Chapter 2 / Atomic Structure and Interatomic Bonding
Chapter 2 / Atomic Structure and Interatomic Bonding
Chapter 2 / Atomic Structure and Interatomic Bonding
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
22 ● <strong>Chapter</strong> 2 / <strong>Atomic</strong> <strong>Structure</strong> <strong>and</strong> <strong>Interatomic</strong> <strong>Bonding</strong><br />
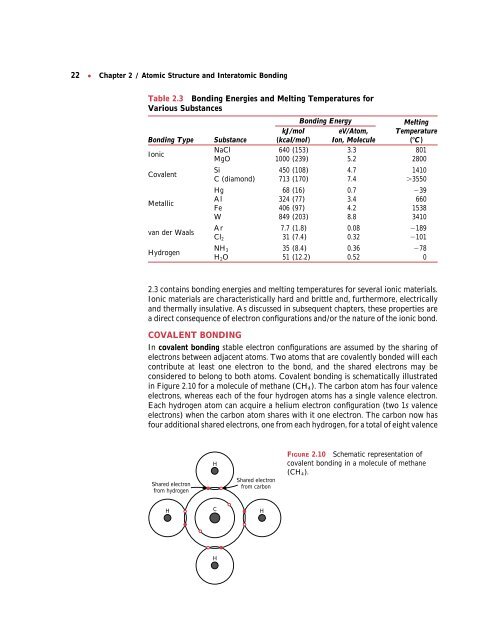
Table 2.3 <strong>Bonding</strong> Energies <strong>and</strong> Melting Temperatures for<br />
Various Substances<br />
<strong>Bonding</strong> Energy Melting<br />
kJ/mol eV/Atom, Temperature<br />
<strong>Bonding</strong> Type Substance (kcal/mol) Ion, Molecule (C)<br />
Ionic<br />
NaCl<br />
MgO<br />
640 (153)<br />
1000 (239)<br />
3.3<br />
5.2<br />
801<br />
2800<br />
Covalent<br />
Si<br />
C (diamond)<br />
450 (108)<br />
713 (170)<br />
4.7<br />
7.4<br />
1410<br />
3550<br />
Hg 68 (16) 0.7 39<br />
Metallic<br />
Al<br />
Fe<br />
324 (77)<br />
406 (97)<br />
3.4<br />
4.2<br />
660<br />
1538<br />
W 849 (203) 8.8 3410<br />
van der Waals<br />
Hydrogen<br />
Ar 7.7 (1.8) 0.08 189<br />
Cl2 31 (7.4) 0.32 101<br />
NH3 35 (8.4) 0.36 78<br />
H2O 51 (12.2) 0.52 0<br />
2.3 contains bonding energies <strong>and</strong> melting temperatures for several ionic materials.<br />
Ionic materials are characteristically hard <strong>and</strong> brittle <strong>and</strong>, furthermore, electrically<br />
<strong>and</strong> thermally insulative. As discussed in subsequent chapters, these properties are<br />
a direct consequence of electron configurations <strong>and</strong>/or the nature of the ionic bond.<br />
COVALENT BONDING<br />
In covalent bonding stable electron configurations are assumed by the sharing of<br />
electrons between adjacent atoms. Two atoms that are covalently bonded will each<br />
contribute at least one electron to the bond, <strong>and</strong> the shared electrons may be<br />
considered to belong to both atoms. Covalent bonding is schematically illustrated<br />
in Figure 2.10 for a molecule of methane (CH4). The carbon atom has four valence<br />
electrons, whereas each of the four hydrogen atoms has a single valence electron.<br />
Each hydrogen atom can acquire a helium electron configuration (two 1s valence<br />
electrons) when the carbon atom shares with it one electron. The carbon now has<br />
four additional shared electrons, one from each hydrogen, for a total of eight valence<br />
Shared electron<br />
from hydrogen<br />
H<br />
H<br />
C<br />
H<br />
Shared electron<br />
from carbon<br />
H<br />
FIGURE 2.10 Schematic representation of<br />
covalent bonding in a molecule of methane<br />
(CH4).