REVIEW ARTICLE TUBERCULOSIS: EPIDEMIOLOGY ... - rbrs
REVIEW ARTICLE TUBERCULOSIS: EPIDEMIOLOGY ... - rbrs
REVIEW ARTICLE TUBERCULOSIS: EPIDEMIOLOGY ... - rbrs
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
JBR–BTR, 2006, 89: 243-250.<br />
<strong>REVIEW</strong> <strong>ARTICLE</strong><br />
<strong>TUBERCULOSIS</strong>: <strong>EPIDEMIOLOGY</strong>, MANIFESTATIONS, AND THE VALUE OF<br />
MEDICAL IMAGING IN DIAGNOSIS<br />
A. I. De Backer 1 , K. J. Mortelé 2 , B. L. De Keulenaer 3 , P. M. Parizel 4<br />
Mycobacterial infections have been shown to be increasing in number worldwide, mainly due a global increase in<br />
developing countries, the increased number of patients with HIV infection and AIDS disease worldwide, an increasing<br />
number of elderly patients and the emergence of multidrug resistant tuberculosis. Inhalation is the predominant<br />
pathway of Mycobacterium tuberculosis (M. tuberculosis) infection, making pulmonary tuberculosis the most common<br />
form of tuberculosis.Tuberculosis may arise either from a recent infection with M. tuberculosis, or from the reactivation<br />
of dormant bacilli, years or decades after initial infection. Extrapulmonary tuberculosis mainly results from<br />
reactivation of a tuberculous focus after hematogenous dissemination or lymphogenous spread from a primary, usually<br />
pulmonary focus. Tuberculosis may demonstrate a variety of radiological features depending on the organ site<br />
involved and may mimick other pathologies.The final diagnosis of tuberculous disease mainly depends on the detection<br />
of the causative organism on histopathological examination, culture and polymerase chain reaction-based assay<br />
for mycobacterial DNA on material obtained during bronchoscopic washings, fine needle aspiration cytology (FNAC)<br />
or biopsy.<br />
Key-word: Tuberculosis.<br />
Tuberculosis, a disease caused by<br />
M. tuberculosis, has been recorded<br />
in history since the Greco-Roman<br />
and Egyptian civilizations, with evidence<br />
of spinal tuberculosis being<br />
recorded as long ago as 3400 BC.<br />
Ancient Indian scriptures also mention<br />
this disease (1), with the first<br />
known description of tuberculous<br />
spondylitis being written in Sanskrit<br />
sometime between 1500 and<br />
700 BC. However, the modern name<br />
of the disease has been attributed to<br />
Laennec in the 1800s (2).<br />
It has been postulated that<br />
M. tuberculosis existed as an unimportant<br />
pathogen to man until the<br />
coming of the industrial revolution<br />
(3). With resulting urbanisation<br />
and propinquity of living, a new epidemic,<br />
described as ‘a great white<br />
plague’, evolved. In the newly industrialised<br />
countries, the incidence of<br />
tuberculosis probably increased<br />
sharply from the mid 1700s with<br />
subsequent pandemic spread<br />
throughout Western Europe over the<br />
next century and a peak incidence<br />
around 1800 (4). Migration probably<br />
resulted in spread to the United<br />
States, central Africa and also to<br />
South and South-east Asia. As<br />
recently as 1950 tuberculosis has<br />
affected previously completely uninfected<br />
and, therefore, non-immune<br />
populations, such as the Inuit<br />
Eskimos of Northern Canada and<br />
the natives of the highlands of<br />
Papua New Guinea (5, 6).<br />
It has been stated that as tuberculosis<br />
moves through a nonimmune<br />
population, natural selection would<br />
eventually resulted in a resistant<br />
population and subsequent gradually<br />
decline of the disease pandemic.<br />
In most persons, infection with M.<br />
tuberculosis is initially contained by<br />
host defences, and the infection<br />
remains latent. However, latent<br />
tuberculous infection has the potential<br />
to develop into tuberculosis at<br />
any time, and persons with active<br />
tuberculosis become sources of<br />
new infections (7).<br />
Today, tuberculosis remains<br />
endemic in most of the developing<br />
countries. In common with many<br />
other developed countries, Belgium<br />
faces a resurgence of tuberculosis.<br />
After declining for more than a century,<br />
notification rates began to<br />
increase in the mid 1980s and the<br />
long-term downward trend in mortality<br />
also shows signs of levelling<br />
off (8). Several factors may have<br />
contributed to these trends, includ-<br />
From: 1. Department of Radiology, General Hospital Sint-Lucas, Ghent, Belgium, 2.<br />
Department of Radiology, Division of Abdominal Imaging and Intervention, Brigham<br />
and Women’s Hospital, Harvard Medical School, Boston, USA, 3. Intensive Care Unit,<br />
Royal Darwin Hospital, Rocklands, Northern Territory, Australia, 4. Department of<br />
Radiology, Universitair Ziekenhuis Antwerpen, Edegem, Belgium.<br />
Address for correspondence: Dr A. I. De Backer, M.D., PhD, Department of Radiology,<br />
