BioSep: - sonosep
BioSep: - sonosep
BioSep: - sonosep
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Technical Data Sheet<br />
STS90<br />
Duration: 0.5-1 weeks<br />
Cell density: 2 x 10 6 c/ml<br />
Finish: no more nutrients<br />
Feed<br />
Addition of concentrated nutrients<br />
=> higher product concentration.<br />
Duration: 1-1.5 weeks<br />
Cell density: 2.5 x 10 6 c/ml<br />
Finish: Viability < 50%<br />
Cell retention device<br />
Feed<br />
BATCH<br />
FED - BATCH<br />
PERFUSION<br />
Harvest<br />
Addition of nutrients with<br />
cell retention.<br />
Duration: 1-3 months<br />
Cell density: 20 x 10 6 c/ml<br />
Cell suspension<br />
<strong>BioSep</strong>:<br />
the advanced acoustic cell retention device<br />
With the progression of the genomics initiative, increasing numbers of proteins will need<br />
to be produced rapidly.<br />
The growing demand for novel proteins has motivated the development of more efficient<br />
and reliable mammalian cell culture production technologies. This currently is resulting in<br />
a spreading use of simpler, more productive processes.<br />
Perfusion is the technology to use, providing:<br />
• high cell density<br />
• high (volumetric) productivity<br />
• cost-effective operation<br />
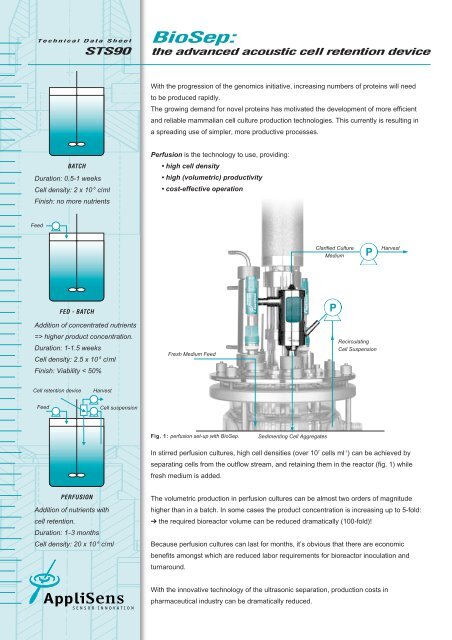
Fresh Medium Feed<br />
Fig. 1: perfusion set-up with <strong>BioSep</strong>.<br />
Sedimenting Cell Aggregates<br />
Clarified Culture<br />
Medium<br />
P<br />
P<br />
Recirculating<br />
Cell Suspension<br />
Harvest<br />
In stirred perfusion cultures, high cell densities (over 107 cells ml-1 ) can be achieved by<br />
separating cells from the outflow stream, and retaining them in the reactor (fig. 1) while<br />
fresh medium is added.<br />
The volumetric production in perfusion cultures can be almost two orders of magnitude<br />
higher than in a batch. In some cases the product concentration is increasing up to 5-fold:<br />
➔ the required bioreactor volume can be reduced dramatically (100-fold)!<br />
Because perfusion cultures can last for months, it’s obvious that there are economic<br />
benefits amongst which are reduced labor requirements for bioreactor inoculation and<br />
turnaround.<br />
With the innovative technology of the ultrasonic separation, production costs in<br />
pharmaceutical industry can be dramatically reduced.
