole watershed for three winters are illustrated<br />
in Figure 1. The results indicate the concentration of MRP in the surface<br />
runoff is in the range of 34 to 60 microgrammes MRP/1, which is considerably<br />
higher than the 6 microgrammes MRP/l estimated for biogeochemical sources.<br />
The loading of diffuse sources was then calculated as the product of the<br />
estimated concentration at 100% surface runoff multiplied by the estimated<br />
amount of surface runoff (5 12 to 15% of total discharge). Finally, the<br />
point source inputs were estimated by difference.<br />
To summarize the calculations, the biogeochemical inputs were<br />
estimated as the product of total flow times average concentration MRP (or of<br />
TPS) in wells and stream flow from watersheds devoid of human activity. The<br />
inputs associated with human activity were estimated as the difference between<br />
total loading and biogeochemical loading. The diffuse source loading (associated<br />
with human activity) was calculated as the product of estimated concentration<br />
in surface runoff times estimated amount of surface runoff (% 12-<br />
15% of total flow in the watershed studied). Finally the point source inputs<br />
were estimated as the remainder.<br />
Samples of the results of this analysis are presented in Table 2.<br />
Clearly one can raise many questions about the validity of this procedure.<br />
We defend it to the extent that it represents a reasonable first approximation<br />
and simultaneously suggest a more precise and less arbitrary procedure<br />
should be developed.<br />
The third aspect of our analysis is associated with the implications<br />
of results of loading of phosphorus to the biota of lakes. The phosphorus<br />
Carried in streams is distributed among several chemical forms and physical<br />
states. Consideration of the nature and behaviour of these several components<br />
suggests that their effect on the biology of lakes differs. A major fraction<br />
of the total phosphorus carried out of a watershed by a stream is associated<br />
with the particulate matter suspended in water, the much of which is delivered<br />
during a relatively short period of time when the discharge rate is very high<br />
and hence the velocity of flow is sufficient to transport particles of varying<br />
sizes and densities. A smaller fraction of the total phosphorus is dissolved<br />
in the water. For example, during storm periods Fall Creek water often<br />
contained <strong>50</strong>0 to 1000 microgrammes of phosphorus per litre associated with<br />
particulate matter and 20 to 40 microgrammes per litre of dissolved phosphorus.<br />
Upon reaching the lake these fractions behave very differently; the larger<br />
particles soon settle to the bottom because water movement in the lake is no<br />
longer sufficient to keep them in suspension. At least in the lakes in<br />
central New York, a few days after large a storm which delivers large amounts<br />
of suspended solids, the water in the lake (even near stream outlets) becomes<br />
relatively clear and only the fine particles remain in suspension plus, of<br />
238
TABLE 2.<br />
ALLOCATION OF MOLYBDATE REACTIVE PHOSPHORUS (MRP)<br />
IN WATER AT LOCATIONS 15, 16 AND 1 FOR THE PERIOD<br />
MAY 1974 THROUGH AUGUST 1975.<br />
BGC 11<br />
Human Activity<br />
Point Diffuse Total<br />
Location kg x<br />
kg x<br />
kg x kg<br />
15<br />
16<br />
1<br />
"Biogeochemical<br />
45<br />
1260 41<br />
n<br />
a<br />
x 00<br />
394 22<br />
1100 61<br />
885 20<br />
590 47 12<strong>50</strong> 95 8 565<br />
310 17<br />
I I I<br />
025 0<strong>50</strong> 0.75 1.0<br />
SURFACE RUNOFF + TOTAL DISCHARGE<br />
Figure 1. The regressions of MRP concentration on fraction of<br />
the total flow as surface runoff at location 1 for<br />
three winters.<br />
239<br />
30<br />
1800<br />
3060 930
course, the dissolved constituents. Lake samples seldom contain more than a<br />
total of 10 to <strong>50</strong> microgrammes of phosphorus per litre in all forms. Thus<br />
the phosphorus remaining in the lake water is very different in nature and<br />
amount from that delivered by the stream during storm periods.<br />
According to the reasoning which follows, the amount of particulate<br />
phosphorus delivered by the stream has only a minor effect on the biology of<br />
lakes, while the dissolved phosphorus has a major effect.<br />
First, the bottom of the lake is covered with sediment delivered by<br />
past events from the lake basin. If the phosphorus in the sediment delivered<br />
by past events was essentially in the same forms as that delivered by current<br />
events, accumulation of more of the same seems unlikely to change the effect<br />
of the bottom sediments on lake biology; that is, adding a few millimetres<br />
of sediment to several metres of sediment seems unlikely to have an important<br />
effect on lake biology.<br />
Second, questions can be raised about the chemical activity of the<br />
particulate phosphorus; for example, if samples of suspended solids were<br />
leached with phosphorus-free water, how rapidly would the solid phase phosphorus<br />
dissolve and what would be the concentration of phosphorus in the leaching<br />
water? The results of such studies show that there is a small amount (usually<br />
considerably less than 10%) of the total phosphorus which dissolves fairly<br />
rapidly and that the concentration of leachate decreases as leaching continues.<br />
Such phosphorus is referred to as labile or sorbed phosphorus. Once this<br />
labile phosphorus is removed, the remainder dissolves very slowly and concentrations<br />
in solution are only few a microgrammes per litre. Furthermore,<br />
this is consistent with the fact that most of the phosphorus associated with<br />
the suspended solids has persisted through long geological periods; its<br />
resistance to dissolution must be considerable or it would have been leached<br />
out of the unconsolidated mantle and carried to the sea long ago. The results<br />
of studies with Fall Creek sediments demonstrate that the particulate matter<br />
contains about 1.1 milligrammes of total phosphorus per gram and about 0.04<br />
milligrammes (4%) of this is in a labile or sorbed form.<br />
Further evidence that soluble, not adsorbed, phosphorus is the<br />
critical factor determining phytoplankton concentrations is provided by<br />
comparative studies of the way lakes respond to phosphorus loadings. Detailed<br />
investigations on a group of New York lakes revealed that average summer<br />
standing crops of phytoplankton were better correlated with loadings calculated<br />
as soluble phosphorus than with those determined in the form of total<br />
phosphorus. Models resulting from this work have subsequently been extended<br />
to a global basis for temperate latitude lakes. These and other arguments<br />
lead us to formulate the following hypotheses about the effects of these fractions<br />
for temperate systems with soils and bedrock similar to those of the New<br />
York lakes: 1) probably 100% of the molybdate reactive phosphorus (MRP) and<br />
most of the total soluble phosphorus (TSP) is available to organisms in<br />
lakes. 2) probably the labile or sorbed fraction of particulate phosphorus<br />
is of limited importance to the biology of lakes. 3) probably the remainder<br />
of the particulate phosphorus is of no consequence to the biology of lakes.<br />
A most important aspect of the preceding paragraphs is to at least<br />
emphasize the question of biological effects of the different phosphorus<br />
fractions. There seems to be no reasonable defense for the hypothesis that<br />
all fractions are of equal biological importance. Thus measurements of<br />
loading based on the single parameter of total P delivered by streams seem to<br />
240
e of limited value in terms of estimating impact on lake biology. However,<br />
total P in the photic zone of the lake is an important parameter, but it<br />
should be noted that total P delivered by the stream and total P in lake<br />
water are entirely different; in fact, MRP the and TSP fractions measured<br />
in stream flow are probably comparable in many respects to total P in lake<br />
samples.<br />
Major portions of the work reported here were performed in cooperation<br />
with an interdisciplinary team funded in part by the Rockefeller<br />
Foundation, FT70103. A more comprehensive summary of the project is to be<br />
found in the following book:<br />
Porter, Keith, ed. Nitrogen and Phosphorus: Food Production,<br />
Waste and the Environment. Ann Arbor Sci. Publications,<br />
Inc. Ann Arbor, 1975.<br />
241
DISCUSSION<br />
Dr. T. Logan: You mentioned the method that you used for estimating the<br />
labile phosphorus by using the equilibrium phosphorus concentration and<br />
extrapolating to the amount that is desorbed. We have been doing more or<br />
less the same type of procedure to find what fraction of the particulate<br />
phosphorus might be bio-available. The only thing that bothers me about<br />
that method is that it is time-dependent. It is the amount that you are<br />
going to desorb in a given time which you pretty well define by your experimental<br />
conditions. But if you consider the situation that might be<br />
occurring in the lake, it is possible that you can regenerate additional<br />
labile phosphorus if you let the sediment rest or put it through a rest<br />
period. We have done some of this and in fact you can regenerate a considerable<br />
amount of labile phosphorus. I want to know how we try and resolve<br />
these types of problems.<br />
Dr. D. Bouzdin: The argument I would use is that we are interested in the<br />
incremental effect on the phosphorus associated with the sediments we that<br />
are adding. We assume that the sediments already there contain the phosphorus<br />
that they inherited from the geological materials and that adding a fresh<br />
batch really is not going to have too much influence on the lake. The thing<br />
is, the new sediment coming in has this labile fraction that in may fact<br />
have a much larger impact on the lake than the old sediment. That is our<br />
rationale, and to be sure I would not say that what we did was the best.<br />
It is a crude estimate and you can go into a lot of refinement with that,<br />
and I think you probably should. The only point I am trying to make Is that it is not a very large fraction of the total sediment phosphorus that<br />
we are dealing with. It is a relatively small fraction in our particular<br />
case and I do not think this is something that you extrapolate everywhere.<br />
Dr. T. Logan: The other point is, do you think that this labile phosphorus<br />
is a sediment parameter? Do you see it changing with land use OK fertilization<br />
practices, manure practices, this type thing, of or is it more or<br />
less determined by the soil characteristics?<br />
Dr. D. BouZdin: I think it should very much depend on the kinds of treatment.<br />
I would think the suspended solids derived from a non-agricultural landscape<br />
are quite different in nature from those derived from a barnlot. The amount<br />
243
of labile phosphorus on the barnlot sediments is quite high. Let me say<br />
one other thing, I am just convinced from everything I see and all the<br />
analysis of the data I can carry out, that well over <strong>50</strong>% of the total suspended<br />
solids in the Fall Creek Watershed are simply derived from stream bank<br />
erosion, and this is not the kind of erosion that is the reworking old sediments.<br />
In one case in the stream sub-watershed that gives me the highest<br />
loading of suspended solids, the stream is clearly eating its way through an<br />
old terminal moraine. It has not got there yet. It is still chewing away on<br />
that and you can see that in very many places in the watershed. We have got<br />
a little bit of suspended solids from the barnlots mixed up with a lot of<br />
stuff that has never seen phosophorus or manure or anything else. It is<br />
ground up glacial debris that has been sitting there 10,000 for years.<br />
244
INTRODUCTION<br />
Geochemistry of Sediment/Water Interactions of Nutrients,<br />
Pesticides and Metals, Including Observations on Availability.<br />
Pesticides<br />
J. B. Weber<br />
Pesticides used in agricultural production eventually reach the<br />
soil where they are acted upon by various processes. The chemicals may<br />
be decomposed by chemical, biological, or photochemical degradation.<br />
They are also acted upon by transfer processes such as adsorption, exudation,<br />
retention and accumulation by organisms, adsorption by soil<br />
colloids, and other surfaces, and movement in the vapour, liquid and<br />
solid state through the atmosphere, soil, and water (1. 2, 3). In this<br />
paper, we are concerned primarily with pesticides and particulate matter<br />
that end up as sediment in water.<br />
SEDIKNT<br />
Soil sediment is defined as the solid material, both mineral<br />
and organic, that is in suspension, is being transported, or has been<br />
moved from its site of origin by air, water, gravity, or ice and has come<br />
to rest on the earth's surface either above or below sea level (4). It<br />
is by far the United States' single largest water pollutant, exceeding the<br />
sewage load by some <strong>50</strong>0 to 700 times (5). About half of all sediment<br />
originates on agricultural land (6) and some of it contains adsorbed<br />
pesticides (7).<br />
The properties of sediment influence its effect on the environment.<br />
Physical properties, including size, density, and shape of the particles<br />
affect the sediment movement. Coarse sediment is relatively inert, but fine<br />
sediments composed of silt, clay, and organic materials are chemically<br />
active and may absorb or desorb chemicals from solution. The bulk of the<br />
sediment problem, however, is caused by the colloidal fractions of sediment.<br />
The colloidal material may be a mixture of clay minerals, of the expanding<br />
or nonexpanding type, and of organic materials ranging from relatively<br />
hydrophilic and undecomposed to highly lipophilic and highly decomposed.<br />
Little is known about the chemistry of sediment in field situations, but<br />
information is available on the interaction of pesticides with various<br />
soil colloids and specific adsorbents (3,8). It is also known that<br />
radioactive materials from fallout selectively erode with soil sediment<br />
and may result in higher concentrations of radioactivity in reservoir<br />
deposits (7) and there is a good possibility that an analogous situation<br />
is occurring with pesticides. A recent survey along tributaries of the<br />
lower Mississippi River revealed isolated bottom sediment deposits containing<br />
as much as 0.5 ppm DDT (9).