General Hospital Sint-Lucas, Groenebriel 1, B-9000 Ghent, Belgium. E-mail:<br />
adelard.debacker@azstlucas.be.<br />
ing immigration from countries with<br />
a high prevalence and the epidemic<br />
of HIV and AIDS. In addition, other<br />
underlying diseases (diabetes mellitus,<br />
chronic renal failure, chronic<br />
obstructive pulmonary disease, liver<br />
cirrhosis, leukemias and lymphomas)<br />
and numerous sociological<br />
factors contributed to the re-emergence<br />
of tuberculosis: a growing<br />
elderly population; overcrowded<br />
prisons; poor living facilities; poor<br />
nutrition status, alcohol and drug<br />
abuse; persons in long-term care<br />
facilities and homelessness (9, 10).<br />
Among health care workers, the risk<br />
of occupational tuberculosis varies<br />
among and within institutions, but<br />
workers involved in autopsies and<br />
cough-inducing procedures seem to<br />
be at higher risk (11). Finally, it is<br />
known that immigrants visiting their<br />
country of origin can “bring back”<br />
tuberculosis on their return (12).<br />
Tuberculosis may arise in two different<br />
ways: either from a recent<br />
infection with M. tuberculosis; or<br />
from the reactivation of dormant<br />
tubercle bacilli years or decades<br />
after initial infection resulting in<br />
tuberculous disease. As a consequence,<br />
the present level of tuberculosis<br />
comprises both individuals<br />
with “new” exogenous infections<br />
and those with a reactivation of<br />
“old” endogenous disease. The<br />
annual risk of developing pulmonary<br />
tuberculosis following a<br />
recent primary infection is estimated<br />
to be 300 times greater than the<br />
risk of disease from endogenous<br />
reactivation (13). However, older
244 JBR–BTR, 2006, 89 (5)<br />
people having lived through a period<br />
of high tuberculosis incidence<br />
are very likely to have been infected<br />
with M. tuberculosis and now comprise<br />
a growing population group.<br />
In contrast, younger people who<br />
have acquired primary infections<br />
have done so during a period of<br />
much lower incidence and consequently<br />
comprise a smaller subgroup.<br />
Therefore, it has been stated<br />
that disease in the elderly largely<br />
consists of endogenous reactivation<br />
whilst most tuberculosis in younger<br />
people is the result of new exogenous<br />
infection (13).<br />
HIV/AIDS and tuberculosis<br />
It is well established that the<br />
impairment of the immune system<br />
as a result of human immunodeficiency<br />
virus (HIV) infection predisposes<br />
to the development of tuberculosis<br />
and the disease is now<br />
regarded as a “sentinel” manifestation<br />
of the progression from HIV to<br />
AIDS (14-16). The specific targeting<br />
of the CD4 helper cells by the HIV<br />
carries a greater risk of endogenous<br />
reactivation of any latent tuberculous<br />
infection. However, in patients<br />
infected with HIV, opportunistic<br />
infection with M. tuberculosis most<br />
commonly occurs as a result of<br />
exogenous infection (15). The risk of<br />
developing progressive primary<br />
tuberculosis within the first year in<br />
HIV-infected persons is almost 30%<br />
in contrast with the 3% risk of the<br />
non-HIV-infected persons (17).<br />
Infection with M. tuberculosis has<br />
been reported as one of the most<br />
pathogenic of the HIV/AIDS opportunistic<br />
infections (15). Foley et al.<br />
(18) described an increase in the<br />
proportion of tuberculous patients<br />
infected with HIV whilst the total<br />
number of TB notifications remains<br />
largely unchanged and suggested a<br />
direction of causality from the wider<br />
population to the AIDS group.<br />
Tuberculosis as the primary cause of<br />
death has also been reported in<br />
patients suffering from AIDS and<br />
tuberculosis (19, 20).<br />
It is unlikely that HIV will directly<br />
cause a rise in tuberculous rates in<br />
the indigenous population of the<br />
developed world because the incidence<br />
of infection with tuberculosis<br />
amongst the younger population,<br />
which is the one most at risk of<br />
acquiring HIV, is low. However, in<br />
the developing world with a high<br />
prevalence of infection with tuberculosis<br />
in the young adult population,<br />
AIDS-related tuberculosis may<br />
increase dramatically over the next<br />
decade (21). It is likely that the high<br />
and rising rates of tuberculosis in<br />
many developing countries with a<br />
high HIV prevalence will indirectly<br />
make an impact on developing<br />
countries as long as rates of tuberculosis<br />
amongst migrants to the<br />
developed world remain high or<br />
increase further (3). During the past<br />
15 years, alongside the dramatic<br />
rise in HIV, Africa has experienced a<br />
concomitant increase in the tuberculosis<br />
rate (18). In the United<br />