Purely based on sound, an<br />
invisible energy mesh is created:<br />
the <strong>BioSep</strong>, a filter that never<br />
will foul.<br />
Result of the invisible and<br />
harmless energy mesh<br />
Cells appear as evenly<br />
spaced visible vertical lines<br />
in the viewing window of the<br />
<strong>BioSep</strong> chamber.<br />
They are held by ultrasonic<br />
forces against the upward flow<br />
of the culture medium.<br />
The acoustic forces form a<br />
barrier to the cells, eliminating<br />
the need for mesh or<br />
membrane filters.<br />
Acoustic Energy Field<br />
Transducer<br />
Reflector<br />
The <strong>BioSep</strong> from AppliSens is the first reliable and economical solution for the realization<br />
of mammalian cell perfusion processes.<br />
The acoustic separation technology of the <strong>BioSep</strong> can be applied on research, pilot and<br />
production scale.<br />
Perfusion processes using the <strong>BioSep</strong> acoustic separator typically involve continuous<br />
addition of fresh medium to the bioreactor, while cells are filtered from the harvest<br />
stream by the <strong>BioSep</strong> chamber and returned to the bioreactor. The <strong>BioSep</strong> chamber can<br />
directly be mounted onto the bioreactor head plate.<br />
Fresh Feed<br />
clarified<br />
culture medium<br />
concentrated<br />
Cell recycle<br />
harvest<br />
Cell<br />
suspension<br />
Fig. 2: Typical configuration of the acoustic cell retention system.<br />
cell settling<br />
Several modes of operation are available making acoustic perfusion generally applicable<br />
for suspended mammalian and animal cell culture, but also for anchorage dependent cell<br />
lines, or for the perfused culture of plant cells (see literature reference list).<br />
The Biosep separation principle is purely based on gentle acoustically induced loose<br />
aggregation followed by sedimentation. In contrast to other cell separation techniques,<br />
the acoustic energy mesh created within the Biosep constitutes a “virtual”, thus superior<br />
non-contact, non-fouling, non-moving filtration means. The technology allows for up to<br />
thousands of hours of continuous operation. As a result, greatly increased steady state<br />
cell density, productivity, and product quality is obtained.<br />
<strong>BioSep</strong> acoustic filters are not designed to ultra-purify the harvest stream from any cells.<br />
In contrast, a small escape rate allows for controlled cell bleeding and positively<br />
contributes to the viability of the culture (see publications).<br />
Typical separation efficiency of the <strong>BioSep</strong> ranges from 90-99%.
The <strong>BioSep</strong> chamber is mounted above the bioreactor head plate. The cell suspension<br />
is pumped into the chamber by the recirculation pump. The flow is then split into the<br />
harvest flow and the return flow. The flow rate through the <strong>BioSep</strong> is controlled by the<br />
harvest pump. The ultrasonic forces in the <strong>BioSep</strong> aggregate and hold the suspended<br />
cells stationary against the harvest flow, thereby clarifying the harvest stream. The<br />
planar aggregates appear as parallel lines when seen from the side through the viewing<br />
window. Aggregated cells that settle from the resonator are rapidly recycled to the<br />
bioreactor in the return stream where they are dispersed by the impeller.<br />
Conventional cell retention devices include filters, settlers and centrifuges. Regardless of<br />
their design, the filter surfaces are susceptible to fouling. Settling chambers and<br />
centrifuges solely rely on the difference in density between cells and medium.<br />
Settling chambers<br />
require, a large settling area<br />
and long settling times due to<br />
the small difference in density.<br />
This leads to prolonged exposure<br />
of the cells to an uncontrolled<br />
environment.<br />
Centrifugation<br />
the sedimentation process is<br />
enhanced by centrifugal forces<br />
many times the force of gravity.<br />
The separation efficiency<br />
of a centrifuge is a function of a<br />
multitude of operating parameters.<br />
Mechanical systems such as<br />
centrifuges are susceptible to<br />
failure and cells are exposed to<br />
high shear forces.<br />
The <strong>BioSep</strong><br />
simple and compact<br />
non-mechanical device<br />
in which only harmless<br />
sound waves are<br />
exploited to separate<br />
the cells from the<br />
suspending medium.<br />
<strong>BioSep</strong> 10 L <strong>BioSep</strong> 50 L <strong>BioSep</strong> 200 L<br />
Cell retention device<br />
Feed<br />
PERFUSION<br />
Harvest<br />
Cell suspension<br />
Compared to technologies such<br />
as filters, centrifuges and<br />
settlers, the <strong>BioSep</strong> offers an<br />
economic separation technique<br />
in perfusion cultures:<br />
• surprisingly simple<br />
• highly reliable<br />
The <strong>BioSep</strong> chamber assembly<br />
is entirely solid state and is<br />
unaffected by fouling, rendering<br />
it reliable for thousands of hours<br />
of continuous operation.<br />
The <strong>BioSep</strong> 10L is designed to<br />
operate at a perfusion harvest<br />
rate between 1 and 10L/day.<br />
The <strong>BioSep</strong> 50L operation range<br />
is between 5 and 50L/day<br />
The <strong>BioSep</strong> 200L is designed<br />
for both pilot- and production<br />
scale. The operating range is<br />
between 20L and 200L/day.