Complete elimination of soil erosion is impossible, so guidelines<br />
are being developed to define acceptable levels of erosion and sedimentation<br />
(10). Models for predicting runoff (11) and pesticide movement through<br />
soils (12) are being developed. An excellent bibliography on the effects of<br />
agricultural practices or environmental quality is also available (13).<br />
PESTICIDE I~RACTION~<br />
Herbicides, insecticides, and fungicides vary greatly in their<br />
physical and chemical properties and in their behaviour in the environment<br />
(1, 3, 14, 15, 16). Of the many properties that regulate pesticide behaviour,<br />
at least four are of primary importance. They include: (a) ability to ionize;<br />
(b) presence of complexing elements (P, As); (c) water solubility; and<br />
(d) ability to vapourize (3). A classification of pesticides based on the overall<br />
characteristics of these four properties of selected pesticides is shown<br />
in Table 1. Chemical names for the compounds are given in Table 2.<br />
PESTICIDE FROPERTIES<br />
The ability of a pesticide to ionize determines whether or not the chemical<br />
will have an ionic charge in aqueous solution. Cationic pesticides, such as<br />
the herbicides cyperquat, difenzoquet, diquat, and paraquat, are readily<br />
adsorbed by negatively charged surfaces of colloidal soil sediment (3).<br />
They are completely removed from aqueous solution by colloidal sediment and<br />
move with the sediment (3). Anionic pesticides, such as the acidic herbicides<br />
dalapon, dicamba. picloram, and 2,4-D are adsorbed in only small<br />
amounts by colloidal soil sediment and are readily desorbed in aqueous<br />
systems (3). They are, therefore, found primarly in the solution phase in<br />
waters. Basic pesticides, such as the 6-triazine herbicides ametryn,<br />
atrazine, prometon, and prometryn are adsorbed by acidic colloidal surfaces<br />
in relatively large amounts and by neutral or basic colloidal surfaces in<br />
much smaller amounts. Since their adsorption is dependent on the acidity of<br />
the system, the amount that is retained or released by soil sediment depends<br />
on the pH of the system (3, 17). They are, therefore, in equilibrium with<br />
the sediment and the solution phase. Nonionic pesticides are adsorbed by<br />
colloidal surfaces primarily through physical adsorption forces the and<br />
amount that is retained by soil sediment is dependent to a large extent upon<br />
the water solubility of the pesticide and the hydrophilic/lipophilic characteristics<br />
of the sediment (3). Relatively water soluble, nonionic pesticides,<br />
such as aldicarb, CDAA, dimethoate, molinate, carbetamide, fenuron,<br />
methomyl, and propoxur, are adsorbed soil by colloids in very small amounts<br />
and are readily desorbed in dynamic, aqueous systems. Pesticides of very<br />
low water solubility such as aldrin, BHC, lindane, trifluralin, DDT, dieldrin,<br />
endrin, and oryzalin, are adsorbed by highly lipophilic organic colloidal<br />
sediments but not by hydrophilic clay colloids. They are in equilibrium<br />
with the surrounding aqueous phase, but because of their low solubilities<br />
desorb only slightly. Nonionic pesticides of moderate water solubilities<br />
are intermediate between the low and high water soluble compounds in their<br />
reaction with colloidal sediments. Pesticides which possess complexing<br />
elements such as phosphate, (demeton, disulfoton, glyphosate, parathion, and<br />
phorate) or arsenic (DSMA, MAMA and MSMA) readily complex with clay colloids<br />
and move with these sediments.<br />
246
N<br />
c.<br />
U<br />
TABLE 1.<br />
CLASSIFICATION OF SELECTED PESTICIDES BASED ON OVEMLL THE CHARACTERISTICS OF<br />
IONIZABILITY, WATER SOLUBILITY, PRESENCE OF COMPLEXING ELEMENTS, AND VAPOURIZABILITY<br />
Ionic Pesticides<br />
I With<br />
Complexing High<br />
Nonionic Pesticides<br />
Water Moderate Water Low Water<br />
Elements Solubility Solubility Sol<br />
Cationic Basic Acidic Non- Non- Non-<br />
Pesticides Pesticides Pestieides Volatile Volatile Volatile Volatile Volatile Volatile Volatile Volatile<br />
cyperquat ametryn dalapon demeton DSMA aldicarb carbetamide CDEC butachlor aldrin DDT<br />
difenzoquat atrazine dicamba disulfoton glyphosate CDAA fenuron EPTC diuron BHC dieldrin<br />
diquat prometon picloram parathion MAMA dimethoate methomyl pebulate fluometuron lindane endrin<br />
paraquat prometryn 2,4-D phorate MSMA molinate propoxur vernolate monuron trifluralin oryzalin<br />
~~
Common<br />
Name<br />
cyperquat<br />
difenzoquat<br />
diquat<br />
paraquat<br />
one t ryn<br />
atrazine<br />
prometon<br />
prometryn<br />
dalapon<br />
dicamba<br />
picloram<br />
2,4-0<br />
demeton<br />
disulfoton<br />
parathion<br />
phorate<br />
DSMA<br />
glyphosate<br />
"A<br />
MSMA<br />
aldicarb<br />
Table 2.<br />
NAMES AND PROPERTIES SELECTED PESTICIDES<br />
Chemical Name<br />
Water<br />
solubility<br />
(wd<br />
1-methyl-4-phenylpyridinium ion >lo5<br />
1.2-dimethyl-3.5-diphenylpyrazium ion >lo5<br />
6.7-dihydrodipyrido[1,2-a:2',1'-c]pyrazinediium ion>105<br />
l,l'-dimethyl-4,4'-bipyridinium ion >lo5<br />
2-(ethylamino)-4-(isopropylamino)-6-(methylthio) 185<br />
-s-triazine<br />
2-chloro-4-(ethylamino)-6-(isopropylamino)-s- 33<br />
triazine<br />
2,4-bis(isopropylamino)-6-(methoxy)-s-triazine 7<strong>50</strong><br />
2.4-bis(isopropylamino)-6-(methylthio)-s-triazine 48<br />
2,2-dichloropsopionic acid<br />
3,6-dichloro-o-anisic acid<br />
4-amino-3,5,6-trichloropicolinic acid<br />
2,4-dichlorophenoxy acetic acid<br />
0,O-diethyl-o(and S)-(Z-(ethylthio)ethyl)<br />
phosphorothioates<br />
0,O-diethyl-S-(2-ethy1thio)ethyl)phosphoro-<br />
dithioate<br />
0,O-diethyl-0-p-nitrophenyl phosphorothioate<br />
0,O-diethyl-S-(ethy1thio)-methyl phosphoro-<br />
dithioate<br />
disodium methanearsonate<br />
N-phosphonomethylglycine<br />
monoammonium methanearsonate<br />
monosodium methanearsonate<br />
2-methy1-2-(methylthio)-propionaldehyde-O-<br />
(methylcarbamoy1)oxine<br />
248<br />
>lo5<br />
4<strong>50</strong>0<br />
430<br />
900<br />
2<strong>50</strong><br />
66<br />
24<br />
<strong>50</strong><br />
>lo5<br />
>lo5<br />
loG<br />
lo6<br />
6000<br />
Vapour<br />
pressurc<br />
(mm Hg @ 21<br />
< 10-<br />
(10-6<br />
2 x 10-6<br />
5 x<br />
3 x 10-6<br />
a0-6<br />
1 x 10"~<br />
1.8 x<br />
2.3 x<br />
1 x<br />
10"<br />
Common<br />
Name<br />
CDlZA<br />
dimethoate<br />
molinate<br />
carbetamide<br />
f enuron<br />
methomyl<br />
propoxur<br />
CDEC<br />
EPTC<br />
pebulate<br />
vernolate<br />
butachlor<br />
diuron<br />
fluometuron<br />
monuron<br />
aldrin<br />
BHC<br />
lindane<br />
trifluralin<br />
DDT<br />
dieldrin<br />
endrin<br />
oryzalin<br />
Chemical Name<br />
N,N-diallyl-2-chloroacetamide<br />
0,O-dimethyl-S-(-methylcarbamoylmethyl)<br />
phosphorodithioate<br />
S-ethylhexahydro-1H-azepine-1-carbothioate<br />
D-N-ethyl-2-(phenylcarbamoyloxy)-propioamide<br />
l,l-dimethyl-3-phenylurea<br />
Water<br />
solubility<br />
(ppm)<br />
>lo5<br />
25,000<br />
800<br />
3<strong>50</strong>0<br />
3000<br />
S-methyl-N-((methylcarbamoy1)oxy)thioacetimidate 10,000<br />
2-isopropylphenyl-N-methylcarbamate<br />
2-chloroallyl diethyldithiocarbamate<br />
S-ethyl dipropylthiocarbamate<br />
S-propyl butylethylthiocarbamate<br />
S-propyl dipropylthiocarbamate<br />
N-butoxymethyl-2-chloro-2',6'-diethylacetanilide<br />
3-(3,4-dichlorophenyl)-l,l-dirnethyurea<br />
1,l-dimethyl-3-(a,a,a-trifluoro-m-tolyl)urea<br />
3-(p-chlorophenyl)-l,l-dimethylurea<br />
1,2,3,4,10,10-hexachloro-l,4,4a,5,8,8a-hexahydro-<br />
1,4-endo-exo-5,8-dimethanonaphthalene<br />
1,2,3,4,5~,6-hexachlorocyclohexane<br />
gamma isomer of 1,2,3,4,5,6-hexachlorocyclohexane<br />
2000<br />
92<br />
370<br />
60<br />
90<br />
23<br />
42<br />
90<br />
230<br />
0.2<br />
a,a,a-trifluor-2,6-dinitro-N,N-dipropyl-p-toluidine 0.05<br />
dichlorodiphenyltrichlorethane 0.001<br />
1,2,3,4,10,10-hexachlor-exo-6,7-epoxy-l,4,4a,5,6, 0.25<br />
7,8,8a-octahydro-l,4-endo-exo-5,8-dimethano-<br />
naphthalene<br />
1,2,3,4.10,10-hexachloro-6,7-epoxy-1,4.4a.5.6.7 0.23<br />
8,8a,-octahydro-l,4-endo,endo-5,8-dirnethanonaphthalene<br />
3,5-dinitro-N4,N'-dipropylsulfanilamide 2.4<br />
249<br />
10<br />
10<br />
Vapour<br />
pressure<br />
(mm Hg @ 25'C)<br />
1 x io-'<br />
5.6 x<br />
(10-<br />
2.2 x 10-3<br />
3.4 x<br />
3.5 x<br />
1.0 x<br />
4.5 x<br />
5 x<br />
10-<br />
5 X<br />
1.3 x 10"<br />
1.9 X<br />
10-<br />
2.7 X loq6<br />
2 x 10"
Pesticides with vapour pressures higher than 1 x mm of Hg at<br />
25' C are considered to be volatile and generally need to be incorporated<br />
into the soil to be effective (3). Generally the higher the vapour pressure,<br />
the more volatile is the chemical. Such pesticides, including those listed<br />
in Table 1 and the esters of the phenoxy acid herbicides, are adsorbed by<br />
colloidal soil sediments, but they are readily desorbed in dynamic aqueous<br />
systems; in addition the vapours escape from the aqueous phase into the<br />
atmosphere.<br />
PESTICIDE b m ~ ~<br />
Studies on the movement of pesticides from agricultural lands<br />
showed that the chemicals were transported both in the runoff water and<br />
adsorbed to suspended materials (18, 19, 20, 28). The amounts transported<br />
in runoff depended on climatic factors such as frequency, intensity, and<br />
duration of rain; chemical properties of the pesticides; and properties of<br />
the soils such as soil type, slope, and moisture content and surface condition.<br />
Barnett % &. (18) found that ester formulations of 2,4-D were<br />
more susceptible to runoff than the more soluble amine salt forms. The<br />
ester forms adsorbed to soil particles and moved with the eroding soil,<br />
whereas the amine forms dissolved and leached into the soil.<br />
Surface runoff of 2,4-D and picloram was found to be greater from<br />
sod than from fallow soil surfaces in studies by Trichell (19). The opposite<br />
was found by Edwards (30) to be the case for atrazine. Runoff of 2,4,5-T was<br />
about equal under the same conditions (3). Herbicide runoff increased with<br />
increasing slope and rate of application and concentration decreased when<br />
runoff passed over untreated sod.<br />
In studies where trifluralin, DDT, toxaphene, or methylparathion<br />
was applied to cotton, less than 1%, 3.12%, 1%, and 0.25%, respectively, was<br />
recovered in the runoff (26, 26). Of the four pesticides recovered, 84%,<br />
96%, 75%, and 12%, respectively was associated with the sediment.<br />
The highest concentrations of diuron, summitol, 2,4-D, 2,4,5-T,<br />
and picloram observed in surface runoff waters were 1.8 ppm, 0.5 ppm, 4.2<br />
ppm. 1.2 ppm, and 2.7 ppm respectively, in ditchbank weed control studies<br />
in Utah (29). The greatest herbicide movement occurred during intial storms<br />
following herbicide application.<br />
Fluometuron movement in runoff from studies conducted in five<br />
southern states, and Puerto Rico, ranged from 0.10 to 0.87 ppm in the first<br />
runoff and decreased to less than 0.2 ppm in later runoff (21). Most of the<br />
herbicide was found distributed in the 0 to 30 cm zone (22), but in two<br />
sandy soils it was found in the 15 to 30 cm zone in high enough concentrations<br />
in injure bioassay plants (23).<br />
In a study conducted in areas where forest vegetation had been cut<br />
two years before spraying, less than 0.1% of the 2,4-D, and less than 1% of<br />
the picloram applied was lost in runoff from the first seven runoff events<br />
after herbicide application (24).<br />
2<strong>50</strong>
Sheets st. (25) found, where 2,4-D, 2,4,5-T, picloram, or<br />
dicamba was applied to small plots in a mountain watershed, maximum con-<br />
centrations of 2.55 ppm, 0.68 ppm, 4.19 ppm, and 0.16 ppm, respectively,<br />
occurred in surface runoff during the first OK second event after application.<br />
BIOACTIVITY OF ADSOREED PESTICIDES<br />
Biological availability of bound pesticides is dependent upon the<br />
type of pesticide and colloidal sediment involved (2, 17). Pesticides that<br />
are weakly adsorbed to the surfaces of colloidal sediment, such as the<br />
phenoxy acid herbicides, are readily desorbed into the surrounding aqueous<br />
environment and dissipate primarily through dilution. Pesticides, such as<br />
diquat and paraquat, that are adsorbed on the internal surfaces of expanding<br />
types of clay colloids are not released to the aqueous phase and are not<br />
available to organisms. Pesticides that are very water insoluble are normally<br />
adsorbed to the surface of lipophilic sediment. They are in equilibrium<br />
with the surrounding aqueous environment and are released or degraded chemically<br />
OK biologically, except in those cases where the chemicals have<br />
become part of the organic collodial matrix. The majority of pesticides are<br />
intermediate in biological degradability in the bound state. They are in<br />
equilibrium with the surrounding dynamic media which tends to desorb them<br />
and make them available for degradation.<br />
GMCLUS IONS<br />
The extent to which pesticides applied to agricultural lands may<br />
pollute runoff water and the eroding sediment depends on the physical,<br />
chemical, and biological properties of the chemicals, the intensity and<br />
duration of the rainfall, and edaphic properties such as soil cover, soil<br />
type, and slope.<br />
LITERATURE CITED<br />
A Primer on Agricultural Pollution. (1971)., J. Soil and Water<br />
Conservation, g,(2); 44-65.<br />
Bailey, G. W., R. R. Swank, Jr., and H. P. Nicholson. (1974).,<br />
Predicting Pesticide Runoff from Agricultural Land: A<br />
Conceptual Model. J. Environ. Qual., 2,95-102.<br />
Barnett, A. P., E. H. Hauser, A. W. White, and J. H. Holladay.<br />
(1967)., Loss of 2,4-D in Washoff from Cultivated Fallow Land.<br />
Weeds, x, 133-137.<br />
Barthel, W. F., J. C. Hawthorne, J. H. Ford, G. C. Bolton,<br />
L. L. McDowell, E. H. Grissinger, and D. A. Parsons.<br />
(1969)., Pesticide Residue in Sediments of the Lower Mississippi<br />
River and its Tributaries. Pesticides Monitoring J., 2(1),<br />
8-66.<br />
251
Bradley, J. R., Jr., T. J. Sheets, and M. D. Jackson. (1972).,<br />
DDT and Toxaphene Movement in Surface Water from Cotton Plots.<br />
J. Environ. Qual., 1. 102-105<br />
Control of Water Pollution from Cropland. (1975) Vol. 1-A Manual for<br />
Guideline Development. ARS, USDA, and Office of Research<br />
and Development, EPA Report No. ARS-H-5-1, Superintendent of<br />
Documents, Washiongton, D. C.<br />
Davidson, J. M., G. H. Brusewitz, D. R. Baker, and A. L. Wood.<br />
(1975)., Use of Soil Parameters for Describing Pesticide Movement<br />
Through Soils. Environmental Protection Agency Report No.<br />
660/2-75-009, National Environmental Research Centre, Office<br />
of Research and Development, EPA, Corvallis, Oregon. 149 pp.<br />
Edwards, W. M. (1972)., Agricultural Chemical Pollution as Affected<br />
by Reduced Tillage Systems. Proc. No-Tillage Systems Symposium,<br />
February 21-22, 1972, Columbus, Ohio., pp. 30-40.<br />