states, HIV infection has also been<br />
implicated in the rapid increase in<br />
tuberculosis notifications or young<br />
men (18). The extent to which HIV is<br />
implicated in the resurgence of<br />
tuberculosis in Belgium will depend<br />
on the overlap between the populations<br />
infected with these two conditions.<br />
However, this overlap seems<br />
to be small.<br />
Manifestations of tuberculosis<br />
Inhalation is the predominant<br />
route of M. tuberculosis infection,<br />
making pulmonary tuberculosis the<br />
commonest form of tuberculous<br />
infection (22). The organism gains<br />
access to the blood stream via the<br />
lymphohematogenous route and<br />
may then affect any organ. The incidence<br />
of extrapulmonary tuberculosis<br />
is increasing, especially because<br />
of HIV (23). In patients infected with<br />
HIV M. tuberculosis usually involves<br />
multiple extrapulmonary sites<br />
including the skeleton, abdominal<br />
organs, and central nervous system.<br />
Tuberculosis may demonstrate a<br />
variety of clinical and radiological<br />
features depending on the organ<br />
site involved and as a consequence<br />
may mimic other pathologies. It is<br />
important to be familiar with the<br />
various radiological features of<br />
tuberculosis to obtain a presumptive<br />
diagnosis as early as possible<br />
(24).<br />
Pulmonary tuberculosis<br />
Pulmonary tuberculosis is classically<br />
divided into primary and postprimary<br />
(reactivation) tuberculosis.<br />
However, a considerable overlap in<br />
the radiological presentations of<br />
those entities may be seen.<br />
Although primary tuberculosis is<br />
the most common form of pulmonary<br />
tuberculosis in infants and<br />
children, it has also been increasingly<br />
encountered in adult patients.<br />
Primary tuberculosis<br />
Primary disease accounts now for<br />
23%-34% of all adult cases of tuber-<br />
culosis (25). Primary pulmonary<br />
infection occurs when an uninfected<br />
person inhales an infectious droplet,<br />
which successfully establishes<br />
infection in a terminal airway or<br />
alveolus (22). The resultant primary<br />
parenchymal (Ghon) focus usually<br />
drains via local lymphatics to the<br />
regional lymph nodes. The combination<br />
of the Ghon focus, local lymphangitis<br />
and regional lymph node<br />
involvement is known as the Ranke<br />
complex. Sometimes, associated<br />
pleural reaction overlying a peripheral<br />
Ghon focus may be present. The<br />
formation of the Ghon complex is<br />
often subclinical and a random<br />
chest radiography following primary<br />
infection is often normal or reveals<br />
only a single component, mostly<br />
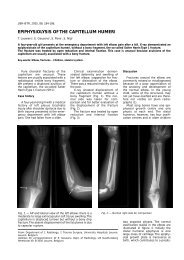
hilar adenopathy (Fig. 1) (22).<br />
Disease progression may occur at<br />
the site of the Ghon focus, within<br />
the regional lymph nodes or as a<br />
result of lymphatic drainage with<br />
hematogenous dissemination or<br />
after local penetration across<br />
anatomical boundaries (26). Penetration<br />
may occur into an adjacent<br />
anatomical space or structure, into<br />
an airway with additional intrabronchial<br />
spread or into a blood vessel<br />
with hematogenous dissemination.<br />
Two main types of hematogenous<br />
spread of M. tuberculosis<br />
are differentiated, but dissemination<br />
via the hematogenous route represents<br />
a condition of infinite gradation.<br />
Following dissemination, bacilli<br />
lodge in small capillaries where<br />
they may progress locally and give<br />
rise to further hematogenous<br />
spread. In the other type, disease<br />
progression may result in a caseous<br />
focus eroding into a blood or lymph<br />
vessel (Fig. 2). Except for immunocompromised<br />
patients, the first<br />
type, contrary to the second one,<br />
rarely progresses to disseminated<br />
disease (27).<br />
Primary tuberculosis typically<br />
manifests radiologically as parenchymal<br />
disease, lymphadenopathy,<br />
pleural effusion, miliary disease, or<br />
atelectasis, which may be either<br />
lobar or segmental. Parenchymal<br />
disease in primary tuberculosis<br />
affects the areas of greatest ventilation.<br />
Most commonly, the middle<br />
lobe, the lower lobes, and the anterior<br />
segment of the upper lobes are<br />
involved (11). Atelectasis is usually<br />
the consequence of bronchial<br />
obstruction by an enlarged hilar<br />
adenopathy.<br />
Postprimary tuberculosis<br />
Postprimary tuberculosis usually<br />
results from reactivation of a previ-
Fig. 1. — Ranke complex. Contrast-enhanced chest CT shows<br />
a well-delineated, solitary nodular lesion in the apical segment<br />
of the right lower lobe (straight arrow) and tuberculous right<br />
hilar lymphadenopathy (curved arrow).<br />
ously dormant primary infection in<br />
90% of cases. In a minority of cases,<br />