The <strong>BioSep</strong> features<br />
• Cell filtration by ultrasonic<br />
resonance field<br />
• Continuous operation<br />
• Low shear environment<br />
• Simple design<br />
• Compact autoclavable<br />
device<br />
• Compact In-Situ-<br />
Sterilizable device<br />
• Scalable system<br />
The <strong>BioSep</strong> advantages<br />
• No physical filter surfaces<br />
to foul<br />
• No mechanical parts to fail<br />
(no moving parts)<br />
• Small retention volume<br />
• Rapid turn around<br />
(low hold-up times)<br />
• Increased efficiency<br />
• Wide flow range<br />
• High cell viability<br />
• Consistent culture<br />
environment<br />
• Easy installation<br />
• Easy automation<br />
• Minimal operator<br />
involvement<br />
• Clean-In-Place<br />
The main production systems which are used today for Mab production are stirred tanks.<br />
Homogeneous systems like a stirred tank represent the biggest unit reactor volume<br />
attainable today and with the highest unit production capacities. This capacity can be<br />
increased drastically with the use of a cell retention device.<br />
The <strong>BioSep</strong> will typically remove between 90 and 99% of the cells in the harvest stream at<br />
a reactor cell concentration of up to 20 million cells/ml. The separation efficiency of the<br />
<strong>BioSep</strong> system is defined as:<br />
( )<br />
SE = 100 % 1 – Ch<br />
Cb<br />
• SE is the separation efficiency<br />
• Ch is the cell concentration in the harvest<br />
• Cb is the cell concentration in the bioreactor<br />
The separation efficiency of the <strong>BioSep</strong> system (10L, 50L and 200L) is controlled<br />
by adjusting the acoustic power input to the resonator and the run/stop cycle time ratio<br />
for the <strong>BioSep</strong> and harvest pump.<br />
Pump control cable<br />
Compressed air<br />
Output<br />
cable<br />
Harvest<br />
Recirculation pump<br />
Feed<br />
Effects on the economics of an acoustic perfused process as compared<br />
to a batch process:<br />
• improved efficiency in medium use (e.g. lower serum concentrations in growth medium),<br />
up to 5 times more efficient. The trapped and returned cells use only nutrients to maintain<br />
their metabolism and for biosynthesis of products.<br />
• a factor 10-20 higher viable cell concentration<br />
• antibody production per reactor volume per day with a factor 10-100 fold higher than<br />
in a batch (high volumetric productivity).<br />
• better on-line control due to steady state condition<br />
• reduced exposure of the products to proteases<br />
• antibody concentration in harvest improved with a factor 2-5 fold compared to batch process<br />
• downstream processing of the secreted product is reduced by one step.