Environmental Quality: An Annotated Bibliography of Papers,<br />
Published in the Soil Science Society of America Proceedings,<br />
(1962-1971)., Soil Science Society of America, Madison, Wisconsin,<br />
1975.<br />
Epstein, E. and W. J. Grant., (1968). Chlorinated Insecticides in<br />
Runoff water as Affected by Crop Rotation. Soil Sci. SOC.<br />
Amer. Proc., 32, 423-426.<br />
Evans, J. 0. and D. R. Duseja. (1973)., Herbicide Contamination of<br />
Surface Runoff Waters. Environmental Protection Agency Report<br />
R2-73-266. Superintendent of Documents, U.S. Government,<br />
Washington, D. C., 99 pp.<br />
Glymph, L. M. and C. W. Carlson. (1966)., Cleaning Up Our Rivers and<br />
Lakes, Paper No. 66-711. American Society Agricultural<br />
Engineering, St. Joseph, Michigan. 43 pp.<br />
Glymph, L. M. and H. C. Storey. (1967)., Sediment--its Consequences<br />
and Control. Pub. 85. American Association Advancement<br />
of Science, Washington, D. C., pp. 205-220.<br />
Peeper, T. F. and J. B. Weber. (1974)., Vertical Fluometuron Movement<br />
in Runoff Studies of the Southern Region. Proc. So. Weed Sci.<br />
SOC., 27, 324-332.<br />
Resource Conservation Glossary., (1970) Soil Conservation Society of<br />
America, Ankeny, Iowa.<br />
Savage, K. E. (1975)., S-78 Regional Research Project--Movement and<br />
Persistence of Fluometuron in the South: I. Persistence and<br />
Vertical Movement in Soil. Proc. So. Weed Sci. SOC., 8,32<br />
(abstract)<br />
Sheets, T. J., J. R. Bradley, Jr., and M. D. Jackson. (1973).,<br />
Movement of Trifluralin in Surface Water. Proc. So. Weed<br />
Sci. SOC., 26, 376 (abstract).<br />
252
Suffling, R., D. W. Smith, and G. Sirons. (1974)., Lateral LOSS of<br />
Picloram and 2,4-D from a Forest Podzol During Rainstorms.<br />
Weed Res., 2, 301-304.<br />
Trichell, D. W., H. L. Morton, and M. G. Merkle. (1968)., Loss of<br />
Herbicides in Runoff Water. Weed Sci., 16, 447-449.<br />
Weber, J. B. (1972)., Interaction of Organic Pesticides with Particulate<br />
Matter in Aquatic and Soil Systems. In R. F. Gould (ed.) Fate<br />
of Organic Pesticides in the Aquatic Environment, Chapter 4,<br />
pp. 55-120, American Chemical Society, Washington, D. C.<br />
Weber, J. B. (1975)., Agricultural Chemicals and Their Importance<br />
as a Non-point Source of Water Pollution. Proc. S. E. Regional<br />
Conf. Non-Point Sources of Water Pollution, Virginia Polytechnic<br />
Institute and State University, May 1-2, 1975, pp. 115-129.<br />
Weber, J. 8. (1970)., Mechanisms of Adsorption of s-triazines by<br />
Clay Colloids and Factors Affecting Plant Availability. &<br />
F. A. Gunther (ed.) The Triazine Herbicides, pp. 93-130,<br />
Springer-Verlag, N. Y.<br />
Weber, J. B., Environmental Safety of Pesticides: The Old VS. the<br />
New. Submitted for publication in Environ. Sci. and Tech.,<br />
June, (1976).<br />
Weber, J. B. and S. B. Weed. (1974)., Effects of Soil on the Biological<br />
Activity of Pesticides. Chapter 10, pp. 223-256, W. P.<br />
Guenzi (ed.) Pesticides in Soil and Water, Soil Science Society<br />
of America, Inc., Madison, Wisconsin.<br />
Weber, J. B., S. B. Weed, and T. J. Sheets. (1972)., Pesticides: How<br />
They Move and React in the Soil. Crops and Soils Magazine, z,(l),<br />
14- 17.<br />
Weber, 3. B., T. J. Monaco, and A. D. Worsham. (1973)., What Happens<br />
to Herbicides in the Environment? Weeds Today,<br />
4,(1),16-17,22.<br />
Weed, S . B. and J. B. Weber. (1974)., Pesticide-Organic Matter Inter-<br />
actions, Chapter 3, pp. 39-66, W. D. Guenzi (ed.) Pesticides in<br />
Soil and Water, Soil Science Society of America, Inc., Madison,<br />
Wisconsin.<br />
White, A. W., A. P. Barnett, B. G. Wright, and J. H. Holladay. (1967)..<br />
Atrazine Losses from Fallow Land Caused by Runoff and Erosion.<br />
Environ. Sci. Tech., 1. 740-744.<br />
Wiese, A. F. (1975). S-78 Regional Research Project--Movement and<br />
Persistence of Fluometuron in the South: 11. Lateral Movement<br />
in Runoff Water. Proc. So. Weed Sci. SOC., 28, 322, (abstract).<br />
253
INTRODUCTION<br />
Geochemistry of SedimentlWater Interactions of Nutrients,<br />
Pesticides and Metals, Including Observations on Availability.<br />
Metals<br />
I. Jonasson<br />
A knowledge of an element's behaviour at a sediment-water interface<br />
is of prime concern in the development of an understanding of what may be the<br />
significant chemical controls on that element's dispersion and perhaps upon<br />
its fixation into sediments.<br />
Allied research on the transport of elements in solution as complexed<br />
species has received wide attention in the geochemical literature.<br />
Pitwell (1973) has concluded that the most common complexing ligands present<br />
in groundwaters are water itself, carbonate, chloride, fluoride and some<br />
organic acids. Taken to their extreme limits, processes by which metals are<br />
mobilized, transported, precipitated and remobilized are considered important<br />
steps in the formation of sedimentary sulphide orebodies. Saxby (1973) has<br />
suggested that on diagenesis, metal-organic complexes may eventually form<br />
concentrations of metallic sulphides.<br />
However, this discussion is concerned primarily with the nature of<br />
the dynamic interactions, mainly chemical, which affect the cycling of a<br />
given trace metal ion or its compounds through fluvial systems.<br />
Firstly it is necessary to deal with organic matter and its involvement<br />
in such interactions, and then with the influences of certain hydrous<br />
metal oxides. In nature, these might be regarded as two relatively pure<br />
systems representing end-members of a full spectrum of interactions in which<br />
practically any substance present can be regarded as a participant in dispersion<br />
processes.<br />
For example, organic matter predominates in the flat-lying terrain<br />
of the tree covered section of the Canadian Shield and Interior Lowlands.<br />
Streams are commonly fed by waters from swamps, marshes and muskeg. The<br />
residence time of waters within organic trash ensures considerable dissolution<br />
of humic materials. Silts and clays are often covered with finely<br />
divided organic matter. By contrast, stream sediments from the far north of<br />
Canada and from the alpine Cordilleran regions are generally clean of organic<br />
matter; waters derived mainly from snow melt contain very little dissolved<br />
organics and adsorption of metals directly into clays, rock flour and hydrous<br />
metal oxides and dissolution of mineral particles are the predominant watersediment<br />
interactions.<br />
TAL INTERACTIONS<br />
(a) Interactions Involving Organic Matter<br />
Within surficial waters the most significant form of metal-organic<br />
interaction is generally accepted to involve chelation of trace elements<br />
255
with the complex, polymeric organic compounds which comprise humic matter<br />
(Saxby 1969, Bowen, 1966). Experimental studies have shown that, in comparison<br />
with model organic compounds likely to find their way into natural<br />
waters, soluble humic matter has the greatest potential to form soluble,<br />
stable metal complexes in competition with hydrolytic and other precipitating<br />
reactions. Solid humic matter in a fluvial system exhibits exceptionally<br />
strong cation exchange properties thus concentrating trace metals from<br />
solution onto a solid phase of bed a sediment.<br />
The trace element distribution between sediment and natural water,<br />
and the chemical factors influencing this are of prime concern. The dominant<br />
interactions here are suggested to be cation hydrolysis, cation exchange<br />
reactions and related adsorption-desorption processes, as well as complex<br />
species formation involving both cations and anions.<br />
Of the organic species involved, humic acids, fulvic acids and<br />
humins are the most abundant. A major problem in studies on the nature of<br />
metal-complex speciation centres in the fact that all of the above compounds<br />
form part oE an extremely heterogeneous mixed polymer system where the<br />
differences between the sub-divisions are due to variations in elemental<br />
composition, acidity, degree of polymerization and range of molecular weights.<br />
Thus, humins comprise the highest molecular weight groups; highly polymerized<br />
and largely insoluble in aqueous solution. Humic acids are from the middle<br />
molecular size range, also very complex and, in part, soluble under certain<br />
conditions of acidity. Fulvic acids are likely the main transporters of<br />
metals in solution and comprise hydrophilic polyhydroxy aliphatic and aromatic<br />
acids. At the low molecular weight end of the range are the so-called yellow<br />
organic acids commonly observed in swamp waters or in stream waters of certain<br />
arid zones, particularly where considerable carbonate is present. These<br />
substances are probably also important in pore waters and interstitial waters<br />
of bed sediments representing as they do end members of humic matter degradation.<br />
Micro-organisms are, in part, responsible for the decomposition of<br />
the higher weight organic acids (Kuznetsov, 1975) producing the smaller more<br />
soluble fragments. Micro-organisms are also important in mobilizing certain<br />
metals from sediments by means of very specific biochemical interactions.<br />
These have been well discussed in the literature and will not be discussed<br />
further here (Wong et&., 1975 for Pb; Chau et&., 1976 for Se; Wood, 1974,<br />
1975 for Sn, Hg, As and Se).<br />
It should also be noted that humic and fulvic acids behave as<br />
negatively charged species in solution, due to the ionization of their acidic<br />
carboxyl and hydroxyl groups. Neutralization of this charge, perhaps by<br />
interacting metal ions, metal oxide colloids, or adsorption onto clay particles<br />
can lead to flocculation of the colloids and subsequently further<br />
coprecipitation of metals. Mechanisms by which metallic ions from natural<br />
waters are absorbed or complexed by such organic matter, have been discussed<br />
extensively by many authors (e.g. Krauskopf, 1955; Schnitzer and Khan, 1972;<br />
Curtis, 1966). The attractive interactions of ions with soluble, colloidal<br />
or particulate organic material range from weak forces leaving the ion easily<br />
replaceable (physical adsorption) to strong forces indistinguishable from<br />
chemical bonds and often not replaceable (i.e., chemisorption, or specific<br />
adsorption).<br />
The problems involved in studying metal-sediment-water interactions<br />
either in laboratory models or in the field are many. Several useful reaction<br />
schemes have been proposed as a basis for laboratory studies. That of Curtis<br />
(1966) is shown in Figure 1. According to Curtis' idealistic scheme, metal<br />
ions can show a positive correlation with organic carbon in a sediment when<br />
256
4* or M(H~o)~+<br />
Y<br />
y<br />
metal-organic<br />
compounds or ions<br />
adsorption of<br />
metal-organic<br />
material onto<br />
clay particles<br />
(he metal-C correlation)<br />
no interaction (no element4 correlation)<br />
CP: clay particles<br />
ORG: organic matter<br />
x organic matter and element<br />
competing for clay particles<br />
(-ve element - C correlation)<br />
Figure 1: Generalized metal-organic-solid reaction scheme as proposed by<br />
Curtis (1966).<br />
251
metal ions interact in solution with dissolved organic matter that is in<br />
turn concentrated by adsorption onto particulates such as clay minerals.<br />
Zero correlations may result when there is no solution phase interaction and<br />
the dissolved organic matter alone adsorbs onto the particulates. If there<br />
is competition between metal ions and dissolved organics for adsorption sites,<br />
then negative carbon-metal correlations are the result. Ong and Bisque<br />
(1968) and Ong 4. (1970) have refined the Curtis scheme to consider<br />
colloids as possible transporting agents of metal ions (Figure 2).<br />
The ability of solid humic matter to physically and chemically<br />
adsorb metals from aqueous solution has been well documented. For example,<br />
Rashid (1974) investigated metal adsorption onto sedimentary and peat humic<br />
acids. A solution containing equal amounts of Co, Cu, Mn, Ni and Zn ions was<br />
brought into contact with humic acid; Cu was preferentially adsorbed.<br />
Leaching with various agents showed that Cu was held far more firmly than the<br />
other metals. From these and similar experiments described in the literature,<br />
it has proved possible to indicate a probable order of binding strength for a<br />
*<br />
number of metal ions onto humic or fulvic acids, e.g., UOn > Hg* > Cu* ><br />
ft<br />
Pb* > Zn* > Nift > Co*. Ca* is considered to bind as strongly as Pb .<br />
Similar experiments by Rashid to adsorb base metals from seawater<br />
were not nearly so successful probably due to the very low contents of these<br />
metals in seawater and the dominating presence of alkali and alkaline earth<br />
cations, which although likely more weakly adsorbed are in much greater<br />
abundance when in competition for adsorption sites. This an is important<br />
parameter to be considered in all field studies of freshwater systems as<br />
well.<br />
The importance of relative binding strengths should not be overlooked,<br />
nor should the fact that the order of metal-organic matter stabilities<br />
will vary somewhat according to the nature and complexity of the organics and<br />
to conditions such as acidity and redox potential.<br />
Another major function of organic matter in water-sediment interactions<br />
concerns the ability of soluble organic acids to chemically leach and<br />
even dissolve minerals. Thus particulates trapped in organic-rich sediments,<br />
perhaps as a result of coprecipitation or surface adsorption, will slowly<br />
undergo a prolonged attack by sorbed fulvic or humic acids which can extract<br />
a variety of elements including metals. For example Baker (1973) found that<br />
humic acids rapidly decompose sulphides such as chalcopyrite, chalcocite,<br />
sphalerite, galena or pyrite and silicates such as clays, micas or chlorite<br />
and also metal oxides such as pyrolusite or geothite; the last being an<br />
observation of some relevance to stream systems. The carbonates calcite,<br />
dolomite and malachite are also vulnerable to dissolution by humic acids. On<br />
the other hand, Sequi &. (1976) have reported that the alkali solubility<br />
of soil organic matter, common in stream sediments, is greatly dependent on<br />
the nature of metal ions present in the stream.<br />
Therefore interactions among metal ions, minerals, organic<br />
sediments and water seem to be mutually destructive of all solid species.<br />
The ultimate result of the breakdown of a mineral or metal-sediment complex<br />
by organic acids must be to promote the remobilization of that metal ion<br />
until the adsorption, flocculation, polymerization, precipitation cycle takes<br />
it out of solution once again.
