ANTIMICROBIAL EFFECT OF FERROCENYL DIARYL BUTENES AGAINST OLIVE PLANTLET DISEASES
These compounds efficiently inhibited the growth of these microorganisms in culture media and in inoculated plantlets which did not contract the corresponding diseases, especially when treated with P8. These findings shed light on the potential utilisatio
These compounds efficiently inhibited the growth of these microorganisms in culture media and in inoculated plantlets which did not contract the corresponding diseases, especially when treated with P8. These findings shed light on the potential utilisatio
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
656 Ferrocenyl diaryl butenes against olive plantlets diseases Journal of Plant Pathology (2011), 93 (3), 651-657<br />
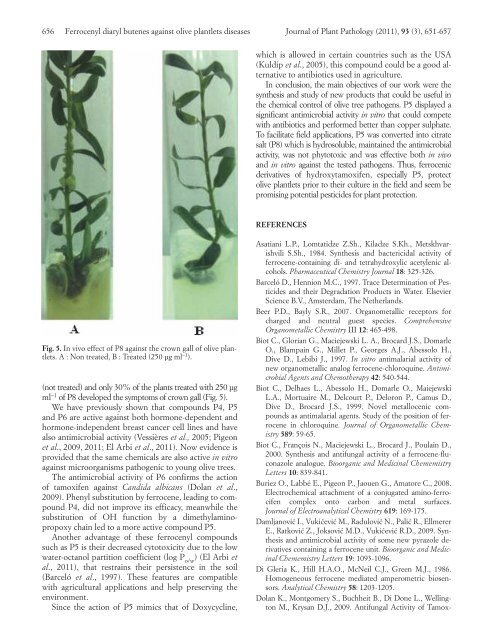
Fig. 5. In vivo effect of P8 against the crown gall of olive plantlets.<br />
A : Non treated, B : Treated (250 µg ml –1 ).<br />
(not treated) and only 30% of the plants treated with 250 µg<br />
ml –1 of P8 developed the symptoms of crown gall (Fig. 5).<br />
We have previously shown that compounds P4, P5<br />
and P6 are active against both hormone-dependent and<br />
hormone-independent breast cancer cell lines and have<br />
also antimicrobial activity (Vessières et al., 2005; Pigeon<br />
et al., 2009, 2011; El Arbi et al., 2011). Now evidence is<br />
provided that the same chemicals are also active in vitro<br />
against microorganisms pathogenic to young olive trees.<br />
The antimicrobial activity of P6 confirms the action<br />
of tamoxifen against Candida albicans (Dolan et al.,<br />
2009). Phenyl substitution by ferrocene, leading to compound<br />
P4, did not improve its efficacy, meanwhile the<br />
substitution of OH function by a dimethylaminopropoxy<br />
chain led to a more active compound P5.<br />
Another advantage of these ferrocenyl compounds<br />
such as P5 is their decreased cytotoxicity due to the low<br />
water-octanol partition coefficient (log P o/w ) (El Arbi et<br />
al., 2011), that restrains their persistence in the soil<br />
(Barceló et al., 1997). These features are compatible<br />
with agricultural applications and help preserving the<br />
environment.<br />
Since the action of P5 mimics that of Doxycycline,<br />
which is allowed in certain countries such as the USA<br />
(Kuldip et al., 2005), this compound could be a good alternative<br />
to antibiotics used in agriculture.<br />
In conclusion, the main objectives of our work were the<br />
synthesis and study of new products that could be useful in<br />
the chemical control of olive tree pathogens. P5 displayed a<br />
significant antimicrobial activity in vitro that could compete<br />
with antibiotics and performed better than copper sulphate.<br />
To facilitate field applications, P5 was converted into citrate<br />
salt (P8) which is hydrosoluble, maintained the antimicrobial<br />
activity, was not phytotoxic and was effective both in vivo<br />
and in vitro against the tested pathogens. Thus, ferrocenic<br />
derivatives of hydroxytamoxifen, especially P5, protect<br />
olive plantlets prior to their culture in the field and seem be<br />
promising potential pesticides for plant protection.<br />
REFERENCES<br />
Asatiani L.P., Lomtatidze Z.Sh., Kiladze S.Kh., Metskhvarishvili<br />
S.Sh., 1984. Synthesis and bactericidal activity of<br />
ferrocene-containing di- and tetrahydroxylic acetylenic alcohols.<br />
Pharmaceutical Chemistry Journal 18: 325-326.<br />
Barceló D., Hennion M.C., 1997. Trace Determination of Pesticides<br />
and their Degradation Products in Water. Elsevier<br />
Science B.V., Amsterdam, The Netherlands.<br />
Beer P.D., Bayly S.R., 2007. Organometallic receptors for<br />
charged and neutral guest species. Comprehensive<br />
Organometallic Chemistry III 12: 465-498.<br />
Biot C., Glorian G., Maciejewski L. A., Brocard J.S., Domarle<br />
O., Blampain G., Millet P., Georges A.J., Abessolo H.,<br />
Dive D., Lebibi J., 1997. In vitro antimalarial activity of<br />
new organometallic analog ferrocene-chloroquine. Antimicrobial<br />
Agents and Chemotherapy 42: 540-544.<br />
Biot C., Delhaes L., Abessolo H., Domarle O., Maiejewski<br />
L.A., Mortuaire M., Delcourt P., Deloron P., Camus D.,<br />
Dive D., Brocard J.S., 1999. Novel metallocenic compounds<br />
as antimalarial agents. Study of the position of ferrocene<br />
in chloroquine. Journal of Organometallic Chemistry<br />
589: 59-65.<br />
Biot C., François N., Maciejewski L., Brocard J., Poulain D.,<br />
2000. Synthesis and antifungal activity of a ferrocene-fluconazole<br />
analogue. Bioorganic and Medicinal Chememistry<br />
Letters 10: 839-841.<br />
Buriez O., Labbé E., Pigeon P., Jaouen G., Amatore C., 2008.<br />
Electrochemical attachment of a conjugated amino-ferrocifen<br />
complex onto carbon and metal surfaces.<br />
Journal of Electroanalytical Chemistry 619: 169-175.<br />
Damljanović I., Vukićević M., Radulović N., Palić R., Ellmerer<br />
E., Ratković Z., Joksović M.D., Vukićević R.D., 2009. Synthesis<br />
and antimicrobial activity of some new pyrazole derivatives<br />
containing a ferrocene unit. Bioorganic and Medicinal<br />
Chememistry Letters 19: 1093-1096.<br />
Di Gleria K., Hill H.A.O., McNeil C.J., Green M.J., 1986.<br />
Homogeneous ferrocene mediated amperometric biosensors.<br />
Analytical Chemistry 58: 1203-1205.<br />
Dolan K., Montgomery S., Buchheit B., Di Done L., Wellington<br />
M., Krysan D.J., 2009. Antifungal Activity of Tamox-