Due to their higher acoustic contrast, viable cells are retained by the acoustic filter with<br />
higher efficiency than dead cells and cell debris.<br />
This results in a significantly higher escape rate for non-viable cells.<br />
This effect limits the accumulation of non-viable biomass in the bioreactor.<br />
This effect is beneficial for perfusion strategies:<br />
➔ it will selectively retain the producing cells and remove<br />
the non productive dead cells from the bioreactor.<br />
Cell concentration<br />
(10 6 cell/ml)<br />
45 110<br />
40 100<br />
35 90<br />
30 80<br />
25 70<br />
20 60<br />
15 50<br />
Viable cells<br />
10 40<br />
Total cells<br />
5 30<br />
0<br />
0 100<br />
Economic impact<br />
Viable cells<br />
200 300 400 500 600 700 800 900 1000<br />
20<br />
1100<br />
Time (h)<br />
Results <strong>BioSep</strong> 50L CHO cell perfusion culture<br />
Average total proteine concentration in harvest 272 µg/ml, courtesy: A.O.A. Miller<br />
Annual production of 1 kilogram Mouse-IgG antibody production using Mouse Hybridoma 2E11<br />
Batch FED-Batch Perfusion at 3 volumes/day<br />
Bioreactor volume (active) 500L 350L 7L<br />
Cell concentration 2 x 10 6 /ml (peak) 2 x 10 6 /ml (average) 20 x 10 6 /ml (steady state)<br />
Runs per year 40 20 4<br />
Duration per run 1 week 2 weeks 10 weeks<br />
Consumption of medium per year 20,000 L 7,000 L 7,000 L<br />
MAb concentration in harvest 50 µg/ml 150 µg/ml 150 µg/ml<br />
MAb conc.[ g/ml]<br />
Cumulative Monoclonal Antibody (g)<br />
Cell Concentration (Cells/mL)<br />
Selective retention<br />
Cell concentration<br />
10 8<br />
10 7<br />
10 6<br />
10 5<br />
Antibody Production<br />
180<br />
160<br />
140<br />
120<br />
100<br />
80<br />
60<br />
40<br />
20<br />
0<br />
0<br />
10<br />
8<br />
6<br />
4<br />
2<br />
0<br />
0<br />
0<br />
Batch<br />
100 200 300 400 500 600 700 800<br />
Time (h)<br />
Batch<br />
Perfusion<br />
200 400 600 800<br />
Time (h)<br />
200 400 600 800<br />
Time (h)<br />
Perfusion<br />
(viable cells)<br />
Perfusion<br />
Successive<br />
Batch Cultures
Technical description <strong>BioSep</strong> Chamber 10L<br />
Air inlet<br />
BNC<br />
connector<br />
BNC<br />
connector<br />
Recirculation<br />
inlet<br />
Adapter<br />
Return tube<br />
Air inlet<br />
Harvest outlet<br />
Resonator body<br />
Gasket<br />
Cuvette<br />
O-ring<br />
Recirculation inlet<br />
Hexagon screw<br />
Return tube<br />
Harvest outlet<br />
Resonator<br />
body<br />
Gasket<br />
Cuvette<br />
Gasket<br />
O-ring<br />
Hexagon<br />
screw<br />
Mechanical:<br />
Material: Body: SS 316L<br />
Window: Pyrex glass<br />
Gasket: Silicone<br />
O-ring: Silicone<br />
Finish: Interior - Electro polish, Ra < 0.8 µm<br />
Weight: 0.5 kg<br />
Total volume: 24 ml<br />
Resonator volume: 7 ml<br />
Height above headplate: 150 mm<br />
Height: 344 mm<br />
Max. width: 79 mm<br />
Exterior - Electro polish/mechanical polish<br />
Head plate connection: Through a 12 mm diameter (pH/mV) sensor holder<br />
Insertion length: 190 mm<br />
Clarified medium outlet: 4 mm barbed fitting<br />
Medium inlet: 7 mm barbed fitting<br />
Air inlet: 4 mm barbed fitting<br />
Temperature: 130˚C max.<br />
Test pressure (internal): 4 bars<br />
Electrical:<br />
Operating frequency: 2.1 MHz<br />
Power consumption: 10 W max.<br />
<strong>BioSep</strong> Chamber 50L<br />
Mechanical:<br />
Material: Body: SS 316L<br />
Window: Pyrex glass<br />
Gasket: Silicone<br />
O-ring: Silicone<br />
Finish: Interior - Electro polish, Ra < 0.8 µm<br />
Weight: 1.5 kg<br />
Total volume: 150 ml<br />
Resonator volume: 50 ml<br />
Height above headplate: 177 mm<br />
Height: 344 mm<br />
Exterior - Electro polish/mechanical polish<br />
Max. width: 110 mm (72 mm housing)<br />
Return tube connection: 0.5” Tri-clamp (tube: ø 9.53)<br />
Clarified medium outlet: 6 mm barbed fitting<br />
Medium inlet: 10 mm barbed fitting<br />
Air inlet: 6 mm barbed fitting<br />
Temperature: 130˚C max.<br />
Test pressure (internal): 4 bars<br />
Electrical:<br />
Operating frequency: 2.1-2.15 MHz<br />
Power consumption: 10 W max.