Organic acids<br />
(hydrophilic,<br />
negatively charged<br />
colloids)<br />
+ M+<br />
Metal-humates Physical<br />
(precipitates) aspect<br />
predominates<br />
M+<br />
(neutral colloids) *-.<br />
Chemical Metal-organic<br />
aspect<br />
complexes<br />
predominates (hydrophilic + M'<br />
colloids)<br />
Metal-organic complexes<br />
(hydrophobic colloids)<br />
Figure 2: Metal-organic interaction scheme as proposed by Ong etas. (1970).<br />
259
Organic matter is also capable of acting as a reducing agent. In<br />
this way ferrous ion can be stabilized in oxygenated solution by complexation<br />
with certain organic acids (Theis and Singer, 1973) such as tannic or gallic,<br />
as well as by naturally occurring humic acid compounds (Rashid and Leonard,<br />
ft<br />
1973). It is possible that other metal ions such UO;? as , or oxy-anions of<br />
no, V or As could also be stabilized in solution in a lower oxidation state<br />
when complexed by humic matter; but there does not yet appear to be general<br />
agreement on this (Bloomfield and Kelso, 1973). However, the solubilization<br />
of hydrous Fe and Mn oxides attributed to a reduction process following<br />
complexation by fulvic acids (Zajicek and Pojasek, 1976); (Koshy g &.,<br />
1967). The fate of metal ions whose dispersion in stream sediments is<br />
controlled by adsorption and desorption from Fe Mn and oxide surfaces is<br />
therefore strongly influenced by the operation of such reduction processes<br />
with humic matter.<br />
One very specific example of how metal mobilization can be directly<br />
effected by humic acid reduction concerns the production of Hgo from mercuric<br />
-4-k +t<br />
(Hg ) ion (Alberts &. , 1974). By this means, Hg , which is normally<br />
considered to be very strongly bound to humic matter and not easily displaced<br />
by other metal ions (Strohal and Huljev, 1970), is freed from its ligand upon<br />
reduction to elemental Hg which in turn is held by weak absorptive forces<br />
only. In this way it can easily pass back into solution from reducing sed-<br />
iment s .<br />
The influence of sulphide has not been considered to date. A<br />
number of authors have conducted laboratory tests to investigate competition<br />
between organic ligands and sulphide ion for dissolved metal ions. Timperley<br />
and Allan (1974) attempted to determine the forms of binding of Cu, Fe and Zn<br />
in organic-rich reducing muds. One of the main conclusions drawn was that Cu<br />
was held mainly by sulphide precipitation, whereas organic complexing was<br />
more important for Zn and Fe. Their work suggests that there may be some<br />
value in using the solubility data for simple metal sulphides as a basis for<br />
predicting metal-sediment interaction behaviour in such sediments. For<br />
example, Cd might behave like Zn but As and Hg might be largely fixed as<br />
sulphide or organic-sulphide complexes or adducts.<br />
In this context, the work of Boulegue and Michard (1974) is relevant.<br />
They noted that inorganic sulphur is present in sediments as polymeric hydrosulphides<br />
which are good complexing agents in their own right. They also can<br />
act as both redox and acidity buffers. Interactions between these and metal<br />
ions would be expected to be strong.<br />
One of the major problems in studying water-sediment interactions<br />
relates to a dearth of information on the nature of metal-ligand speciation.<br />
However, some powerful analytical tools have come into use recently whereby<br />
metal ion speciation in natural waters now can be studied.<br />
Anodic stripping voltametry (e.g. Zirino and Healy, 1972) can be<br />
used to demonstrate the importance of complexation reactions and perhaps to<br />
measure the apparent stability constants for strong complexes formed between<br />
metal ions dissolved in lake (Chau &., 1974) and river waters (Ramamoorthy<br />
and Kushner, 1975). But as Langford and Gamble (1974) have pointed out, this<br />
application is limited to relatively "non-labile" complexes with large organic<br />
ligands (e.g., fulvic acids). They suggest that a study of the kinetics of<br />
formation of such complex species would be informative in that kinetic<br />
260
mechanisms of reaction can help to predict solution equilibria. Faster reactions<br />
involving the formation of kinetically more labile complexes could be studied<br />
by means of techniques such as stopped-flow spectroscopy.<br />
By using anodic stripping voltammetry or pulse polarography or<br />
electrophoresis methods in combination with other experimental techniques,<br />
considerable data on elemental speciation could be gathered (Raspov &.,<br />
1975). Bilinski gal. (1976) have measured stability constants for some<br />
hydroxo- and carbonato-complexes of Pb (IT), Cu (II), Cd (II), and Zn (11);<br />
important species in most surficial waters and certainly in groundwaters.<br />
Benes and Steinnes (1974) have described in situ dialysis experiments which<br />
permit determination of truly dissolved and colloidal forms of trace elements<br />
in natural waters. The more such research which can be done on site, the<br />
better.<br />
Field studies on the effects that interactions between trace metals<br />
and organic matter have on dispersion processes are not commonly encountered.<br />
Dc (:root (1971) and de Groot 1. (1971) have published extensive data on<br />
the Rhine River system. The Rhine has been subjected to industrial pollution<br />
and as a consequence, the reported Hg, Zn, Cr, Pb, Cu and As levels of the<br />
fluvial sediments were high. But downstream from the freshwater tidal area,<br />
a pronounced remobilization of these elements into solution was observed.<br />
It was considered that the change in chemical conditions, e.g., oxygenation<br />
and increased salinity, prompted intensive decomposition of the sediment<br />
organic materials which were retaining these metals. From this de Groot<br />
concluded that the formation of soluble metal-organic complexes was mobilizing<br />
the pollutant metals from the sediments. It is interesting to observe in de<br />
GrooL's data that the order of remobilization oE the metals, Hg > Cu > Zn ><br />
Pb Cr > As > Co > Fe, is roughly the reverse of the solubilities of simple<br />
sulphides. Therefore it seems likely that the greater complexing affinities<br />
of e.g., Hg (IT) and Cu (11) towards soluble organic matter are more important<br />
kinetically than their sulphide solubilities. It has also been suggested that<br />
dilution by incoming sediments carried by ocean tides is partly responsible<br />
for the apparent mobilization effects. This may be true, but does not account<br />
for the observed sequence of elemental mobilization.<br />
The release of heavy metals from river bottom sediments has been<br />
studied in the laboratory mainly with the use of radioisotopes. For example,<br />
Co-60 has been used in the Danube River (Radosavjevric &., 1973); Mn-54,<br />
211-65, Sc-46, Co-60 and Hg-203 have been used in the Columbia River, (Lenaers,<br />
1971; Bothner and Carpenter, 1973).<br />
One of the more interesting studies of metal-sediment interactions<br />
in stream systems is described by Jackson (1975). He designed model laboratory<br />
experiments over a pH range to 4 9 to simulate interactions in the system:<br />
dissolved metal ion-clay -dissolved natural organic acids. The objective was<br />
to compare results with data from field studies within carefully selected<br />
stream systems. Interferences due to the presence of dissolved carbonate<br />
were also observed. The ability of soil-derived humic and fulvic acids to<br />
desorb metal previously adsorbed on clay and also metal from metal hydroxide<br />
precipitates, was investigated. Cu (11), Pb (II), Zn (11) and Ni (11) cations<br />
were chosen for study.<br />
261
Results indicated that Cu was strongly stabilized in solution, but<br />
W,IS the most kinetically hindered in desorption reactions. Pb was least<br />
retained in solution by organic complexing but was effectively removed by<br />
inducing colloidal coagulation of organic matter. The solubilities of Zn and<br />
Ni were enhanced by organic complexing but were found to be pH dependent with<br />
hydrolysis leading to decreased solubilities. Furthermore, Ni solubility was<br />
also dccreased by carbonate precipitation.<br />
Jackson, (1975) also embarked upon a field study of selected streams<br />
in which similar processes were thought to prevail. Predicted behaviour was<br />
compared with observed hehaviour. He found that the natural dispersion of Pb<br />
and Fe seemed to be favoured by organic complexing. Cu dispersion was strongly<br />
governed by sorption to organic-rich sediments. The hehaviour of Ni was<br />
strongly controlled by interference, reactions - viz., with carbonate and<br />
llydroxide at alkaline pH. The model reaction scheme was found to be inadequate<br />
for Zn. .Jackson suggested that these laboratory experiments be continued<br />
with solid organic phases included in the scheme.<br />
b) 1-nteractions Involving HydrousLetal Oxides<br />
Fe and Mn oxides are certainly important species in organic systems<br />
but their role as direct adsorbers of metal ions is overshadowed by competition<br />
from the more reactive humic acids and organo-clays or obscured by<br />
coatings uf u~gduie matter. Moreover, these oxides are unstable in certain<br />
organic-rich sediments, particularly if at all reducing. However, in fluvial<br />
systems, such as those found in the Canadian sub-arctic or in alpine regions,<br />
the role of organic matter in water-sediment interactions is relatively<br />
unimportant compared with the influences of hydrous Fe and Mn oxides. The<br />
same situation may also be true of more rugged regions of the Canadian Shield.<br />
Quite a number of studies have been made on the functions of these<br />
oxides in fluvial systems (e.g., Perhac, 1972; Crasselly and Hetenyi, 1971).<br />
ft<br />
The relevant chemistry of Mn , in aqueous solution has also been described<br />
(Handa, 1969; Benes, 1967). For example it has been shown that the oxides<br />
U<br />
strongly adsorb ions such as Hg , (Lockwood and Chen, 1973; 1974) and Co*,<br />
H<br />
Ni* and Zn (McKenzie, 1972). The behaviour of Mn* in water and speciation<br />
between pH 2-8 has been documented by Angino &. (1971). Most common<br />
species are Mn(@H):, ElnC!!; XI! prcdomizantly, Mn(H20) g* ion. Micro-organisms<br />
*<br />
are capable of oxidizing Mn to Mn (IV) in natural waters (Schweisfurth,<br />
1972).<br />
Sylva (1972) has summarized the hydrolysis chemistry of Fe (111)<br />
along with its polymerization reactions in which colloids and sols are formed.<br />
The formation of polynuclear species of Fe and Mn oxides is likely a very<br />
important process in the remobilization of Fe (11) and Mn (11) from reduced<br />
sediments. Such colloidal species are quite reactive towards trace metal<br />
ions.<br />
To illustrate the dominant role Fe and Mn oxides can have in certain<br />
fluvial systems some studies of arctic drainage by Cameron and co-workers<br />
(Cameron, 1974; Allan al., 1974; Cameron and Ballantyne, 1975) can be<br />
summarized.<br />
262
The drainage in the barren grounds of the Hackett River area of the<br />
N.W.T. resembles, at first sight, a lake system rather than fluvial-type<br />
drainage, However chains of lakes are connected by overflow streams in<br />
spring and early summer during which time most metal dispersion takes places.<br />
Because of the virtual absence of dissolved organic matter in waters and of<br />
colloidal or dispersed suspensions of humic material, these watersheds can<br />
be regarded as inorganic systems in which chemical processes follow predictable<br />
paths described by pH, metal ion hydrolysis and simple complex ion formation.<br />
For example, the stgble forms of Cu in these waters of near-neutral pH are<br />
expected to be CuOH , CuCO3' and [Cu(C03)~]-. In fact, in certain lakes<br />
where Cu is quite abundant in surrounding rocks, the carbonate malachite<br />
precipitates onto the bed sediments. The water bodies are found to be in<br />
near equilibrium with these precipitates when Cu, OH- and CO1- are determined<br />
in the lake waters. Under the same conditions, Zn is likely present in the<br />
+<br />
waters as ZnOH and [Zn(H,O)6]* ions.<br />
Two watersheds supplied by metal ions from similar buried volanogenic<br />
Cu-Zn sulphide mineralization can be compared. The first situation,<br />
at Hackett River, N.W.T. demonstrates the degree of dispersion of Zn and Cu<br />
when waters near the source of the metal ions retain a pH in excess of 7.<br />
This was found (Cameron and Ballantyne, 1975) to be due to the presence in<br />
the catchment of considerable exposures of carbonate-enriched (limy) volcanic<br />
rocks. Inspection of Figure 3 reveals that dispersion of Zn from the East<br />
Cleaver zone falls off rapidly over a distance of about 6 miles. Cu is lost<br />
from solution far more rapidly, reaching its natural background levels (for<br />
this region) of 1 ppb after about 3 miles dispersion. Dissolved Fe or Mn<br />
was generally less than 5 ppb, even at source. The second situation, at<br />
Agricola Lake, N.W.T., is virtually identical to the Hackett River watershed<br />
except that the underlying volcanic rocks are devoid of carbonate<br />
minerals. The effects on pH are immediately apparent (Figure 4) at source,<br />
pH is commonly between 3 and 4 with Fe reaching 800 ppb and Mn reaching 90<br />
ppb. As well dissolved Zn falls from about 1000 ppb to about 2 ppb over 5<br />
miles. Figure 4 also displays the change in pH down-drainage.<br />
The work of Jenne, (1968) has shown that the main controls on Cu<br />
and Zn dispersion relate to their fixation in hydrous Fe and Mn oxides<br />
coatings or precipitates. Cameron and co-workers observed that when Fe and<br />
Mn were initially present at very low levels in the alkaline lake waters of<br />
the Hackett River area near source, due to the presence of limy rocks restricting<br />
mobility of those ions, the dispersion of soluble Zn and Cu through chains<br />
of lakes was more extensive. This is attributed to the absence of an oxide<br />
scavenger coprecipitating trace metals down-drainage. Thus dispersion can<br />
be said to be enhanced in spite of the intially high water pH.<br />
By contrast, in the Agricola Lake watershed where there is<br />
little limy material to suppress acidity, initially high<br />
accompanied into solution by initially high dissolved<br />
hydrolysis of these ions resulted in the coprecipitation<br />
Zn and Cu were<br />
Fe and Mn. Rapid<br />
of Cu and Zn (and<br />
As, Figure 5) into lake sediments as the pH increased with dilution.<br />
