First results of the Phase II TITAN trial: anti-von Willebrand ... - Ablynx
First results of the Phase II TITAN trial: anti-von Willebrand ... - Ablynx
First results of the Phase II TITAN trial: anti-von Willebrand ... - Ablynx
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
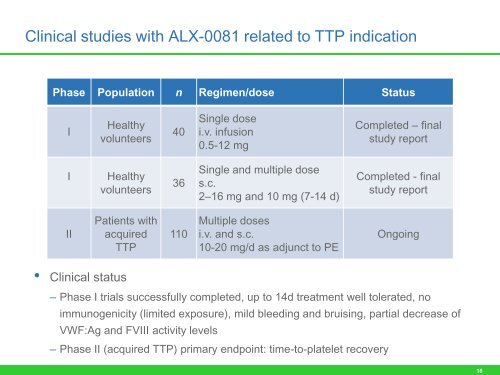
Clinical studies with ALX-0081 related to TTP indication<br />
<strong>Phase</strong> Population n Regimen/dose Status<br />
I<br />
Healthy<br />
volunteers<br />
I Healthy<br />
volunteers<br />
<strong>II</strong><br />
Patients with<br />
acquired<br />
TTP<br />
• Clinical status<br />
40<br />
36<br />
110<br />
– <strong>Phase</strong> I <strong>trial</strong>s successfully completed, up to 14d treatment well tolerated, no<br />
immunogenicity (limited exposure), mild bleeding and bruising, partial decrease <strong>of</strong><br />
VWF:Ag and FV<strong>II</strong>I activity levels<br />
Single dose<br />
i.v. infusion<br />
0.5-12 mg<br />
Single and multiple dose<br />
s.c.<br />
2–16 mg and 10 mg (7-14 d)<br />
Multiple doses<br />
i.v. and s.c.<br />
10-20 mg/d as adjunct to PE<br />
– <strong>Phase</strong> <strong>II</strong> (acquired TTP) primary endpoint: time-to-platelet recovery<br />
Completed – final<br />
study report<br />
Completed - final<br />
study report<br />
Ongoing<br />
16