APS 990 <strong>BioSep</strong> Controller for <strong>BioSep</strong> chamber 10L and 50L<br />
The APS 990 controller consists of a frequency generator and a power amplifier.<br />
The internal control automatically optimises the frequency and amplitude of the output<br />
signal for best separation performance.<br />
The adjustable timers do set the run/stop cycle times for the harvest pump and<br />
the acoustic field. The display indicates the frequency and the LED bar does indicate<br />
the power output in percentages.<br />
Frequency indication<br />
Power knob<br />
APS990 <strong>BioSep</strong> controller<br />
Mechanical:<br />
Dimensions: W x H x D = 130mm x 130mm x 305mm<br />
Weight: 3.5 kg<br />
Electrical:<br />
Power supply: 110 -240VAC, 50/60 Hz<br />
Power consumption: Max. 150W<br />
Coaxial cable: Length 2 meters<br />
Frequency range: 2.1 – 2.15 MHz<br />
Output power: 10 W max.<br />
Output voltage: 30 Vpp max.<br />
Internal timer: Run time: 1 – 15 minutes<br />
On/off toggle<br />
Stop time: 3 –10 sec.; 5 – 15 minutes<br />
Power indication (%)<br />
Run time knob<br />
Stop time knob<br />
Automatic power/frequency<br />
adjustment<br />
Minimal operator involvement<br />
Simple human interface<br />
• The output power is<br />
increased automatically<br />
during scanning<br />
• Scanning stops at<br />
the resonance peak<br />
Interface I/O port:<br />
• Status output<br />
24 VDC / 100 mA max.<br />
switching load<br />
• Interrupt output<br />
24 VDC / 100 mA max.<br />
switching load<br />
• DC source<br />
max load: 20 mA<br />
• Remote on/off input<br />
max 15 VDC / 4 – 20 mA<br />
Auxiliary port:<br />
• Engaged when aucoustic<br />
field is off<br />
12 VDC<br />
max. load: 1A
The <strong>BioSep</strong> 200L Acoustic<br />
Perfusion System is a simple,<br />
effective and reliable cell<br />
separation system designed<br />
expressly for cell retention during<br />
perfusion of high-density stirred<br />
suspension cultures.<br />
The system consists of a<br />
resonator chamber with a 20 -<br />
200 L/day harvest rate capacity<br />
and the APS 991 controller.<br />
The chamber, where cell<br />
separation takes place, is<br />
compact and simply installed<br />
on or above the bioreactor head<br />
plate. The system is easy to<br />
operate and provides robust,<br />
continuous operation, whatever<br />
the desired cell culture duration.<br />
Using this device existing batch<br />
or fed-batch reactors can be<br />
conveniently adapted to highproductivity<br />
perfusion.<br />
Scale-up of acoustic perfusion: The <strong>BioSep</strong> 200L<br />
The 200L acoustic perfusion system consists of a <strong>BioSep</strong> chamber and the <strong>BioSep</strong><br />
controller APS991.<br />
The <strong>BioSep</strong> chamber assembly is entirely solid state and is essentially unaffected by<br />
fouling, rendering it reliable for thousands of hours of continuous operation.<br />
It is designed to operate at a perfusion harvest rate between 20 and 200L/day.<br />
The separation efficiency of the <strong>BioSep</strong> 200L system is controlled by adjusting the<br />
power input to the resonator and the run/stop cycle time ratio for the acoustic field<br />
and harvest pump.<br />
Cell concentration<br />
(10 6 cells/ml)<br />
45<br />
40<br />
35<br />
30<br />
25<br />
20<br />
15<br />
10<br />
5<br />