Therefore dispersion was found to be much more limited than in the Hackett<br />
River situation in spite of the initially high concentrations of Cu and Zn<br />
and the very low water pH. Figure 4 also indicates that Zn begins to<br />
appear in lake sediments when the pH exceeds 4.5, about 2 miles down-drainage,<br />
i.e., when Fe and Mn oxides are forming as suspensions. Further transport<br />
of Zn is probably in particulate form.<br />
263
Figure 3. Distribution of zinc and copper in ppb, and pH in lake waters<br />
from the Hackett River area, N.W.T.<br />
(after Cameron & Rallantvne. 1975)
265
1<br />
N<br />
0<br />
N<br />
- Zn (log pprnl ~n sediments<br />
0<br />
I<br />
R
ORGANIC-INORGANIC INTERACTIONS<br />
It is clear that the presence of organic matter in these systems<br />
would have several predictable effects. Complexation of metal ions would<br />
inhibit hydrolysis of Fe and Mn, redissolve oxide precipitates, and restrict<br />
the coprecipitation of Cu and Zn. Humic matter is also an efficient acidity<br />
buffer, therefore it is likely that high acidity as observed in the Agricola<br />
Lake area would be suppressed at the source and much more localized. What is<br />
less predictable are the scavenging effects colloidal organic matter would<br />
have on free metal ions, complexed metal ions and on the interactions<br />
between all of these species and bottom sediments.<br />
LITEXATURE CITED<br />
Alberts, J. J., Schindler, J. E., Miller, R. W. and Nutter, D. E.,<br />
(1974), Elemental Mercury Evolution Mediated by Humic Acid.<br />
Science, 184, (4139), 879-898.<br />
Allan, R. J., Cameron, E. M., and Jonasson, I. R.,<br />
(1974), Mercury and Arsenic Levels in Lake Sediments From the<br />
Canadian Shield. In "Primero Congreso Internacional de<br />
Mercurio" Barcelona, Tom 11, Publ. (1975), Fab. Nac. Moneda<br />
y Timbre, Madrid, pp. 93-119.<br />
Angino, E. E., Hathways, L. R., Worman, T.,<br />
(1971), Identification of Manganese in Water Solutions by<br />
Electron Spin Resonance, Adv. Chem. Ser., No. 106,<br />
299-308.<br />
Baker, W. E.,<br />
(1973), The Role of Humic Acids From Tasmanian Podzolic Soils in<br />
Mineral Degradation and Metal Mobilization, Geochem. Cosmochim.<br />
Acta, 21, 269-281.<br />
Benes, P. and Steinnes, E.,<br />
(1974), In Situ Dialysis for the Determination of the State of<br />
Trace Elements in Natural Waters, Water Research, 8, 947-<br />
953.<br />
Benes, P.,<br />
(1967), On the State of Manganese and Gold Traces in Aqueous<br />
Solution, J. Inorg. Nucl. Chem., 29, 2889-2898.<br />
Bilinski, H. Huston, R. and Stumm, W.,<br />
(1976), Determination of the Stability Constants of Some Hydroxo<br />
and Carbonato Complexes of Pb (II), Cu (II), Cd (11) and Zn (11)<br />
in Dilute Solutions by Anodic Stripping Voltammetry and<br />
Differential Pulse Polarography, Analyt. Chim. Acta, $,(I),<br />
157.<br />
Bloomfield, C., and Kelso, W. I.,<br />
(1973), The Mobilization and Fixation of Mo, V and U by Decomposing<br />
Plant Matter, J. Soil. Sci., g, 368-379.<br />
261
Ik~tI~n~~r, FI. 11. .mi (:,lrpentc-r, K.,<br />
(1')73), Sc,rllt ion-llr~sorption Keartions of Mercury with Suspended<br />
blntter in tlw Columbia Kiver. Itadioact. Contam. Marine Environ.,<br />
l'roc. Svml~., pp. 73-87.<br />
l~oulep,tle, J., and Nichard, (:. ,<br />
(19741, Intcxractions Between Sulphur-Polysulphide System and<br />
Organic Material in Reducing Media, C. R. Acad. Sci., Ser.<br />
I). 279, (l), 13-15.<br />
Uowen, 11. .I. M.,<br />
(1966), Trace Elements in Biochemistry, Academic Press, London.<br />
HoyLt,, I
Groot, A. J. de, Goeij, J. J. M. de and Zegers, C.,<br />
(1971), Contents and Behaviour of Mercury as Compared With Other<br />
Heavy Metals in Sediments from the Rivers Rhine and Ems., Geol.<br />
Mijnbouw, <strong>50</strong>, 393-398.<br />
Handa, B. K. ,<br />
(1969), Chemistry of Manganese in Natural Waters, Chem. Geol.,<br />
- 5, 161-165.<br />
Jackson, K. S.,<br />
(1975), Geochemical Dispersion of Elements Via Organic Complexing,<br />
Unpubl. Thesis, Carleton Univ., Ottawa, Canada, 344 pp.<br />
Jenne, E. A.,<br />
(1968), Controls on Mn, Fe, Co, Ni, Cu and Zn Concentrations in<br />
Soils and Waters: The Significant Role of Hydrous Fe and Mn<br />
Oxides. In “Trace Inorganics in Water”, Adv. Chem. Ser., No. 73.,<br />
Amer. Chem. Soc., 337-387.<br />
Koshy, E., Desai, M. V. M. and Ganguly, A. K.,<br />
(1967), Studies on Organo-Metallic Interactions in the Marine<br />
Environment. I. Interaction of Some Metallic Ions with<br />
Dissolved Organic Substances in Seawater, Curr. Sci., 2,<br />
555.<br />
Krauskopf, K. B.,<br />
(1955), Sedimentary Deposits of Rare Metals, Econ. Geol.,<br />
<strong>50</strong>th Anniv. Vol., 411-463.<br />
Kusnetsov, S. I.,<br />
(1975), Role of Micro-Organisms in the Formation of Lake Bottom<br />
Deposits and Their Diagenesis, Soil Sci., =(I), 81-88.<br />
Langford, C. H. and Gamble, D. S.,<br />
(1974), Dynamics of Interaction of Cations and Fulvic Acids, Proc.<br />
Int. Conf. Transport of Persistent Chemicals in Aquatic Ecosystems,<br />
Publ. 1974 N.R.C. Ottawa, 11, 37-39.<br />
Lenaers, W. M.,<br />
(1971), Photochemical Degradation of Sediment Organic Matter.<br />
Effect on Zn-65 release. Report (1971) RLO-2227-T-12-32 64 pp.<br />
Nucl. Sci. Abstr., %,(20), (1972), No. 48038.<br />
Lockwood, R. A. and Chen, K. Y.,<br />
(1973), Adsorption of Hg (11) by Hydrous Manganese Oxides,<br />
Environ. Sci., Technol., 1, (IT), 1028-1034.<br />
Lockwood, R. A., and Chen, K. Y.,<br />
(1974), Adsorption of Hg (11) by Ferric Hydroxide, Environ.<br />
Letts., 4, (3), 151-166.<br />
McKenzie, R. M.,<br />
(1972), Sorption of Some Heavy Metals by the Lower Oxides of<br />
Manganese, Geoderma, g,(l), 29-35.<br />
Ong, H. L. and Bisque, R. E.,<br />
(1968), Coagulation of Humic Colloids by Metal Ions, Soil. Sci.,<br />
106, 220-224.<br />
269
Ong, fl. L., Swanson, V. E., and Bisque, R. E.,<br />
(.L970), Natural Organic Acids as Agents of Chemical Weathering,<br />
U.S. Geol. Surv., Prof. Paper 700-C, pp. 130-137.<br />
Perhac, R. M.,<br />
(19721, Distribution of Cd, Co, Cu, Fe, Mn, Ni, Pb and Zn in<br />
Dissolved and Particulate Solids From Two Streams in Tennessee,<br />
.I. Hydrol., 15, 177-186.<br />
Pitwell, I,. R.,<br />
(1973), Some Coordination Effects in Orebody Formation,<br />
Chrm. Geol., 12, 39-49.<br />
Radosavl.jevic, R., Tasovac, T., Draskovic, R., Zaric, M. and<br />
Markovic, V.<br />
(1973), Complex Behaviour of Cobalt in the Danube River,<br />
Arch. Hydrobiol., Suppl., 43,(2), 241-248.<br />
Knmamoc~rthy, S. and Kushner, I). .J.,<br />
(19751, Heavy Metal Binding Sites in River Water, Nature,<br />
(London), 255, (5516), 399-401.<br />
kashld, M. A. and Leonard, J. D.,<br />
(19731, Modifications of the Solubility and Precipitation Behaviour<br />
of Various Metals as a Result of Their Interactions With Sedimentary<br />
Humic Acid., Chem. Geol., ll, 89-97.<br />
Rashid, M. A.,<br />
(1974), Adsorption of Metals on Sedimentary and Peat Humic Acids,<br />
Chem. Geol., 12, 115-123.<br />
Raspov, B., Valenta, P. and Nurnberg, H. W.,<br />
(1975), The Formation of Cd(I1) - NTA Complex in Seawater,<br />
Int., Conf., Heavy Metals in the Environment, Toronto (1975),<br />
Abstr. NO. D-5.<br />
Saxby, J. D.,<br />
(1969), Metal-Organic Chemistry of the Geochemical Cycle, Rev.<br />
Pure. Appl. Cilrlu., &, i31-1<strong>50</strong>.<br />
Saxby, .J. D.,<br />
(1973), Diagenesis of Metal-Organic Complexes in Sediments:<br />
Formation of Metal Sulphides From Cysteine Complexes, Chem.<br />
Geol., 12, 241-288.<br />
Schnitzer, M. and Khan, S. U.,<br />
(1972), Humic Substances in the Environment, Marcel Dekker Inc.,<br />
New York, N.Y.<br />
Schweisfurth, R.,<br />
(1972), Manganese Oxidizing Micro-Organisms in Drinking Water<br />
Supply Sytems, Gas - Wasserfach, Wasser - Abwasser, u,(l2),<br />
562-572.<br />
Sequi, P., Guidi, C. and Petruzzelli, G.,<br />
(1976), Influence of Metals on Solubility of Soil Organic Matter,<br />
(koderma, 12, (3), 153-162.<br />
2 70
Strohal, P. and Huljev, D.,<br />
(1970), Mercury Pollutant Interaction With Humic Acids by Means<br />
of Radioisotopes, Nucl. Techniques Environ. Pollut., Proc.<br />
Symp., I'ubl. I.A.E.A. Vienna, (1971), 439-446.<br />
Sylva, K. N.,<br />
(1972), The Hydrolysis<br />
22, 115-132.<br />
of Iron (III), Rev. Pure. and Appl. (:hem.,<br />
~~<br />
'Theis, T. L. and Singer, P. C.,<br />
(1973). The Stabilization of Ferrous Lon by Organic Cornpomds<br />
in Natural Waters. In "Trace Metals and Metal-Organic 1ntcrac:tions<br />
in Natural WaLers", ed. Singer, P.C., Ann Arbor Publ., Inc.,<br />
Mich., 703-320.<br />
Timp
EidDVEi 8<br />
IMPLICATIONS FOR<br />
THE MEAT MKES
INTRODUCTION<br />
J. R. Vullentyne<br />
Here are the three questions posed by the Governments of the United<br />
States and Canada to the <strong>International</strong> <strong>Joint</strong> <strong>Commission</strong> in the Reference on<br />
Pollution from Land Use Activities:<br />
(1) Are the boundary waters of the Great Lakes System being<br />
polluted by land drainage (including ground and surface<br />
runoff and sediments) from agriculture, forestry, urban<br />
and industrial land development, recrcational and park<br />
land development, utility and transportation systems and<br />
natural sources?<br />
(21 If the answer to the foregoing question is in the affirm-<br />
ative, to what extent, bll what causes, and in what<br />
Ldcalities is the pollution taking place?<br />
(3) If the Comission should find that pollution of the<br />
character just referred to is taking place, what<br />
remedial measures would, in its judgement, be most<br />
practicable and what would be the probable cost thereof?<br />
Dr. D. R. Bouldin commented this morning that if this Workshop, which<br />
has been arranged to help answer these questions, had been held three ago years<br />
when PLUARG was just beginning its work, there would have been to nothing talk<br />
about: the relevant information was not on hand. 1 think he is right. But<br />
even with the new data, talents and personalities that have been brought to<br />
bear on the problem within PLUARG and in this Workshop, the issues still remain<br />
complex and resistant to quantitative interpretation.<br />
This complexity worries me. I see it as a persistent threat to<br />
the defendability of proposed remedial measures requested in Question 3 of the<br />
Reference. If a remedial measure is not defendable it will not be accepted<br />
by the <strong>Commission</strong>, by the two Governments, or by the affected people and<br />
organizations in the two countries. The most important component of any<br />
strategy for action is therefore a strategy for defence. For this reason,<br />
the order of attention given by PLUARG is answering the three questions should<br />
be the reverse of the order in which they were posed.<br />
From information presented at this Workshop is it apparent that the<br />
most important factors contributing to the complexity of the problems are:<br />
(1) the virtual impossibility of tracing the sources and<br />
ultimate fates of pollutants once they have entered<br />
into stream transport;<br />
275
(2) the variety and large number of independent sources<br />
requiring separate control;<br />
(3) the variability of climatic factors affecting land runoff,<br />
their low frequency and unpredictability in time;<br />
(4) local variations of soil and topographic factors affecting<br />
runoff ;<br />
(5) persistent questions concerning the availability to plants,<br />
or toxicity in the environment of chemicals in different<br />
phases or forms.<br />
It will not he easy for PIJJARG, for the <strong>Commission</strong> or for regulatory<br />
agencies in Canada and the United States to formulate and defend remedial<br />
measures perhining to a large number of control points whose effects are<br />
not easily traceable to boundary waters. Because of this complexity it will<br />
he impnrtant to stress two aspects of defensive strategy in the technical report<br />
LO the <strong>Commission</strong>.<br />
I, ACCENT TE LONG TERM<br />
As the time base lengthens the rate of delivery of persistent<br />
pollutants to boundary waters increases. Al.so, it must he stressed to the<br />
<strong>Commission</strong> that pollution of boundary waters arising from Land use activities<br />
is not going to be solved or even quantitatively understood overnight. The<br />
need lor changes in human attitudes and hehavioural practices in regard to<br />
land use activities suggests that it may be optimistic to think in terms of<br />
Etfrcting solutions in less than a generation's time. I hope that PLIIARG will<br />
mnkc reference to the need for controliing the rate of growth of human population<br />
and technology in the Great Lakes i3asin; it is already controlling<br />
us, as individuals, to an extent we do not realize. I believe that the<br />
<strong>Commission</strong>, the two governments and the people and organizations involved<br />
wi1.l understand the accent on Lhe long term if it is properly presented. Hut<br />
this accent should not he used as an excuse for inaction or delay.<br />
War is not an appropriate analogy in the present context; nevertheless,<br />
a quote from E. Machell-Cox's translation of Sun Tzu's, "The Principles<br />
of War," written 2300 years ago, is not out of place:<br />
"Once hostilities have begun, if victory he long deferred,<br />
weapons will grow blunt and ardour dampened; if the army<br />
attack a city, it will exhaust its strength; if it he !ong<br />
in the field, state supplies will be insufficient. Now,<br />
when weapons are blunted, ardour dampened, strength exhausted<br />
and treasure gone, the other princes will rise and take<br />
advantage of your extremity and, though you were a very Sage,<br />
you should not then remedy the consequences. So it is that<br />
chough you shall hear of stupid haste in war yet you shall<br />
IicBvc'r liear tllat long campaigns are clever. There has never<br />
x'c't heen a country that has benr€ited by a long war."<br />
276
11. AVOID GENERALITIES 1 .<br />
AT bCE<br />
1 THE ~ ~ PLAGUE: Do NOT TRY TO SOLVE EVERYTHING<br />
Certain land use activities are more likely tc, affect boundary<br />
waters than others; for example, those in close proximity to boundary waters.<br />
Because of their small size, large surface area and charge, fine-grained<br />
particles are likely to be more important that coarse-grained particles in<br />
transporting pollutants from distant sites to boundary waters. Steep slopes<br />
are likely to be more susceptible to erosion than flat land with porous<br />
soils. Let there bt- an heirarchy of priorities in space and time. Over-<br />
generalization will only antagonize those whose cases do not easily fit into<br />