it may result from the continuation<br />
of primary disease (28). Reinfection<br />
is very rare. Reactivation of mycobacterial<br />
disease is almost exclusively<br />
seen in adolescence and<br />
adulthood. Reactivation occurs as<br />
the result from numerous causes<br />
such as poor nutrition status, neoplasia,<br />
infection or increasing age.<br />
Post-primary tuberculous lesions<br />
show a slow progressive course<br />
resulting in high morbidity and mortality<br />
if not adequately treated (29).<br />
The radiologic features as seen in<br />
postprimary tuberculosis are the<br />
result from a continuous interaction<br />
between the individual patient, with<br />
his own immune status, and M.<br />
tuberculosis (30). The radiologic<br />
features may be classified as parenchymal<br />
disease with cavitation,<br />
airway involvement, and pleural<br />
extension.<br />
Parenchymal pulmonary disease<br />
may show caseous and liquefaction<br />
necrosis and communicate with the<br />
tracheobronchial tree to form cavities<br />
(Fig. 3). A predilection for the<br />
apical or posterior segment of the<br />
upper lobes or the superior segment<br />
of the lower lobes has been reported<br />
(28). Mostly, two or more segments<br />
are involved, and bilateral<br />
upper lobe involvement may also be<br />
noted (11). Most commonly, cavities<br />
occurs within areas of consolidation,<br />
are multiple, and show thick<br />
irregular walls. However, thin and<br />
smooth cavity walls may also be<br />
seen. An air-fluid level within the<br />
cavity is an uncommon finding, and<br />
IMAGING FEATURES OF <strong>TUBERCULOSIS</strong> — DE BACKER et al 245<br />
may reflect superimposed bacterial<br />
or fungal infection (31).<br />
Bronchogenic spread is a common<br />
complication in postprimary<br />
tuberculosis and represents a chronic<br />
granulomatous infection in which<br />
active organisms spread via airways<br />
after caseous necrosis of bronchial<br />
walls. Endobronchogenic spread is<br />
characterized by multiple, ill-defined<br />
micronodules, distributed in a segmental<br />
or lobar distribution, distant<br />
from the site of the cavity formation<br />
and typically involving the lower<br />
lung zones (30) (Fig. 4). If untreated,<br />
end stage disease may lead to lobar<br />
Fig. 2. — Contrast-enhanced chest CT demonstrates caseous<br />
hilar lymphadenopathy eroding into branches of the right superior<br />
pulmonary vein with subsequent obliteration and dilatation<br />
(curved arrows).<br />
Fig. 3. — Postprimary pulmonary tuberculosis with cavity formation.<br />
CT obtained with lung windowing demonstrates irregular<br />
defined cavities accompanied by mural bronchial wall<br />
thickening and endobronchial spread of tuberculous disease in<br />
both lungs.<br />
or complete lung opacification and<br />
destruction (Fig. 5). However, with<br />
chronic disease, fibroproliferative<br />
lesions composed of nodular opacities<br />
and clearly defined, medium to<br />
coarse reticular areas, may develop.<br />
Most often associated poorly marginated<br />
areas of increased density<br />
may be present (Fig. 6).<br />
A marked fibrotic response is a<br />
common finding after postprimary<br />
tuberculosis and may result in<br />
atelectasis of the upper lobe, retraction<br />
of hilum, compensatory lower<br />
lobe hyperinflation, mediastinal<br />
shift towards the fibrotic lung and
246 JBR–BTR, 2006, 89 (5)<br />
Fig. 4. — Endobronchial spread of tuberculosis (same patient<br />
as Fig. 3). CT obtained with lung windowing shows severe<br />
changes of bronchiolar dilatation and impaction. Bronchiolar<br />
wall thickening (curved arrow) and mucoid impaction of<br />
contiguous branching bronchioles produce a tree-in-bud<br />
appearance (straight arrow).<br />
Fig. 6. — Chronic tuberculous disease characterized by fibroproliferative<br />
lesions. Nodular opacities, coarse reticular areas<br />
and poorly marginated areas of increased density are present.<br />
apical pleural thickening (30) (Fig. 7).<br />
Central airway involvement in<br />
tuberculosis may be the result of<br />
direct extension from tuberculous<br />
lymph nodes, endobronchial spread<br />
of infection, or lymphatic dissemination<br />
to the airway (32). Bronchial<br />
stenosis may result in segmental or<br />
lobar collapse, lobar hyperinflation,<br />
obstructive pneumonia, or mucoid<br />
impaction. A common complication<br />
of endobronchial tuberculosis con-<br />
sists of bronchiectasis resulting from<br />
pulmonary destruction and fibrosis,<br />
and central bronchostenosis.<br />
Pleural effusions in postprimary<br />
tuberculosis are usually small and<br />
associated with parenchymal disease.<br />
A loculated pleural fluid collection<br />
with parenchymal disease and<br />
cavitation may indicate tuberculous<br />
empyema and air-fluid levels in the<br />
pleural space indicate bronchopleural<br />