APS991 controller<br />
Resonator chamber<br />
Medium feed<br />
Bioreactor<br />
Total cells<br />
Water bath<br />
Medium<br />
pump<br />
pump control cable<br />
Output cable<br />
Typical configuration of the 200L <strong>BioSep</strong> acoustic perfusion system.<br />
0<br />
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18<br />
Culture time (days)<br />
Harvest<br />
<strong>BioSep</strong> 200L CHO perfusion (courtesy 4C, Belgium)<br />
Recirculation<br />
pump<br />
Recirculation<br />
cell suspension<br />
Resonator<br />
support
<strong>BioSep</strong> Chamber 200L<br />
Mechanical:<br />
Material: Body: 1.4404 SS 316L<br />
Transducer plate: Pyrex glass<br />
Gasket: Silicone<br />
O-ring: Silicone<br />
Finish: Interior - Electro polish, Ra < 0.8 µm<br />
Weight: Approximately 13 kg<br />
Total volume: 1.0 L<br />
Resonator volume: 290 ml<br />
Height: 317 mm<br />
Max. width without sensor port: 193 mm<br />
Diameter housing 158 mm<br />
Clarified medium outlet: 0,5” Triclamp<br />
Medium inlet: 0,5” Triclamp<br />
Return outlet: 0,5” Triclamp<br />
Water bath inlet: 0,5” Triclamp<br />
Water bath outlet: 0,5” Triclamp<br />
Temperature: 130˚C max.<br />
Pressure range (internal): 3.2 barg<br />
Exterior - Electro polish/mechanical polish<br />
Electrical:<br />
Operating frequency range: 2.10 - 2.13 MHz<br />
Power consumption: 100 W max.<br />
APS 991 Controller<br />
Mechanical:<br />
Dimensions: W x H x D = 450 x 150 x 350mm<br />
Weight: 15 kg<br />
Electrical:<br />
Power supply: 110-120/220-240 VAC, 50/60 Hz<br />
Fuses: 3.15 A Slow blow 250V<br />
Power consumption: Max. 570 VA<br />
Coaxial HF cable length: 2 m<br />
Frequency range: 2.10 - 2.19 MHz<br />
Output power: 100 W max.<br />
Output voltage: 100 Vpp max.<br />
Internal Timer: Run time: 10 - 600 s<br />
Stop time: 1 - 60 s<br />
Top<br />
plate<br />
Cuvette<br />
Water<br />
bath inlet<br />
Recirculation inlet<br />
Harvest outlet<br />
Sensor<br />
port<br />
Water bath<br />
outlet<br />
Funnel<br />
Return<br />
outlet
Fresh feed<br />
Recirculation pump<br />
• speed ±3 x harvest flow<br />
Fresh feed<br />
Clarified culture<br />
medium<br />
Harvest pump<br />
• variable speed with remote<br />
control input<br />
• variable speed with remote<br />
control input and prime rate<br />
reverse action<br />
Clarified culture<br />
medium<br />
Concentrated<br />
cell recycle<br />
Concentrated<br />
cell recycle<br />
Harvest<br />
Cell<br />
suspension<br />
Harvest<br />
Prime rate<br />
reverse pump<br />
Cell<br />
suspension<br />
Pumps and other hardware to complete a perfusion set-up<br />
The harvest pump is a variable speed pump. The pump is stopped (remotely) by the<br />
<strong>BioSep</strong> controller during field off times, facilitating the settling of aggregates. This means<br />
that the harvest flow is stopped for a few seconds, arresting the flow in the chamber.<br />
The stop time of a few seconds will affect the overall harvest flow rate by a small<br />
amount. This pump can be controlled directly by a connecting cable between the <strong>BioSep</strong><br />
controller and the pump.<br />
In cultures of sticky cells or too large aggregates, the variable speed pump with prime<br />
rate reverse action (code Z288010020) is a recommended option.The pump has the<br />
capability to reverse the harvest flow at full pump speed (prime), during field off time.<br />