the general scheme; and the loss of their support will only weaken the cause.<br />
In this context it is worth re-emphasizing the importance of a defensive<br />
strategy to PLUARG with another quote from E. Machell-Cox's translation of<br />
Sun Tzu's, "The Principles of War."<br />
"Just as it is no great sign of strength to lift an<br />
autumn hair, of keen sight to see the sun and moon, or of<br />
sharp hearing to hear thunder, so if the basis of your<br />
victory is apparent and within the understanding of the common<br />
herd, it is not of the highest excellence; nor is it of the<br />
highest excellence if you win a pitched battle and all the world<br />
cry "Ha!" What the ancients called a clever fighter was a man<br />
who won, and won easy victories, which brought neither a name<br />
for wisdom nor credit for courage. His victories came of<br />
making no mistakes; by making no mistakes he had ensured<br />
victory, and needed but to defeat an enemy already overcome.<br />
lhe skillful warrior is one who puts himself in a position such<br />
that he cannot he defeated, and does not then lose the occasion<br />
of defeating the enemy. The victorious army first secures its<br />
victory and then gives battle; the vanquished first engages<br />
and then seeks to securc victory."<br />
!7 1
INTRODUCTION<br />
G. H. Dury<br />
It would be redundant for me to attempt to summarize, or to review,<br />
the Workshop contributions on physical processes. Those attending have been<br />
supplied with abstracts and with papers, have listened to the papers presented<br />
and to the question-and-answer sessions, have laboured with the various work<br />
groups, and have contributed to the recommendations which the work groups<br />
will be making. Readers of the final document will be presented with extended<br />
abstracts of papers, and with the recommendations that emerge from the Workshop<br />
as a whole. Rather than attempting to review, or to repeat, what is said<br />
elsewhere, I propose in these comments to sound certain general themes, to<br />
suggest a possible means of economizing on environmental monitoring, and to<br />
draw attention to some possible dangers and difficulties in attempting to<br />
forecast for the future.<br />
W~RKSKIP ACTIVITIES AND ATTITUDES<br />
Like any good conference, this Workshop has led to the informal<br />
exchange of knowledge and acquaintance. It has also led to, or at least disclosed<br />
the possibilities of, the interfacing of programs-- programs of research,<br />
management, and regulation.<br />
It has also revealed a broad range of viewpoints, particularly<br />
perhaps among those interested mainly in physical processes. At the one<br />
extreme comes the urgent need for practical action, in containing, reducing,<br />
or even eliminating the discharge of sediments, nutrients, and contaminants<br />
into the Great Lakes. At the other extreme comes the desire for long term<br />
basic research, especially research into processes of erosion, transportation,<br />
and discharge or sedimentation. The two corresponding extreme attitudes are<br />
that of eliminating process studies altogether, in favour of implementing<br />
immediate control measures, and that of considering control impossible, until<br />
sources of input are identified and the processes of input are completely<br />
understood.<br />
Somewhat related matters are the high variability of observational<br />
data, and the difficulty of extrapolating to large natural basins the results<br />
obtained from small basins or from trial plots. Although it may very well<br />
prove necessary to do whatever is possible with the data already available,<br />
divergent views on what actually is possible were reflected at the Workshop<br />
in differing opinions on the potential of computer modelling. On the one<br />
hand, soil loss equations can be run through a number of existing computer<br />
programs. On the other hand, some researchers consider that the results<br />
predicted by computer need careful reappraisal against what happens in the<br />
real world. In addition, prediction of soil loss, however accurate, may by<br />
no means foretell what actually will be moved out cf the basin.<br />
279
BAS I 14 STORAG~<br />
'I'ht, rrsidcBnce tinw Cor nutrients and contaminants seems to he<br />
rr~~,isorl,~lllv wc.1 I ~11~11c~rst11m1, in tc3rms of water cllemistry, soil rllemistry,<br />
II~(J( llc,nlistrv, .lnd til
A possible means of economy resides in surrogate observations.<br />
For instance, it can readily be shown that, for the Hurricane Agnes floods in<br />
New York and Pennsylvania in 1972, the total delivery of water and the total<br />
delivery of suspended sediment in a given flood wave can be expressed as a<br />
power function of momentary peak discharge (Dury, press). in This statement<br />
holds, through a range of recurrence intervals from less than 2 to more than<br />
100 years (annual series), and through a range of drainage areas from less<br />
than 1.0 to 16,700 km’. Nearly 97 percent of variation in delivery of suspended<br />
sediment is statistically explained by variation in momentary peak<br />
discharge. This latter variable, once a rating curve has been established,<br />
ran be simply and cheaply recorded by a crest-stage gauge. Once a sediment<br />
rating curve has been established, momentary peak flows of water can be used<br />
to give sediment discharge. Methods of this kind are especially attractive,<br />
since many rivers discharge a very large fraction of total sediment in only a<br />
few days of high flow.<br />
Sundry contributions to the Workshop suggest that, for some streams<br />
at least, discharge of certain nutrients and contaminants can be expressed as<br />
funrtions of discharge of suspended sediment. Phosphorus, nitrogen, carbon,<br />
and pesticides were specifically mentioned. Wherever a firm relationship can<br />
be established between discharge of nutrients/contaminants and discharge of<br />
suspended sediment, and another between discharge of suspended sediment and<br />
discharge of water, then the peak flow/flood wave relationship can be used<br />
to measure total delivery in a particular event of sediment, nutrients, and<br />
contaminants.<br />
SPAT I AL VARIATION<br />
Just as response to sediment input can differ from one part of a<br />
basin to another (*, between upstream and downstream reaches, so variation<br />
is to be expected between one basin and another, and between one region and<br />
another. If surrogate methods of monitoring are to be developed for the<br />
Great 1.akes rivers, possible regional variation needs to he investigated. If<br />
such variation dues occur, then sample streams for the individual regions<br />
could be observed, as against observing all streams throughout the system.<br />
Basin size is likely to prove an additional, and perhaps an influential,<br />
variable. Pilot studies in regional and inter-basin variation would seem to<br />
offer useful possibilities.<br />
PREDICTION FOR THE FUTURE<br />
One attendee remarked that a meeting such as this Workshop would<br />
have been impossible three years ago--the implication being that, in the last<br />
three years, we have already come a very long way in assembling the data<br />
necessary for the tasks in hand. The comment is highly gratifying. At the<br />
same time, a three-year series of Observations is impossibly short for most<br />
purposes of prediction.<br />
281
It is axiomatic, that, for magnitude-frequency analysis of streamflow,<br />
:I 1 -etord nc3eds tu he twice as long as the greatest recurrence interval for<br />
WII i c.11 confident pt-edirlion tan be undertaken. Thus, a 100-year record is<br />
nredcvl for the conlidrnt prdiction of the <strong>50</strong>-year event. In practice,<br />
t,vents o f great m.~gnitudc md low frequency may have to be predicted from<br />
short rc'ror(l scric.s, in which case confidence limits should be stated.<br />
,As ~IS inc~vit.rl,lt~ tilat m~~gnitudt~-f rclquency analysis will appear increasingly<br />
. ~ t t 1r.1c.t ivtl. tiowt,ver, nlucll recent and turrent work has tended to reveal<br />
~ l i ~ ~ ' ~ ~ ~ l t i ~ ~ ~ step ~ ifilnrtii1ns--in t i ~ ~ s - - ~ :i ~ wl1olc ~ m ~ range ~ ~ ~ or r ~ pllenmrna, ~ l<br />
inclilcling those o f rlirnate, weather, and streamflow. It is, for instonce,<br />
c~.nti~-elv p~'ssii11c~ an for abrupt shift to occur in the arca/intensity/duratiorl<br />
1re1.1t ionsilips uf dominant storms,<br />
to a givcrl fluvial system.<br />
and consequently in the input of sediment<br />
1,:ven when very long recor-ds exist, or when the frequency of an<br />
event ot very great magnitude can reasonably be identified from a short<br />
rrcord, it may prove impossible to identify thresholds--such, for example, as<br />
the threshold where severe rhanncl erosion is suddenly initiated, and where<br />
thc, input of sediment into the flowing water is suddenly increased. A case<br />
in point is that of the English rivc'r Nene, which in 1947 experienced ii flood<br />
belonging somewhere between <strong>50</strong>0 and 1000 years on the annual series (Dury,<br />
1974). 'l'llis tlood, however, failed to produce significant efCects, either in<br />
the channcl or on the floodplain. Other rivers are known to respond drastically<br />
to floods of much lower magnitude (comparatively speaking) and of much<br />
greater frequency. Despite the obvious difficulties, the identification of<br />
thresholds of behaviour on the part of stream channels--and, €or that matter,<br />
of farml.lnd soils--is crucial to the prediction and control of sediment<br />
input. Once again, pilot studies and regional assessment are in order.<br />
1. G. H. Dury, in press : Peak Flows, Low Flows, and Aspects<br />
of (:eomorphic Dominance. Expected to appear in "River<br />
Channel Clranges"(ed. K. .I. Gregory): Wiley, for the<br />
British Geomorphological Research Group.<br />
2. (:. H. Dury, (1974): Magnitude Frequency Analysis and Channel<br />
Murphomrtry : in "Fluvial Geomorphology", (ed. M. Morisawa),<br />
State University of New York Publications in Geomorphology,<br />
9 1-121.<br />
2x2
EIiFVEB<br />
4<br />
CONCLUSIONS AND RECOMMENDATIONS<br />
FROM THE WORK GROUPS<br />
RESEARCH NEEUS
RECMNDATIONS OF THE WORK GR~UPS<br />
The work groups established at the workshop were held in four<br />
concurrent sessions. Three groups were each assigned a pollutant related<br />
to the sources and transport of sediment (nutrients, metals and pesticides).<br />
One group dealt with the physical processes involved in sediment generation<br />
and transport. It was made clear to eac.h group that they were to consider<br />
PLUARG's present programme of sediment collection and analysis and make<br />
any recommendations for changes in sampling and/or analysis. Additionally<br />
the groups were to consider the potential impact of ongoing research programmes<br />
and the research needed in the long term to help answer some of the<br />
questions posed during the plenary sessions.<br />
By the very nature of the work groups there was considerable overlap<br />
in the discussions. It would be difficult, for example, to discuss the<br />
relationship of pesticides to sediment without also discussing the physical<br />
processes involved in sediment production and movement. Where recommendations<br />
from work groups have overlapped, they have been edited and combined with<br />
general recommendations for research needs.<br />
The recommendations expressed here have been summarized by the<br />
work group chairmen and are not verbatim accounts of the discussion held.<br />
PHYSICAL PROCESSES<br />
This work group focused upon physical processes in fluvial transport<br />
of nutrients and contaminants although it is recognized that physical, chemical<br />
and biological processes must all be considered together. The work group<br />
examined several questions: (1) What do we need to know? - for PLUARG to<br />
satisfactorily complete its Terms of Reference, - for the Governments to<br />
manage land use activities to achieve satisfactory Great Lakes water quality,<br />
(2) What do we know?, and (3) What recommendations should be made to PLUARG<br />
and to the Research Advisory Board/IJC in terms of recommended actions or<br />
research needs?<br />
The Workshop was partitioned into considerations of sources and instream<br />
transport mechanisms and the discussions of Group 1 are presented here<br />
within the same categories. Research needs are discussed both within the<br />
context of immediate needs of PLUARG and with a view of future research which,<br />
although necessary to answer the PLUARG Terms of Reference, cannot be feasibly<br />
concluded within the life of PLUARG for reasons of cost, available time, data<br />
limitations. etc.<br />
285
Witit reference to the three questions posed in the PLUARG Terms<br />
(>t Reference, it is :rcrrpted tllet tlie boundary waters of the Great Lakes<br />
:;ystfni are being polluted lrom land drainage. Further information needs to<br />
hi, developed to define the extent, CJUS~S and localities in which pollution<br />
is taking place,, and to define the most practical remedial measures. The<br />
items listed helow are a statement of PLUARC, objectives within the context<br />
of (:roup 1 discussions, and necessary to answer the questions posed in the<br />
I’I.UAR(: ‘Terms of Reference:<br />
(1) Estimate contemporary fluvial loadings of sediment-related<br />
nutrients and contaminants into the Great Lakes.<br />
(2) Assess land use and associated loadings into the Great Lakes<br />
in the light of alternative management strategies.<br />
(3) Predict trends of loadings relationships into the future under<br />
different management strategies.<br />
(4) Recommend land use practices (remedial measures) in order to<br />
reduce or eliminate significant contaminant and pollutant<br />
loadings.<br />
It should be noted that the Physical Processes group addressed<br />
~lrr questions of sources of pollutants from land use activities and the<br />
transport and resultant loadings to the Great Lakes; it did not address the<br />
questions of impact analysis on the Great Lakes or the processes and fate<br />
of sediment in lake-river interface areas such as estuaries.<br />
SOURCES<br />
The discussion of land use practice and sediment production,<br />
sediment control and consequent stream loadings of sediment-related quality<br />
p,lrameters (in upstream areas) centered on the efficacy of detailed site and<br />
time-specific cause-effect studies as opposed to a more general mode approach<br />
in which stream loadings either at source of stream terminus are regarded as<br />
functions of classes of land use practices (either within a deterministic or<br />
statistical framework). In view of the timeframe for PLUARG, there was a<br />