fistula.<br />
Fig. 5. — Endobronchial spread of tuberculosis with end<br />
stage disease. CT obtained with mediastinal windowing shows<br />
extensive abnormalities of both lungs with distortion of lung<br />
parenchyma, confluent consolidations, multiple cavities,<br />
pleural thickening and effusions.<br />
Fig. 7. — Lung destruction in postprimary tuberculosis. CT<br />
demonstrates a fibrotic, shrunken left lung with compensatory<br />
overexpansion of the right lung. Bronchiectasis is noted in the<br />
left lung with areas of emphysema and atelectasis. Bilateral<br />
symmetrical interstitial nodules, typical of miliary tuberculosis,<br />
are also present.<br />
Occasionally, pericardial involvement<br />
may be seen with mediastinal<br />
and pulmonary tuberculosis and<br />
may cause calcific pericarditis<br />
(Fig. 8) (33).<br />
Imaging findings of pulmonary<br />
tuberculosis<br />
Chest X-rays<br />
Chest X-rays are effective in<br />
demonstrating airspace disease, the<br />
parenchymal nodule that represents<br />
the Ghon focus, diffuse interstitial<br />
disease and pleural effusions<br />
(Fig. 9A). Revealing the presence of<br />
lymphadenopathy is an important<br />
diagnostic sign. However, chest X-
A<br />
rays have been shown to be insensitive<br />
for the detection of lymphadenopathy<br />
(34). On frontal view,<br />
lymphadenopathy is seen as a lobulated<br />
density occupying the hilum<br />
and obliterating the hilar point.<br />
CT<br />
Despite a high radiation dose and<br />
the need for intravenous contrast<br />
administration, CT has evolved to<br />
the modality of choice for the evaluation<br />
of primary and postprimary<br />
pulmonary tuberculosis (11, 30).<br />
Compared to chest X-ray, CT shows<br />
a higher sensitivity for the demonstration<br />
of tuberculous lymphadenopathy<br />
(34). On contrastenhanced<br />
CT, lymphadenopathy<br />
may appear as circular or ovoid<br />
IMAGING FEATURES OF <strong>TUBERCULOSIS</strong> — DE BACKER et al 247<br />
Fig. 8. — CT and MRI findings in a patient with postprimary tuberculous pericarditis resulting in calcific pericarditis. A. On CT pericardial<br />
thickening with extensive, coarse calcifications are noted. B. Black blood MR image shows calcifications as areas with low<br />
signal intensities. A localised pericardial fluid collection along the wall of the left ventricle is noted.<br />
A<br />
B<br />
B<br />
lesions showing peripheral<br />
enhancement pattern with low-density<br />
centres, heterogeneous, and<br />
homogeneous enhancement. Calcifications<br />
may be present within<br />
these nodes (35). High-resolution CT<br />
is the imaging technique of choice<br />
for demonstration early parenchymal<br />
disease and early endobronchial<br />
spread of disease. Primary<br />
tuberculosis typically manifests as<br />
air-space consolidation that is<br />
dense, homogeneous, and well<br />
defined (22). Typical findings in early<br />
bronchogenic spread of disease are<br />
2- to 4-mm centrilobular nodules<br />
and sharply marginated linear<br />
branching opacities representing<br />
severe bronchiolar impaction, with<br />
clubbing of distal bronchioles<br />
Fig. 9. — Miliary tuberculosis. A. Chest radiograph shows<br />
fine, discrete nodular areas of increased opacity bilaterally.<br />
B. High-resolution CT obtained with lung windowing demonstrates<br />
numerous fine, discrete nodules bilaterally in a random<br />
distribution.<br />
(“tree-in-bud” appearance) (Fig. 4).<br />
In both primary and postprimary<br />
tuberculosis, acute hematogenous<br />
dissemination of M. tuberculosis<br />
may result in innumerable small<br />
tuberculous granulomas in both<br />
lungs. This miliary disease may be<br />
visible on CT before it become radiographically<br />
apparent (Fig. 9B). At<br />
high-resolution CT, a mixture of<br />
both sharply and poorly defined, 1to<br />
4-mm nodules, are seen in a diffuse,<br />
random distribution often<br />
associated with intra- and interlobular<br />
septal thickening (30). On chest<br />
X-ray, the classic miliary pattern<br />
consist of innumerable micronodular<br />
infiltrates, which are all very similar<br />
and diffusely scattered in both<br />
lungs, especially the lung apices.
248 JBR–BTR, 2006, 89 (5)<br />
Fig. 10. — Exudative tuberculous pleuritis demonstrated on<br />
MRI. Contrast-enhanced T1-weighted image shows a right-sided<br />
pleural effusion with enhancement of both visceral and parietal<br />
pleura.<br />
MRI<br />
The use of MRI for the evaluation<br />
of intrathoracic tuberculous lesions<br />
is limited because of technical<br />
limitations, as well as the limited<br />
availability in countries where<br />
tuberculosis is endemic. MRI has<br />
been used for the demonstration of<br />
intrathoracic lymphadenopathy and<br />
pleural effusions (36) (Fig. 10).<br />
Extrapulmonary tuberculosis<br />
Although the predominant form<br />