Sticky cells can adhere to the glass of the resonator chamber. By reversing the flow at<br />
full speed during field off times (for 3 sec) these cells will get a small push back into the<br />
bioreactor. Reversing the flow every 10 min will result in less adherence of cells in the<br />
<strong>BioSep</strong> chamber.<br />
A typical <strong>BioSep</strong> set-up<br />
<strong>BioSep</strong> 10L: • holder to fit resonance chamber into the headplate<br />
• suction tube for recirculation loop<br />
• recirculation pump<br />
• harvest pump (variable speed / remote controlled)<br />
• fresh medium inlet and pump (e.g. controlled via level controller)<br />
<strong>BioSep</strong> 50L: • diptube for return of cells from chamber. (The diptube should be<br />
provided with the proper headplate connector and a 0.5” TC to install<br />
the resonance chamber).<br />
• suction tube for recirculation loop<br />
• recirculation pump<br />
• harvest pump (variable speed / remote controlled)<br />
• fresh medium inlet and pump (e.g. controlled via level controller)<br />
Various adapters are available to fit the <strong>BioSep</strong> 50L to any type or brand of bioreactor.<br />
Adapter M18-0.5”SC-9.53 Adapter PG13.5-0.5”SC-9.53 Adapter D27-0.5”SC-9.53
<strong>BioSep</strong> 200L: Scale-up of acoustic perfusion<br />
There are several options for connecting the return outlet to the reactor,<br />
depending on the chosen way of sterilization.The <strong>BioSep</strong> chamber is<br />
placed on a support above the reactor preferably straight above the<br />
return tube in the top plate to ensure smooth return of the recirculation<br />
medium preventing sedimentation of cells in the tubing.<br />
Additional hardware for a <strong>BioSep</strong> 200L set-up:<br />
• <strong>BioSep</strong> chamber and controller<br />
• <strong>BioSep</strong> support<br />
• Pumps, pump head, pump tubing (harvest, recirculation, feed)<br />
• Water bath (temperature control of resonance chamber)<br />
• Contained additions<br />
)<br />
• Sensors (pH, DO, T) Optional<br />
• Safety clamps pump tubing<br />
Example P&ID for a fully contained <strong>BioSep</strong> in S.I.P.<br />
References:<br />
1. H. Bierau, A. Perani, M. Al-Rubeai, A.N. Emery. A comparison of intensive cell culture bioreactors operating with Hybridomas<br />
modified for inhibited apoptotic response. Journal of biotechnology 62, 195-207, 1998. 2. Gorenflo, V.M., Smith, L, Dedinsky,<br />
B., Persson, B. and Piret, J.M. Scale-up and Optimization of an Acoustic Filter for 200 L/day Perfusion of a CHO Cell Culture.<br />
Biotechnology and Bioengineering, vol 80, no. 4, nov. 2002 3. Miller, A.O.A. Combing cell culture & process operation.<br />
Sonoperfusion allows direct feed with expanded-bed chromatography. GEN Vol. 21, p29, 2001. 4. Pui, P.W.S., Trampler,<br />
F., Sonderhoff, S.A., Groeschl, M., Kilburn, D.G. and Piret, J.M. “Batch and Semi-Continuous Aggregation and Sedimentation of<br />
Hybridoma Cells by Acoustic Resonance Fields”, Biotechnol. Prog. 11: 146-152, 1995. 5. Ryll, T., Dutina, G., Reyes, A., Gunson,<br />
J., Krummen, L., Etcheverry, T., Performance of small-scale CHO perfusion cultures using an acoustic cell filtration device for cell<br />
retention: Characterization of separation efficiency and impact of perfusion on product quality, Biotechnol Bioeng 69: 440-449,<br />
