Wien, 28 - Repository of the LBI-HTA - Ludwig Boltzmann Gesellschaft
Wien, 28 - Repository of the LBI-HTA - Ludwig Boltzmann Gesellschaft
Wien, 28 - Repository of the LBI-HTA - Ludwig Boltzmann Gesellschaft
Erfolgreiche ePaper selbst erstellen
Machen Sie aus Ihren PDF Publikationen ein blätterbares Flipbook mit unserer einzigartigen Google optimierten e-Paper Software.
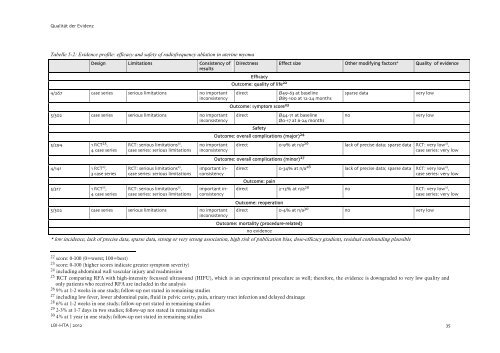
Qualita¨t der Evidenz<br />
Tabelle 5-2: Evidence pr<strong>of</strong>ile: efficacy and safety <strong>of</strong> radi<strong>of</strong>requency ablation in uterine myoma<br />
Design Limitations Consistency <strong>of</strong><br />
results<br />
4/267 case series serious limitations no important<br />
inconsistency<br />
5/302 case series serious limitations no important<br />
inconsistency<br />
5/294 1 RCT 25 ,<br />
4 case series<br />
4/141 1 RCT 25 ,<br />
3 case series<br />
5/317 1 RCT 25 ,<br />
4 case series<br />
RCT: serious limitations 25 ,<br />
case series: serious limitations<br />
RCT: serious limitations 25 ,<br />
case series: serious limitations<br />
RCT: serious limitations 25 ,<br />
case series: serious limitations<br />
no important<br />
inconsistency<br />
important inconsistency<br />
important inconsistency<br />
* low incidence, lack <strong>of</strong> precise data, sparse data, strong or very strong association, high risk <strong>of</strong> publication bias, dose-efficacy gradient, residual confounding plausible<br />
22 score: 0-100 (0=worst; 100=best)<br />
23 score: 0-100 (higher scores indicate greater symptom severity)<br />
24 including abdominal wall vascular injury and readmission<br />
25 RCT comparing RFA with high-intensity focussed ultrasound (HIFU), which is an experimental procedure as well; <strong>the</strong>refore, <strong>the</strong> evidence is downgraded to very low quality and<br />
only patients who received RFA are included in <strong>the</strong> analysis<br />
26 9% at 1-2 weeks in one study; follow-up not stated in remaining studies<br />
27 including low fever, lower abdominal pain, fluid in pelvic cavity, pain, urinary tract infection and delayed drainage<br />
<strong>28</strong> 6% at 1-2 weeks in one study; follow-up not stated in remaining studies<br />
29 2-3% at 1-7 days in two studies; follow-up not stated in remaining studies<br />
30 4% at 1 year in one study; follow-up not stated in remaining studies<br />
Directness Effect size O<strong>the</strong>r modifying factors* Quality <strong>of</strong> evidence<br />
Efficacy<br />
Outcome: quality <strong>of</strong> life 22<br />
direct O/49-63 at baseline<br />
O/85-100 at 12-24 months<br />
Outcome: symptom score 23<br />
direct O/44-71 at baseline<br />
O/0-17 at 6-24 months<br />
sparse data very low<br />
no very low<br />
<strong>LBI</strong>-<strong>HTA</strong> | 2012 35<br />
Safety<br />
Outcome: overall complications (major) 24<br />
direct 0-9% at n/a 26 lack <strong>of</strong> precise data; sparse data RCT: very low 25 ,<br />
case series: very low<br />
Outcome: overall complications (minor) 27<br />
direct 0-34% at n/a<strong>28</strong> lack <strong>of</strong> precise data; sparse data RCT: very low 25 Outcome: pain<br />
,<br />
case series: very low<br />
direct 2-13% at n/a29 no RCT: very low 25 ,<br />
case series: very low<br />
Outcome: reoperation<br />
5/302 case series serious limitations no important<br />
inconsistency<br />
direct 0-4% at n/a30 no very low<br />
Outcome: mortality (procedure-related)<br />
no evidence