of tuberculosis is pulmonary disease,<br />
infection with M. tuberculosis<br />
may be seen in any organ system.<br />
Extrapulmonary tuberculosis mainly<br />
results from hematogenous dissemination<br />
or lymphogenous spread<br />
from a primary, usually a pulmonary,<br />
focus. This hematogenous<br />
spread may occur years before the<br />
onset of progressive tuberculosis,<br />
as foci of latent infection may lie<br />
dormant before reactivation<br />
occurs (37). The precise incidence of<br />
extrapulmonary tuberculosis has<br />
not been determined, but an<br />
increasing incidence has been noted<br />
both in developing countries and in<br />
developed countries since the mid-<br />
1980s (38). Especially in patients<br />
infected with HIV an increased<br />
prevalence of extrapulmonary<br />
tuberculosis has been reported (39).<br />
A<br />
B<br />
In these patients multiple extrapulmonary<br />
sites are often involved (11).<br />
Other factors that have contributed<br />
to the increased prevalence of extra-<br />
Fig. 1. — MRI findings of tuberculosis of the central nervous<br />
system, characterized by solid caseating granuloma. A. T2weighted<br />
MR image shows the center of the lesions as<br />
hypointense, probably reflecting caseous necrosis. A smooth,<br />
peripheral hyperintense rim and perilesional edema is noted<br />
(arrows). B. Gadolinium-enhanced T1-weighted image demonstrated<br />
strong ringlike peripheral enhancement (arrows). The<br />
inner part of the lesion and surrounding edema shows absence<br />
of enhancement. The center of the lesion corresponds to<br />
caseous necrosis.<br />
pulmonary tuberculosis are the<br />
development of drug-resistant<br />
strains of M. tuberculosis, and aging<br />
of the population (11, 40). Finally,
the more widespread use of crosssectional<br />
imaging modalities may<br />
also explain why extrapulmonary<br />
tuberculosis is more commonly<br />
been seen.<br />
The most common sites of extrapulmonary<br />
tuberculosis consist of<br />
lymphatic, genitourinary, bone and<br />
joint, and central nervous system<br />
involvement followed by peritoneal<br />
and other abdominal organ involvement<br />
(Fig. 11) (39). Recently, many<br />
authors focused on the imaging features<br />
of extrapulmonary tuberculosis<br />
using cross-sectional imaging<br />
methods (11, 37, 41, 42).<br />
Diagnosis of tuberculosis<br />
The radiological manifestations<br />
of tuberculosis depend largely on<br />
whether the host is naïve to the<br />
infecting organism, i.e. primary<br />
tuberculosis, or whether there has<br />
been reactivation or re-exposure,<br />
i.e. post-primary tuberculosis. As<br />
mentioned previously a broad spectrum<br />
of radiographic appearances<br />
has been attributed to mycobacterial<br />
infections.<br />
Infection with M. tuberculosis is<br />
indicated by a significant tuberculin<br />
skin test. However, the tuberculin<br />
skin test is not 100 percent sensitive<br />
for infection with M. tuberculosis,<br />
and even among patients with<br />
proven tuberculosis and no apparent<br />
immunosuppression, 10 to<br />
20 percent will have negative results<br />
on tuberculin skin tests (7).<br />
Therefore, the diagnosis of<br />
mycobacterial infection often<br />
depends on the detection on the<br />
causative organism. Detection of<br />
the organism on microscopy provides<br />
the most immediate confirmation<br />
but requires a relatively high<br />
organism load in the tissue or fluid<br />
being examined. Detection of M.<br />
tuberculosis by culture may take up<br />
to 6 weeks. A rapid confirmation of<br />
the presence, and type, of mycobacterial<br />
organisms, with a low organism<br />
load, may be achieved using<br />
polymerase chain reaction-based<br />
assay for mycobacterial DNA (43).<br />
With pulmonary tuberculosis, the<br />
use of sputum culture requires a<br />
high burden of organisms to confirm<br />
diagnosis (44). Identification of<br />
infection with a lower burden of<br />
organisms may be obtained by<br />
other techniques, such as bronchoscopic<br />
washings and lung biopsy.<br />
Extrapulmonary tuberculosis,<br />
however, produces no pathognomonic<br />
imaging signs, and in<br />
advanced stages may mimic other<br />
disease processes. Although diag-<br />
IMAGING FEATURES OF <strong>TUBERCULOSIS</strong> — DE BACKER et al 249<br />
nosis depends largely on the clinical<br />
context, ultrasound, CT and MRI are<br />
valuable tools for early diagnosis<br />
and accurate evaluation of extrapulmonary<br />
tuberculosis. When a mass<br />
lesion is present, fine needle aspiration<br />
cytology (FNAC) or biopsy may<br />
provide material for histopathological<br />
examination, polymerase chain<br />
reaction-based assay for mycobacterial<br />
DNA and culture. Today, FNAC<br />
has become the first-line diagnostic<br />
technique that is highly sensitive<br />