2000. 6. Trampler, F., Sonderhoff, S.A., Pui, P.W.S., Kilburn, D.G. and Piret, J.M. “Acoustic Cell Filter for High Density Perfusion<br />
Culture of Hybridoma Cells”, Bio/Technol. 12: 281-284, 1994. 7. S.M.Woodside, B.D. Bowen, J.M. Piret. Mammalian cell retention<br />
devices for stirred perfusion bioreactor. Cytotechnology 28, 163-175, 1998. 8. Zhang, J., A. Collins, M. Chen, I. Knyazev and<br />
R. Gentz “High-density perfusion culture of insect cells with a <strong>BioSep</strong> ultrasonic filter”, Biotechnol Bioeng 59: 351-359, 1998.<br />
A 100L working volume bioreactor with<br />
a <strong>BioSep</strong> 200L chamber and support.<br />
The <strong>BioSep</strong> technology<br />
is patented (US 5.626.767)
<strong>BioSep</strong><br />
Z099001010 <strong>BioSep</strong> Chamber 10 L/day<br />
Z099005010 <strong>BioSep</strong> Chamber 50 L/day<br />
Z099020010 <strong>BioSep</strong> Chamber 200L/day<br />
Z099020020 <strong>BioSep</strong> Chamber 200L/day with sensor ports<br />
<strong>BioSep</strong> Controller<br />
Z299005020 <strong>BioSep</strong> Controller APS 990 (10-50L/day) 110-240VAC<br />
Z299025010 <strong>BioSep</strong> Controller APS 991 (200L/day) 110-240VAC<br />
Z299025011 Computer interface board APS 991 (optional)<br />
<strong>BioSep</strong> additional hardware<br />
Z199001310 Adapter PG13.5-0.5”SC-9.53<br />
Z199001810 Adapter M18-0.5”SC-9.53<br />
Z199002710 Adapter D27-0.5”SC-9.53<br />
Z199020010 Basic support <strong>BioSep</strong> 200L H=2.0m<br />
Z199020050 Hardware basic P&ID <strong>BioSep</strong> 200L<br />
Z199020060 Hardware contained P&ID <strong>BioSep</strong> 200L<br />
Z230001210 Thermo-circulator <strong>BioSep</strong> 200L (220-240V)<br />
Z230001220 Thermo-circulator <strong>BioSep</strong> 200L (110-120V)<br />
<strong>BioSep</strong> pumps<br />
Z188000010 Pumphead Easyload (10L & 50L)<br />
Z188000030 Pumphead Easyload L/S II (200L)<br />
Z188001410 Tubing norprene 15m, size 14 (harvest 10L)<br />
Z188001610 Tubing norprene 15m, size 16 (recirculation 10L, harvest 50L)<br />
Z188001810 Tubing norprene 15m, size 18 (recirculation 50L)<br />
Z188003510 Tubing norprene 15m, size 35 (recirculation and harvest 200L)<br />
Z288001710 Pump drive fixed speed 17rpm 230V (recirculation 10L/50L)<br />
Z288010010 Pumpdrive var.speed 1-100rpm 230V (recirculation 50L and harvest 10L/50L/200L)<br />
Z288010020 Pumpdrive var.speed prime rate reverse 1-100rpm 230V (harvest 10L/50L/200L)<br />
Z288060010 Pumpdrive var.speed 6-600rpm 230V (recirculation 200L)<br />
Z188141410 Safety clamp for tubing size 14<br />
Z188161610 Safety clamp for tubing size 16<br />
Z188353510 Safety clamp for tubing size 35 (200L)<br />
The <strong>BioSep</strong> acoustic cell retention system:<br />
•a non-fouling perfusion device with increased separation capacity<br />
and improved reliability<br />
• making large-scale perfusion an increasing viable option for cell culture processes<br />
STS90 - VZXV122902 - Subject to modifications - Printed by Applikon Dependable Instruments bv - The Netherlands 10.02<br />
Ordering information<br />
Applikon Dependable Instruments bv<br />
AppliSens<br />
De Brauwweg 13<br />
P.O. Box 149, 3100 AC Schiedam<br />
The Netherlands<br />
Phone: +31 10 298 35 85<br />
Fax: +31 10 437 96 48<br />
E-mail: applisens@applikon.com<br />
Internet: www.applikon.com