Chapter 2 / Atomic Structure and Interatomic Bonding
Chapter 2 / Atomic Structure and Interatomic Bonding
Chapter 2 / Atomic Structure and Interatomic Bonding
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Force F<br />
Attraction<br />
Repulsion<br />
Attraction Repulsion<br />
Potential energy E<br />
+<br />
0<br />
+<br />
0<br />
E 0<br />
r 0<br />
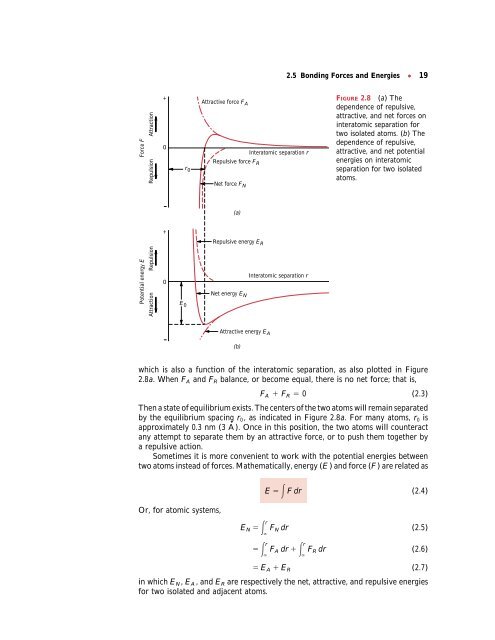
2.5 <strong>Bonding</strong> Forces <strong>and</strong> Energies ● 19<br />
which is also a function of the interatomic separation, as also plotted in Figure<br />
2.8a. When FA <strong>and</strong> FR balance, or become equal, there is no net force; that is,<br />
FA FR 0 (2.3)<br />
Then a state of equilibrium exists. The centers of the two atoms will remain separated<br />
by the equilibrium spacing r0, as indicated in Figure 2.8a. For many atoms, r0 is<br />
approximately 0.3 nm (3 A˚ ). Once in this position, the two atoms will counteract<br />
any attempt to separate them by an attractive force, or to push them together by<br />
a repulsive action.<br />
Sometimes it is more convenient to work with the potential energies between<br />
two atoms instead of forces. Mathematically, energy (E) <strong>and</strong> force (F) are related as<br />
Or, for atomic systems,<br />
Attractive force F A<br />
Repulsive force F R<br />
Net force F N<br />
(a)<br />
Repulsive energy E R<br />
Net energy E N<br />
Attractive energy E A<br />
(b)<br />
<strong>Interatomic</strong> separation r<br />
<strong>Interatomic</strong> separation r<br />
EN r<br />
<br />
r<br />
E Fdr (2.4)<br />
<br />
F N dr (2.5)<br />
FA dr r<br />
FR dr (2.6)<br />
EA ER (2.7)<br />
in which EN, EA, <strong>and</strong> ER are respectively the net, attractive, <strong>and</strong> repulsive energies<br />
for two isolated <strong>and</strong> adjacent atoms.<br />
<br />
FIGURE 2.8 (a) The<br />
dependence of repulsive,<br />
attractive, <strong>and</strong> net forces on<br />
interatomic separation for<br />
two isolated atoms. (b) The<br />
dependence of repulsive,<br />
attractive, <strong>and</strong> net potential<br />
energies on interatomic<br />
separation for two isolated<br />
atoms.