and specific in endemic areas,<br />
where the mere presence of epithelioid<br />
cell granuloma indicates tuberculosis<br />
until proven otherwise.<br />
Furthermore, FNAC provides an<br />
easy way for collecting material for<br />
bacteriological examination.<br />
However, FNAC has several limitations,<br />
especially in the absence of<br />
demonstrable acid-fast bacilli. The<br />
presence of granuloma and/or<br />
necrosis in cytology smears could<br />
not be regarded as definite, especially<br />
in non-endemic areas as they<br />
have several other causes.<br />
Therefore, the combined use of<br />
polymerase chain reaction-based<br />
assay for mycobacterial DNA and<br />
FNAC has been shown to result in<br />
increased sensitivity in FNAC negative,<br />
clinically suspicious cases, and<br />
as a consequence, results in a<br />
reduced need for surgical biopsy<br />
(45).<br />
Conclusion<br />
The clinical and radiologic features<br />
of tuberculosis may mimic<br />
those of many pathologic processes.<br />
A positive culture, polymerase<br />
chain-reaction based assay for<br />
mycobacterial DNA or histologic<br />
analysis on specimens obtained<br />
during bronchoscopic washings for<br />
pulmonary lesions and fine needle<br />
aspiration cytology or biopsy for<br />
mass lesions are still required to<br />
reach a firm diagnosis. In the appropriate<br />
clinical setting, recognition of<br />
the spectrum of imaging features of<br />
tuberculosis may guide diagnostic<br />
work-up and may result in earlier<br />
and adequate treatment.<br />
References<br />
1. Duraiswami P.K., Tuli S.M.: Five thousand<br />
years of orthopaedics in India.<br />
Clin Orthop, 1991, 75: 269-280.<br />
2. Dhillon M.S., Tuli S.M.: Osteoarticular<br />
tuberculosis of the foot and ankle.<br />
Foot Ankle Int, 2001, 22: 679-686.<br />
3. Davies P.D.: Tuberculosis and migration.<br />
The Mitchell Lecture, 1994. J R<br />
Coll Physicians Lond, 1995, 29: 113-<br />
118.<br />
4. Grigg E.R.: The arcane of tuberculosis.<br />
Am Rev Tuberc, 1958, 78: 151-172.<br />
5. Grzybowski S., Styblo K., Dorken E.:<br />
Tuberculosis in Eskimos. Tubercle,<br />
1976, 57: S1-58.<br />
6. Wiggley S.C.: Tuberculosis in Papua<br />
New Guinea. In: Proust AJ (ed).<br />
History of tuberculosis in Australia,<br />
New Zealand and Papua New Guinea.<br />
Canberra: Brogla, 1991, 103-118.<br />
7. Jasmer R.M., Nahid P, Hopewell PC<br />
Latent tuberculosis infection. N Engl<br />
J Med, 2002, 347: 1860-1866.<br />
8. Nisar M., Davies P.D.: Current trends<br />
in tuberculosis mortality in England<br />
and Wales. Thorax, 1991, 46: 438-440.<br />
9. Fain O., Lortholary O., Lascaux V., et<br />
al.: Extrapulmonary tuberculosis in<br />
the northeastern suburbs of Paris:<br />
141 cases. Eur J Intern Med, 2000, 11:<br />
145-150.<br />
10. Lauzardo M., Ashkin D.: Phthisiology<br />
at the dawn of the new century.<br />
Chest, 2000, 117: 1455-1473.<br />
11. Van den Brande P., Vanhoenacker F.,<br />
Demedts M.: Tuberculosis at the<br />
beginning of the third millennium:<br />
one disease, three epidemics. Eur<br />
Radiol, 2003, 13: 1767-1770.<br />
12. McCarthy O.R.: Asian immigrant<br />
tuberculosis-the effect of visiting<br />
Asia. Br J Dis Chest, 1984, 78: 248-<br />
253.<br />
13. Canetti G., Sutherland I., Sandova E.:<br />
Endogenous reactivation and exogenous<br />
reinfection. Their relative<br />
importance with regard to the development<br />
of non-primary tuberculosis.<br />
Bull Int Union Tuberc, 1972, 47: 122-<br />
143.<br />
14. Raviglione M., Norain J.P., Kochi A.:<br />
HIV associated tuberculosis in developing<br />
countries: clinical features,<br />
diagnosis and treatment. Bull World<br />
Health Organ, 1992, 70: 515-526.<br />
15. Festenstein F., Grange J.M.: Tuberculosis<br />
and the acquired immune<br />
deficiency syndrome. J Appl Bacteriol,<br />
1991, 71:, 19-30.<br />
16. Watson J.M., Gill O.N.: HIV infection<br />
and tuberculosis. BMJ, 1990, 300: 63-<br />
65.<br />
17. Zumla A., Malon P., Henderson J.,<br />
Grange J.M.: Impact of HIV infection<br />
on tuberculosis. Postgrad Med J,<br />
2000, 76: 259-268.<br />
18. Kochi A.: The global tuberculosis situation<br />
and the new control strategy of<br />
the World Health Organization.<br />
Tubercle, 1991, 72: 1-6.<br />
19. Elender F., Bentham G., Langford I.:<br />
Tuberculosis mortality in England<br />
and Wales during, 1982-1992: its<br />
association with poverty, ethnicity and<br />
AIDS. Soc Sci Med, 1998, 46: 673-681.<br />
20. McCormick A.: Unrecognised HIV<br />
related deaths. Br Med J, 1991, 302:<br />
1365-1367.<br />
21. Sudre P., ten Dam G., Kochi A.:<br />
Tuberculosis: a global overview of<br />
the situation. Bull World Health<br />
Organization, 1992, 70: 149-159.<br />
22. Marais B.J., Gie R.P., Schaaf H.S., et<br />
al.: A proposed radiological classification<br />
of childhood intro-thoracic<br />
tuberculosis. Pediatr Radiol, 2004,<br />
34: 886-894.