consensus that such tmdelb, which adequately permit a “first-cut‘‘ at landuseloadings<br />
relationships in time and space ought to be tried. It was felt that<br />
within the timeframe of PLUARG, deterministic process linkages between lake<br />
loadings and site-specific land use practices upstream could be not made in<br />
drtail for reasons noted below. Therefore, existing models are the place to<br />
start since they have produced useful information in parts of the United States<br />
tor general categories of land use. Such models are likely adequate in the<br />
short-term for PLUARG purposes, but need to be calibrated with data from the<br />
(:reat Lakes Basin. Although such models may not have a high level of resolution<br />
for a11 tributary basins in the Great Lakes when projected downstream to<br />
lake boundaries, they provide a focus for the experimental and fluvial process<br />
rescarch required to obtain improved relationships between land use activities<br />
and lake loadings.<br />
286
The major deficlency both of existing landuse-loadings models<br />
and of site specific process-response studies of sediment production by<br />
specific land use practice, is the inability to link upstream or source<br />
water quality observations with river mouth loadings. Although the principles<br />
of sediment transport mechanics are well known, the downstream<br />
movement and modification of sediment-related water quality variables are<br />
poorly understood. It is recognized that load modification is noL only<br />
related to physical processes but also includes biological and chemical<br />
interactions. In addition to poorly understood biochemical processes, it<br />
is recognized that contemporary data are inadequate to meet the needs of<br />
instream process research. These data inadequacies pertain both to poor<br />
characterization of variables (e.g., particle-size, inorganic/organic<br />
ratio, speciation of key components, aerosol input, etc.) and to lack of<br />
definition of spatial and temporal variations associated with parameter<br />
flux and with in-stream processes (such as sediment storage-remobilization<br />
mechanisms, time of travel of particle-size classes, short (anthropogenic)<br />
and long-term (climatic) variations in sediment flux and storage, etc.).<br />
It is recognized that these inadequacies must be investigated in order that<br />
extrapolation from loadings in source areas to loadings at river mouths<br />
reflects stream process both in space and in as time the watershed undergoes<br />
natural and anthropogenic perturbations.<br />
DATA<br />
Routine data on water quality and quantity have been collected for<br />
at least a decade on both sides of the Lakes, and therefore a special effort<br />
must be made to assess adequacy of historical data to and fully utilize them<br />
within limits established by the assessment procedure. In conjunction with<br />
the PLUARG (intensive and event-oriented) supplementary data collection,<br />
there is need to examine historical and PLUARG data and collection procedures<br />
for the purpose of:<br />
assessing the loads and the accuracy of load estimates for<br />
sediment, pollutants and contaminants at river mouths (bearing<br />
in mind the often non-linear discharge dependencies illustrated<br />
by many variables);<br />
identifying the watersheds which have a major and deleterious<br />
effect upon Great Lakes water quality;<br />
evaluating sampling technique, in terms of depth and flow dependencies<br />
of problem substances, for identification of temporal<br />
variation in load and the degree to which these data are site<br />
dependent;<br />
updating parameter lists to indicate those recently identified<br />
as either having a deleterious environmental effect or containing<br />
information bearing upon in-stream process mechanisms (e.g.,<br />
particle-size, organic/inorganic, metal speciation);<br />
evaluating sampling network design so that in-stream load modification<br />
from source to mouth may be inferred and the spatial<br />
and temporal resolution of loads and source-area contributions<br />
may be identified.<br />
287
RECOPMENDATIONS AND RESEARCH NEEDS<br />
111 view of the lack of familiarity of many of the workshop part<br />
icipants with the operational organization of PLUARG, recommendations<br />
a~ld r-rsearcti needs are stated on general scientific grounds. Although<br />
rrcommendations may not apply equally to U.S. or Canadian activities,<br />
research nerds reflect inadequacies of state-of-art. Feasibility of recommendations<br />
and research needs must be determined<br />
Research Advisory Board.<br />
by PLUAKG and the<br />
Using river mouth loads, identify all streams that discharge significant<br />
loads to the Great Lakes. Of those streams that are important, conduct<br />
surveys from the mouth (upstream) to identify major problem input sources.<br />
The collective wisdom is that most serious problems at the mouth will<br />
relate to problem areas not far upstream. In such cases, management<br />
scenarios (alternative remedial measures) should be investigated.<br />
Compnrr stream (aquatic) loading to other sources so you know how<br />
important (relatively) the stream loading component is.<br />
Assess adequacy and applicability of existing data. This should<br />
include an assessment of variables and of the efficacy of present<br />
Jata collection strategies for their ability to resolve spatial and<br />
temporal trends in loadings at source and mouth. This recommendation<br />
does not necessarily imply "more" data collection but rather "better"<br />
data collection. Necessary changes should be implemented to correct<br />
data deficiencies.<br />
Apply existing sediment-loadings models both to assess sediment-<br />
prodilrtion at source, and in order to derivc upper-bound values for<br />
stream-mouth loadings by pxtrapolation over large areas.<br />
Identity and rank present management strategies which focus upon<br />
major sources. Models should be used to evaluate probable effects<br />
nf alternative management choices. (The term models, does not<br />
necess,lrily imply computer programs. It may be simple empirical<br />
formulae - although computer models are available and would be<br />
usrful) .<br />
Initiate preliminary field studies of downstream transport mechanisms<br />
and load modifications of both a physical and chemical nature in small,<br />
relativelv simple catchments with transposition to selected Type<br />
rat clmrnts of greater complexity.<br />
'88
Research Needs:<br />
Study of erosion and sediment transfer mechanisms source in areas<br />
with respect to general principles and their spatial and temporal<br />
significance. This should include consideration of "partial area<br />
hydrology" insofar as it relates to probable source areas of sediment.<br />
Establish causal mechanisms for changes in sediment quality and<br />
quantity during stream transport in complex stream systems. This<br />
should include fundamental research into the physical, biological<br />
and chemical process mechanisms. Such data are required to improve<br />
present landuse-loadings models to that the effect of upstream<br />
management alternatives can be assessed in terms of loadings to the<br />
Great Lakes.<br />
Develop and apply improved models<br />
to all tributary basins in which<br />
significant loadings problems exist to facilitate trade-off analyses<br />
of alternative remedial measures. Such application should include<br />
research developments from bl and 112 above.
NUTRIENTS W~RK GROUP<br />
RESEARCH NEEDS ~CDMVENDATIONS<br />
Much more effort should be spent on methods to evaluate the<br />
biological availability of the phosphorus entering the lakes<br />
from the tributaries. A special group should be set up to<br />
concentrate on this aspect.<br />
The kinetics of removal of phosphorus from the water to the<br />
stream and river sediments should be studied in greater detail.<br />
The parameters of riverine storage of phosphorus should be<br />
investigated in detail.<br />
Based on (2) and (3) a weighting system should be devised for<br />
the various tributary sources of phosphorus.<br />
IWEDIATE<br />
~COFF~ENDATIONS FOR PLuAffi<br />
(I) Characterize all pilot watersheds for spatial variability and<br />
attempt to relate this to measured nutrient output based on<br />
partial area hydrology models.<br />
(2) The compatibility of various goals should be investigated, i.e.<br />
is it better to slow erosion to control pesticide transport, or<br />
to allow it to continue to aid in phosphorus de-activation.<br />
(3) One must recognize the divers nature of the Great Lakes area.<br />
Recommendations for control must be made on an almost site<br />
specific basis.<br />
291
PESTICIDES WORK GROUP<br />
RESEARCH NEEDS RECOWENDATIONS<br />
It is essential for the regulatory and other agencies in Canada<br />
and the United States to know precisely the location, names and<br />
quantities of organic substances being used which may be hazardous<br />
to the biota of the Great Lakes Basin wherever that biota is of<br />
economic or peripheral importance. Such knowledge is essential<br />
to establish research priorities and for current and future<br />
surveillance.<br />
A need exists to select and develop standard techniques and<br />
criteria to evaluate the toxic effects of substances on biota<br />
of the greatest importance to the Great Lakes Basin ecosystem.<br />
This will ensure that criteria for all substances in use or<br />
developed for the future are assessed using the same criteria<br />
and at levels likely to be found in the environment.<br />
There is a need to document the case histories of such substances<br />
as DDT and PCBs to ensure that mechanisms and transfers well as<br />
as past analyses are thoroughly understood in order to develop<br />
predictive models for substances of similar nature, but yet to be<br />
developed or used. There is little likelihood that any substances<br />
will be as thoroughly studied and documented as the major pesticides<br />
and derived products used since 1940. The knowledge concerning these<br />
should be so firmly corroborated in respect to fluvial, point source<br />
and aerial inputs and transformations that all future studies can<br />
use the approaches or findings to extrapolate effects before crisis<br />
develop.<br />
There is a need for more information on the relative importance<br />
of the various routes by which organic contaminants enter into the<br />
Great Lakes Basin ecosystem. This information is needed before<br />
recommendations relating to land use can be seriously considered.<br />
The question of open-water disposal of dredged sediments was<br />
raised but appears to be adequately covered by the report from<br />
the <strong>International</strong> Working Group on the Abatement and Control of<br />
Pollution from Dredging Activities.
[“ETALS W~RK GWUP<br />
RESEARCH NEEDS ~COMVENDATIONS<br />
The need,to investigate:<br />
The biological availability of metals in uplands, streams,<br />
lakes, and estuaries under various environmental conditions<br />
i.e., pH, Eh, etc;<br />
The various release mechanisms available for metals entrained<br />
with organic and inorganic sediments in uplands, ground-waters,<br />
streams and lakes;<br />
The metal concentrations and types associated with various size<br />
fractions of organic and inorganic sediments and their chemical<br />
and/or mineralogical composition;<br />
Whether metals are tied to sediment sizes most difficult to<br />
control using conventional and existing soil erosion and sediment<br />
control procedures;<br />
Whether sediment erosion and control practices used in agriculture,<br />
urban areas, mining, construction and related land uses are<br />
adequate to retain these finer-grain sizes. If not, what new<br />
technology may be applied for their control;<br />
The probable costs of these control measures and their benefits<br />
not only with metals but other entrained pollutants, i.e. N, P,<br />
pesticides, etc.;<br />
The processes of chemical fractionation of metals. These processes<br />
may influence metal occurrence, transport and bioavailability.<br />
Chemical fractionation must be coupled to metal release mechanisms<br />
to predict resulting concentrations of metals in aqueous systems;<br />
The organic species (plants, animals, etc.) that tie up metals<br />
that might, in turn, be converted to the elemental form of the<br />
metal ;<br />
The toxicity levels of metals and complexed metals to various<br />
aquatic plants, animals and segments of food chains;<br />
(10) The kinetics of metal complexes as these kinetics will shed light<br />
on metal speciation and metal behaviour within aqueous systems.<br />
The ionic strengths of metal complexes are influenced by salinity<br />
and other factors that must be defined.<br />
295
RECOWENDATIONS FOR PLUARG<br />
(1) 'The reproducibility of results of chemical analyses for<br />
meLals using various sampling times, sampling procedures,<br />
and analytical techniques must be assured through the con-<br />
tinuance of the PLIJARC interlaboratory comparison programme.<br />
(2) PLLJAKG must determine the atmospheric loadings of metals.<br />
Tlw sources and source regions must be found and an estimate<br />
made of possible control measures and costs to reduce this<br />
loading.<br />
296
EilFVEl<br />
Fl<br />
WORKSHOP ORGINIZERS<br />
AND ATTENDEES
WORKSHOP AITENDEES<br />
Dr. John R. Adam<br />
Water Quality Management Program<br />
Toledo Metropolitan Area Council of<br />
(:overnments<br />
Suite 725<br />
420 Madison Avenue<br />
Toledo, Ohio 43604<br />
Ilr. Herbert E. A1lc.n<br />
Assistant: Professor<br />
Illinois Institute of Technology<br />
Chicago, Illinois 60616<br />
Dr. (:arl )s Alonso<br />
[KIM Sedimentation Laboratory<br />
1'. 0. Box 1157<br />
Oxford, Miss. 38655<br />
Dr. Eugene .J. Aubert<br />
Director<br />
Great Lakes Environmental Research<br />
Laboratory<br />
2'300 Waslltenaw Avenue<br />
Ann Arbor-, Michigan 48104<br />
l'rofrsso~ David B. Baker<br />
River Sttldies Laboratory<br />
Heidelberg College<br />
Tiffin, Ohio 44883<br />
Dr. Keith Bedford<br />
Water Resources Centre<br />
The Ohio State University<br />
1791 Neil Avenue<br />
Columbus, Ohio 43210<br />
Professo~ David R. Bouldin<br />
Cornell I!niversity<br />
Department of Agronomy<br />
Bradfielo and Emerson Halls<br />
Ithaca, New York 148<strong>50</strong><br />
299<br />
Mr. J. E. Brubaker<br />
Agricultural Engineering Extensions<br />
Branch<br />
Ontario Ministry of Agriculture<br />
and Food<br />
Guelph, Ontario<br />
Ms. Margery Bynoe<br />
Department of Geography<br />
Queen's University<br />
Kingston, Ontario<br />
Mr. Thomas 1%. Cahill<br />
Resource Management AssociaLes<br />
