Chapter 2 / Atomic Structure and Interatomic Bonding
Chapter 2 / Atomic Structure and Interatomic Bonding
Chapter 2 / Atomic Structure and Interatomic Bonding
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
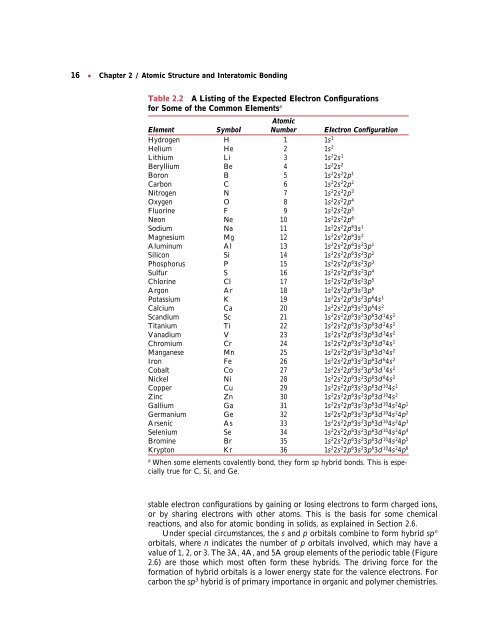
16 ● <strong>Chapter</strong> 2 / <strong>Atomic</strong> <strong>Structure</strong> <strong>and</strong> <strong>Interatomic</strong> <strong>Bonding</strong><br />
Table 2.2 A Listing of the Expected Electron Configurations<br />
for Some of the Common Elementsa <strong>Atomic</strong><br />
Element Symbol Number Electron Configuration<br />
Hydrogen H 1 1s1 Helium He 2 1s2 Lithium Li 3 1s22s 1<br />
Beryllium Be 4 1s22s 2<br />
Boron B 5 1s22s 22p1 Carbon C 6 1s22s 22p2 Nitrogen N 7 1s22s 22p3 Oxygen O 8 1s22s 22p4 Fluorine F 9 1s22s 22p5 Neon Ne 10 1s22s 22p6 Sodium Na 11 1s22s 22p63s 1<br />
Magnesium Mg 12 1s22s 22p63s 2<br />
Aluminum Al 13 1s22s 22p63s 23p1 Silicon Si 14 1s22s 22p63s 23p2 Phosphorus P 15 1s22s 22p63s 23p3 Sulfur S 16 1s22s 22p63s 23p4 Chlorine Cl 17 1s22s 22p63s 23p5 Argon Ar 18 1s22s 22p63s 23p6 Potassium K 19 1s22s 22p63s 23p64s 1<br />
Calcium Ca 20 1s22s 22p63s 23p64s 2<br />
Sc<strong>and</strong>ium Sc 21 1s22s 22p63s 23p63d 14s2 Titanium Ti 22 1s22s 22p63s 23p63d 24s2 Vanadium V 23 1s22s 22p63s 23p63d 34s2 Chromium Cr 24 1s22s 22p63s 23p63d 54s1 Manganese Mn 25 1s22s 22p63s 23p63d 54s2 Iron Fe 26 1s22s 22p63s 23p63d 64s2 Cobalt Co 27 1s22s 22p63s 23p63d 74s2 Nickel Ni 28 1s22s 22p63s 23p63d 84s2 Copper Cu 29 1s22s 22p63s 23p63d 104s1 Zinc Zn 30 1s22s 22p63s 23p63d 104s2 Gallium Ga 31 1s22s 22p63s 23p63d 104s24p1 Germanium Ge 32 1s22s 22p63s 23p63d 104s24p2 Arsenic As 33 1s22s 22p63s 23p63d 104s24p3 Selenium Se 34 1s22s 22p63s 23p63d 104s24p4 Bromine Br 35 1s22s 22p63s 23p63d 104s24p5 Krypton Kr 36 1s22s 22p63s 23p63d 104s24p6 a When some elements covalently bond, they form sp hybrid bonds. This is especially<br />
true for C, Si, <strong>and</strong> Ge.<br />
stable electron configurations by gaining or losing electrons to form charged ions,<br />
or by sharing electrons with other atoms. This is the basis for some chemical<br />
reactions, <strong>and</strong> also for atomic bonding in solids, as explained in Section 2.6.<br />
Under special circumstances, the s <strong>and</strong> p orbitals combine to form hybrid sp n<br />
orbitals, where n indicates the number of p orbitals involved, which may have a<br />
value of 1, 2, or 3. The 3A, 4A, <strong>and</strong> 5A group elements of the periodic table (Figure<br />
2.6) are those which most often form these hybrids. The driving force for the<br />
formation of hybrid orbitals is a lower energy state for the valence electrons. For<br />
carbon the sp 3 hybrid is of primary importance in organic <strong>and</strong> polymer chemistries.