250 JBR–BTR, 2006, 89 (5)<br />
23. Andronikou S., Welman C.J.,<br />
Kader E.: The CT features of abdominal<br />
tuberculosis in children. Pediatr<br />
Radiol, 2002, 32: 75-81.<br />
24. Harisinghani M.G., McLoud T.C.,<br />
Shepard J.O., Ko J.P., Shroff M.M.,<br />
Mueller P.R.: Tuberculosis from head<br />
to toe. RadioGraphics, 2000, 20: 449-<br />
470.<br />
25. Miller W.T., Miller W.T. Jr.: Tuberculosis<br />
in the normal host: radiological<br />
findings. Semin Roentgenol,<br />
1993, 28: 109-118.<br />
26. Marais B.J., Gie R.P., Schaaf H.S., et<br />
al.: The natural history of childhood<br />
intra-thoracic tuberculosis: a critical<br />
review of the literature from the prechemotherapy<br />
era. Int J Tuberc Lung<br />
Dis, 2004, 8: 392-402.<br />
27. Barnes B.F., Bloch A.B., Davidson P.T.,<br />
Snider D.E.: Tuberculosis in patients<br />
with human immunodeficiency virus<br />
infection. N Engl J Med, 1991, 324:<br />
1644-1650.<br />
28. Lee K.S., Im J.G.: CT in adults with<br />
tuberculosis of the chest: characteristic<br />
findings and role in management.<br />
Am J Roentgenol, 1995, 164: 1361-<br />
1367.<br />
29. De Backer A.I., Bomans P.,<br />
Mortele K.J., Leijs J.: Fatal lung<br />
destruction due to phthisis. JBR-BTR,<br />
2004, 87: 203.<br />
30. Van Dyck P., Vanhoenacker F.M., Van<br />
den Brande P., De Schepper A.M.:<br />
Imaging of pulmonary tuberculosis.<br />
Eur Radiol, 2003, 13: 1771-1785.<br />
31. Kuhlman J.E., Deutsch J.H.,<br />
Fishman E.K., Siegelman S.S.: CT<br />
features of thoracic mycobacterial<br />
disease. RadioGraphics, 1990, 10:<br />
413-431.<br />
32. Smith L.S., Schillaci R.F., Sarlin R.F.:<br />
Endobronchial tuberculosis. Serial<br />
fiberoptic bronchoscopy and natural<br />
history. Chest, 1987, 91: 644-647.<br />
33. Taillefer R., Lemieux R.J., Picard D.,<br />
Dupras G.: Gallium-67 imaging in<br />
pericarditis secondary to tuberculosis<br />
and histoplasmosis. Clin Nucl<br />
Med, 1981, 6: 413-415.<br />
34. Delacourt C., Mani T.M., Bonnerot V.,<br />
et al.: Computed tomography with a<br />
normal chest radiograph in tuberculous<br />
infection. Arch Dis Child, 1993,<br />
69: 430-432.<br />
35. Andronikou S., Joseph E., Lucas S.,<br />
et al.: CT scanning for the detection<br />
of tuberculous mediastinal and hilar<br />
lymphadenopathy in children.<br />
Pediatr Radiol, 2004, 34: 232-236.<br />
36. Andronikou S., Wiesenthaler N.:<br />
Modern imaging of tuberculosis in<br />
children: thoracix, central nervous<br />
system and abdominal tuberculosis.<br />
Pediatr Radiol, 2004, 34: 861-875.<br />
37. Vanhoenacker F.M., De Backer A.I.,<br />
Op de Beeck B., et al.: Imaging of<br />
gastrointestinal and abdominal<br />
tuberculosis. Eur Radiol, 2004, 14:<br />
E103-115.<br />
38. Mehta J.B., Dutt A., Harvill L.,<br />
Mathews K.M.: Epidemiology of<br />
extrapulmonary tuberculosis: a com-<br />
parative analysis with pre-AIDS era.<br />
Chest, 1991, 99: 1134-1138.<br />
39. Lupatkin H., Brau N., Flomenberg P.,<br />
Simberkoff M.S.: Tuberculous<br />
abscesses in patients with AIDS. Clin<br />
Infect Dis, 1992, 14: 1040-1044.<br />
40. Engin G., Acunas B., Acunas G.,<br />
Tunaci M.: Imaging of extrapulmonary<br />
tuberculosis. RadioGraphics,<br />
2000, 20: 471-488.<br />
41. Bernaerts A., Vanhoenacker F.M.,<br />
Parizel P.M., et al.: Tuberculosis of the<br />
central nervous system: overview of<br />
neuroradiological findings). Eur<br />
Radiol, 2003, 13: 1876-1890.<br />
42. Moore S.L., Rafii M.: Imaging of musculoskeletal<br />
and spinal tuberculosis.<br />
Radiol Clin North Am, 2001, 39: 329-<br />
342.<br />
43. Hidaka E., Honda T., Ueno I.,<br />
Yamasaki Y., Kubo K., Katsuyama T.:<br />
Sensitive identification of mycobacterial<br />
species using PCR-RFLP<br />
on bronchial washings. Am J Respir<br />
Crit Care Med, 2000, 161: 930-934.<br />
44. Albelda S.M., Kern J.A.,<br />
Marinelli D.L., Miller W.T.: Expanding<br />
spectrum of pulmonary disease<br />
caused by nontuberculous mycobacteria.<br />
Radiology, 1985, 157: 289-<br />
296.<br />
45. Aljafari A.S., Khalil E.A., Elsiddig K.E.,<br />
et al.: Diagnosis of tuberculous lymphadenitis<br />
by FNAC, microbiological<br />
methods and PCR: a comparative<br />
study. Cytopathology, 2004, 15: 44-<br />
48.<br />
POSTGRADUAAT RADIOLOGIE VAN DE VLAAMSE UNIVERSITEITEN<br />
PROGRAMMA 2006-2007<br />
28.09.2006 UA Het postoperatieve gewricht<br />
19.10.2006 UG Neuroradiologie<br />
16.11.2006 KUL Urogenitale Radiologie<br />
14.12.2006 VUB Abdomen<br />
18.01.2007 UA Mammografie<br />
08.02.2007 VUB Radioprotectie<br />
19.04.2007 KUL Angio/Interventionele<br />
17.05.2007 UG Thoraxradiologie