P. 0. Box 740<br />
West Chester, PA 19380<br />
Mr. Wayne Chapman<br />
Soil Conservation Service<br />
c/o EPA<br />
Non Point Sources Branch<br />
WH 554<br />
401 M Street, S.W.<br />
Washington, D. C. 20460<br />
Dr. Y. K. Chau<br />
Canada Centre for Inland Waters<br />
P. 0. Box <strong>50</strong><strong>50</strong><br />
Burlington, Ontario I.7R 4126<br />
Dr. C:. Cllesters<br />
Director<br />
Water Resources Center<br />
University of Wisconsin<br />
1975 Willow Drive<br />
Madison, Wisconsin 53706<br />
Dr. Richard Coote<br />
Engineering Research Services<br />
Agriculture Canada<br />
Ottawa, Ontario KlA OC6
Mr. .I. Culht~rtsm<br />
U.S. 1)ep.lrtrncnt of the Lnterior<br />
L(~ologiva1 Survev<br />
Kt~ston, l'irginia 2209Z<br />
Ur. Trevor Ilic kinson<br />
Engineering Department<br />
University of Guelph<br />
Ouelpll, Ontario N1(: LW1<br />
I'r
Mr. Ken Kemp<br />
Indiana Stream Pollution Control Board<br />
Planning Section<br />
310 W. Michigan Avenue<br />
Indianapolis, Indiana<br />
Dr. .J. Knox<br />
Institute for Environmental Studies<br />
University of Wisconsin-Madison<br />
1042 Warf Building<br />
610 Walnut Building<br />
Madison, Wisconsin 57706<br />
Dr. .I. (;. Konrad<br />
Superviscr of Special Studies<br />
Wisconsir Department of Natural<br />
Resources<br />
Madison, Wisconsin 53701<br />
Dr. A. K. Lebeuvre<br />
Canada Centre for Inland Waters<br />
P. 0. Box <strong>50</strong><strong>50</strong><br />
Burlington, Ontario L7K 4A6<br />
Professor '7'. I.ogan<br />
'The Ohio State Univfrsity<br />
1885 Neil Avenue<br />
Columbus. Ohio 43210<br />
Ms. Elsie MacDonnld<br />
Soil Kescaarch lnstitute<br />
Agriculture Canada<br />
Ottawa, Ontario K1A 0C6<br />
Mrs. Edit.1) MacIntosh<br />
Mayor<br />
City of Kitchener<br />
City Hall<br />
Kitchenei, Ontario<br />
Dr. A. D. McElroy<br />
Ireatmen! and Control Processes Section<br />
Midwest l{esrarch Institute<br />
425 Volktlr Voulevard<br />
Kansas City, Missouri<br />
Mr. William F. Mildner<br />
U.S. Soil Conservation Service<br />
Hyattsville, Maryland 20782<br />
301<br />
Professor M. H. Miller<br />
Department of Land Resource Science<br />
Ontario Agricultural College<br />
University of Cuelph<br />
Cuelph, Ontario N16 2W1<br />
Dr. 11. V. Morley<br />
Agriculture Canada<br />
Room 1113<br />
K. W. Neatby Building<br />
Carling Avenue<br />
Ottawa, Ontario KlA 0C6<br />
Dr. M. D. Mullin<br />
US Environmental Protection Agency<br />
Grosse Ile Laboratory<br />
9311 Groh Koad<br />
Grosse Ile, Michigan 48138<br />
Dr. Carl Nordin<br />
U.S. (:e(,logical Survey<br />
Mailstop 413<br />
Box 2<strong>50</strong>46<br />
Denver Federal Centre<br />
Lakewood, Colorado 80225<br />
Mr. .I. E. O'Neill<br />
Water Kesources Branch<br />
Ministry of the Environnwnt<br />
135 St. Cllir Avenue West<br />
Turonto, Ontario M4V 1P5<br />
Dr. E. D. Ongley<br />
kpartment of Geography<br />
()wen's University<br />
Kingston, Ontario<br />
Mr. R. C. Ostry<br />
Water Quality Management Branch<br />
Ontario Ministry of the Environment<br />
135 St. Clair Avenue West<br />
Toronto, Ontario M4V 1P5<br />
Dr. Richard K. P,irizek<br />
Ilepartment of Geosciences<br />
Pennsylvania State University<br />
University Park, Penn. 16802
Ms. Jean Percival<br />
Department of Geography<br />
Queen's University<br />
Kingston, Ontario<br />
Dr. Harry B. Pionke<br />
Research Leader<br />
U.S. Department of Agriculture<br />
111 Research Building A<br />
University Park, Penn. 16802<br />
Mr. J. Ralston<br />
Water Resources Planning Unit<br />
Ontario Ministry of the Environment<br />
135 St. Clair Avenue West<br />
Toronto, Ontario M4V 1P5<br />
Mr. J. D. Roseborough<br />
Director<br />
Fish and Wildlife Research Branch<br />
Ontario Ministry of Natural Resources<br />
P. 0. Box <strong>50</strong><br />
Maple, Ontario LOJ 1EO<br />
Professor G. K. Rutherford<br />
Department of Geography<br />
Queen's University<br />
Kingston, Ontario K7L 3N6<br />
Professor Joseph Shapiro<br />
Geology & Ecology Department<br />
University of Minnesota<br />
Minneapolis, Minnesota 55455<br />
Dr. Harvey Shear<br />
<strong>International</strong> <strong>Joint</strong> Comisslon<br />
Great Lakes Regional Office<br />
100 Ouellette Avenue<br />
Windsor, Ontario N9A 6T3<br />
Dr. D. B. Simons<br />
Engineering Research Centre<br />
Colorado State University<br />
Fort Collins, Culorado 80523<br />
Dr. P. G. Sly, Chief<br />
Lakes Research Division<br />
Canada Centre for Inland Waters<br />
P. 0. Box <strong>50</strong><strong>50</strong><br />
Burlington, Ontario L7R 4A6<br />
302<br />
Dr. William Sonzogni<br />
Great Lakes Basin <strong>Commission</strong><br />
3475 Plymouth Road<br />
P. 0. Box 999<br />
Ann Arbor, Michigan 48106<br />
Dr. R. L. Thomas<br />
Director<br />
Great Lakes Biolimnology Laboratory<br />
Canada Centre for Inland Waters<br />
P. 0. Box <strong>50</strong><strong>50</strong><br />
Burlington, Ontario L7R 4A6<br />
Mr. Robert E. Thronson<br />
Environmental Protection Agency<br />
Waterside Mall<br />
Room 2417-D<br />
Washington, D. C. 20460<br />
Dr. J. K. Vallentyne<br />
Ocean and Aquatic Affairs<br />
Fisheries 6 Marine Services<br />
Environment Canada<br />
580 Booth Street<br />
Ottawa, Ontario K1A OH3<br />
Dr. F. H. Verhoff<br />
Route 7<br />
Box 307<br />
Morgantown, W. Va. 26<strong>50</strong>5<br />
Mr. Eric Veska<br />
Department of Geological Sciences<br />
Brock University<br />
St. Catharines, Ontario L2S 3A1<br />
Dr. Greg Wall<br />
University of Guelph<br />
Guelph, Ontario N1G 2W1<br />
Dr. D. E. Walling<br />
Department of Geography<br />
Armor Building<br />
Rennes Drive<br />
Exeter, England EX4 4RJ<br />
Dr. A. E. P. Watson<br />
<strong>International</strong> <strong>Joint</strong> <strong>Commission</strong><br />
Great Lakes Regional Office<br />
100 Ouellette Avenue<br />
Windsor, Ontario N9A 6T3
Mr. G. M. Wood<br />
Solid Waste Unit<br />
Ontario Ministry of the Environment<br />
135 St. Clair Avenue Vest<br />
'Toronto. Ontario M4V 1P5<br />
Dr. Stephen Yaksich<br />
Corps of Engineers<br />
Department of the Army<br />
1776 Niagara Street<br />
HufIalo, New York 14207
PWARG ~MBERSHI P<br />
Norman A. Berg (Chairman)<br />
Associate Administrator<br />
Soil Conservation Service<br />
U.S. Department of Agriculture<br />
P. 0. Box 2890<br />
Washington, D. C. 20013<br />
Konald ldaybrant<br />
Water Quality Appraisal Section<br />
Water ()uality Division<br />
Michigan Department of Natural Resources<br />
1’. 0. Box 30028<br />
Lansing, Michigan 48909<br />
I.. Robert Carter<br />
Coordin.ltor of Environmental Programs<br />
Office of the Assistant <strong>Commission</strong>er<br />
for Environmental Health<br />
1330 West Michigan Street<br />
Indianapolis, Indiana 46206<br />
Leo J. Ifetling<br />
Environ~nental Quality<br />
Kesearcll and Development Unit<br />
New York State Department of<br />
Envir(mmenta1 Conservation<br />
<strong>50</strong> Wolf Road<br />
Room 510<br />
Albany, New York 12201<br />
Richard R. Parizek<br />
Professt~r of Geology<br />
Department of Geosciences<br />
The Pennsylvania State University<br />
University Park, Pennsylvania 16802<br />
305<br />
Merle W. Tellekson<br />
Technical Support Branch<br />
Surveillance & Analysis Division<br />
Environmental Protection Agency<br />
Region V<br />
230 South Dearborn Street<br />
Chicago, Illinois 60604<br />
John G. Konrad<br />
Supervisor of Special Studies<br />
Wisconsin Department of Natural<br />
Resources<br />
Madison, Wisconsin 53701<br />
Floyd E. Heft<br />
Division of Soil & Water Districts<br />
Ohio Department of Natural Resources<br />
Fountain Square<br />
Columbus. Ohio 43224<br />
John Pegors<br />
Minnesota Pollution Control Agency<br />
1015 Torrey Building<br />
Duluth, Minnesota 55802<br />
Gerald B. Welsh (Secretary)<br />
Resource Development Division<br />
Soil Conservation Service<br />
U.S. Department of Agriculture<br />
P. 0. Box 2890<br />
Washington, D. C. 20013
Murrav (;. .Johnson (Chairman)<br />
Director General<br />
Ontario Region<br />
Fisheries and brine Service<br />
Ilepartment of Fisheries h Environment<br />
30<strong>50</strong> Harvester Road<br />
tiurlington, Ontario L7N 351<br />
K. I>. l't~omas<br />
Director<br />
(:rc.at Lakes Uiol imnology Laboratory<br />
Canada Ccntrc for Inland Waters<br />
1'. 0. Box <strong>50</strong><strong>50</strong><br />
RurlingLon, Ontario L7R 44.6<br />
Ii. V. Morley<br />
Kesfnr?t? ('or~r,+inntor<br />
(Lnvi rvnment and Krsources)<br />
Agriculture (:anada<br />
K.W. Neathy Building<br />
Room 1111<br />
Carling Avcmue<br />
Ottawa, Ontario KIA 0C6<br />
K. Stiikaze<br />
Chief<br />
Environmental Control Division<br />
Environmental Protection Service<br />
Department of Fisheries & Environment<br />
135 St. Clair Avenue West<br />
2nd Floor<br />
Toronto, Ontario M4V 1P5<br />
K. Code<br />
Ontario Ministry of Natural Resources<br />
Room 6602, Whitney Block<br />
Queen's Park<br />
Toronto. Ontario<br />
306<br />
Donald N. .Teffs<br />
Assistant Llirector<br />
Water Resources Branch<br />
Ontario Ministry of the Environment<br />
135 St. Clair Avenue West<br />
Toronto, Ontario M4V 1P5<br />
(:. Martin Wood<br />
llead, Solid Waste Unit<br />
Ontario Ministry of the Environment<br />
135 St. Clair Avenue West<br />
Toronto, Ontario M4V 1P5<br />
.I. E. Urubaker<br />
Agricultural Engineering Extensions<br />
Branch<br />
Ontario Ministry of Agriculture &<br />
Food<br />
(helph, Ontario N1G 2W1<br />
.John Ralston<br />
Head<br />
Water Resources Planning Unit<br />
Ontario Ministry of the Environmen<br />
135 St. Clair Avenue West<br />
Toronto, Ontario M4V 1P5<br />
John D. Wiebe (Secretary)<br />
Environmental Assessments Coordina<br />
Ontario Region<br />
Environmental Management Service<br />
Department of Fisheries & Environrr<br />
30<strong>50</strong> Harvester Road<br />
Burlington, Ontario L7N 351<br />
Secretariat Responsibilities:<br />
Dr. Harvey Shear<br />
Biologist<br />
<strong>International</strong> <strong>Joint</strong> <strong>Commission</strong><br />
Great Lakes Regional Office<br />
100 Ouellette Avenue, 8th Floor<br />
Windsor, Ontario N9A 6T3
W~RKSI-KIP ORGANIZERS<br />
Dr. Leo J. Hetling<br />
Director, Environmental Quality<br />
Research and Development Unit<br />
New York State Department of<br />
Environmental Conservation<br />
<strong>50</strong> Wolf Road<br />
Albany, New York<br />
Mr. John N. Holeman<br />
Sedimentation Geologist<br />
United States Department of<br />
Agriculture<br />
Soil Conservation Service<br />
Washington, D. C. 202<strong>50</strong><br />
Dr. Dale D. Huff<br />
Iksearch Hydrologist<br />
Environmental Sciences Division<br />
Buildlng 3017<br />
Oak Ridge Nationdl Laboratory<br />
Oak Ridge, Tennessee 37830<br />
Dr. Edwin D. Ongley<br />
Associate Professor<br />
Department of Geography<br />
Queen's University<br />
Kingston, Ontario<br />
307<br />
Dr. Harvey Shear<br />
Aquatic Biologist<br />
<strong>International</strong> <strong>Joint</strong> <strong>Commission</strong><br />
Great Lakes Regional Office<br />
100 Ouellette Avenue<br />
Windsor, Ontario N9A 6T3<br />
Dr. Richard L. Thomas<br />
Director<br />
Lakes Research Division<br />
Canada Centre for Inland Waters<br />
P. 0. Box <strong>50</strong><strong>50</strong><br />
Burlington, Ontario L7R 4A6<br />
Dr. Jack R. Vallentyne<br />
Senior Scientific Advisor to the<br />
Assistant Deputy Minister<br />
Ocean and Aquatic Affairs<br />
Fisheries and Marine Service<br />
Environment Canada<br />
580 Booth Street<br />
Ottawa, Ontario K1A OH3
Dr. D. I. Mount (Chairman)<br />
Director<br />
Environmental Kescarch Laboratory<br />
6201 Congdon Avenue<br />
L)uluth, Minnesota ‘15804<br />
Professor Joseph Shnpiro<br />
Geology Department<br />
University of Minnesota<br />
220 I’illsbury Hali.<br />
Minneapolis, Minnesota 55455<br />
Dr. Eugene J. Aubert<br />
Uirector<br />
Great Lakes Environmental Research<br />
Laboratory<br />
National Oceanographic and Atmospheric<br />
Administration<br />
2300 Washtenaw Avenue<br />
Ann Arbor, Michigan 48104<br />
Professor Leonard 1%. Dworslcy<br />
Civil and Environmental Engineering<br />
Cornell University<br />
302 HolLlster Hall<br />
Itltaca, New York 14853<br />
RESEARCH ADVISORY ~ A R D ~ E R S H I P<br />
(AS OF OCTOBER 1976)<br />
308<br />
Mr. Alvin R. Balden<br />
19 Alina Lane<br />
fiot Springs Village, Arkansas 71901<br />
Mrs. Evelyn Stebbins<br />
Citizens for Clean Air and Water Inc.<br />
705 Elmwood<br />
Rocky River, Ohio 44116<br />
Dr. Herbert E. Allen<br />
Assistant Professor<br />
Department of Environmental<br />
Engineering<br />
Illinois Institute of Technology<br />
Chicago, Lllinois 60616<br />
Professor Archie J. McDonnell<br />
Department of Civil Engineering<br />
Water Resources Research Center<br />
The Pennsylvania State University<br />
University Park, Pennsylvania 16802<br />
Ex Officio<br />
”<br />
.~<br />
Mr. Carlos M. Fetterolf, Jr.<br />
Executive Secretary<br />
Great Lakes Fishery <strong>Commission</strong><br />
1451 Green Road<br />
Ann Arbor, Michigan 48107
Canadian<br />
Dr. A. R. LeFeuvre (Chairman)<br />
Director<br />
Canada Centre for Inland Waters<br />
Environment Canada<br />
P. 0. Box <strong>50</strong><strong>50</strong><br />
Rurlington, Ontario L7R 4A6<br />
Mr. Arnold J. Drapeau<br />
Professor<br />
Ecole Polytechnique<br />
Campus de L'Universite de Montreal<br />
C.P. 6079 - Succursde "A"<br />
Montreal, Quebec H3C 3A7<br />
Mr. H. R. Holland<br />
462 Charlesworth Lane<br />
Sarnia, Ontario N7Y 2R2<br />
Mr. Paul D. Foley<br />
Coordinator<br />
Development and Research Group<br />
Ontario Ministry of the Environment<br />
175 St. Clair Avenue West<br />
'Toronto, Ontario M4V 1P5<br />
Dr. J. C. N. Westwood<br />
Professor & Head of Microbiology and<br />
Immunology<br />
Faculty of Medicine<br />
University of Ottawa<br />
Ottawa, Ontario K1N 6N5<br />
309<br />
Mr. J. Douglas Roseborough<br />
Director<br />
Fish and Wildlife Research Branch<br />
Ontario Ministry of Natural Resources<br />
P. 0. Box <strong>50</strong><br />
Maple, Ontario 1.O.J 1EO<br />
Mrs. Mary Munro<br />
3020 First Street<br />
Burlington, Ontario L7N 1C3<br />
Dr. .J. R. Vallentyne<br />
Senior Scientific Advisor<br />
Ocean & Aquatic Affairs<br />
Fisheries & Marine Service<br />
Environment Canada<br />
580 Booth Street<br />
Ottawa, Ontario K1A OH3<br />
Ex Officio "<br />
Mr. Floyd C. Elder<br />
A/Head, Basin Investigation and<br />
Modelling Section<br />
Canada Centre for Inland Waters<br />
Environment Canada<br />
P. 0. Box <strong>50</strong><strong>50</strong><br />
Burlington, 0ntar:io L7R 4A6<br />
" Secretariat-Responsibilities:<br />
Dr. Dennis E. Konasewich<br />
Scientist<br />
<strong>International</strong> <strong>Joint</strong> <strong>Commission</strong><br />
Great Lakes Regional Office<br />
1.00 Ouellette Avenue, 8th Floor<br />
Windsor, Ontario NYA 6TJ