principles of extraction and the extraction of semivolatile organics ...
principles of extraction and the extraction of semivolatile organics ...
principles of extraction and the extraction of semivolatile organics ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
52 <strong>principles</strong> <strong>of</strong> <strong>extraction</strong><br />
pC<br />
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14<br />
0<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
[H<br />
7<br />
8<br />
9<br />
10<br />
11<br />
12<br />
13<br />
14<br />
2,4-DB [COOH]<br />
+ ] [OH− ]<br />
2,4-DB [COO− pH<br />
]<br />
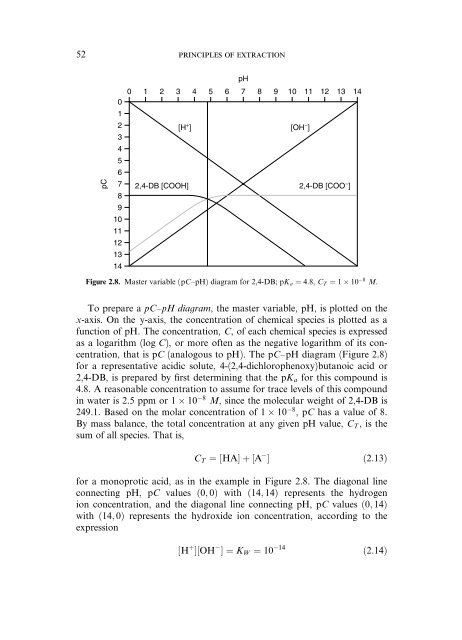
Figure 2.8. Master variable (pC–pH) diagram for 2,4-DB; pK a ¼ 4:8, CT ¼ 1 10 8 M.<br />
To prepare a pC–pH diagram, <strong>the</strong> master variable, pH, is plotted on <strong>the</strong><br />
x-axis. On <strong>the</strong> y-axis, <strong>the</strong> concentration <strong>of</strong> chemical species is plotted as a<br />
function <strong>of</strong> pH. The concentration, C, <strong>of</strong> each chemical species is expressed<br />
as a logarithm (log C), or more <strong>of</strong>ten as <strong>the</strong> negative logarithm <strong>of</strong> its concentration,<br />
that is pC (analogous to pH). The pC–pH diagram (Figure 2.8)<br />
for a representative acidic solute, 4-(2,4-dichlorophenoxy)butanoic acid or<br />
2,4-DB, is prepared by first determining that <strong>the</strong> pKa for this compound is<br />
4.8. A reasonable concentration to assume for trace levels <strong>of</strong> this compound<br />
in water is 2.5 ppm or 1 10 8 M, since <strong>the</strong> molecular weight <strong>of</strong> 2,4-DB is<br />
249.1. Based on <strong>the</strong> molar concentration <strong>of</strong> 1 10 8 ,pC has a value <strong>of</strong> 8.<br />
By mass balance, <strong>the</strong> total concentration at any given pH value, CT, is <strong>the</strong><br />
sum <strong>of</strong> all species. That is,<br />
CT ¼½HAŠþ½A Š ð2:13Þ<br />
for a monoprotic acid, as in <strong>the</strong> example in Figure 2.8. The diagonal line<br />
connecting pH, pC values ð0; 0Þ with ð14; 14Þ represents <strong>the</strong> hydrogen<br />
ion concentration, <strong>and</strong> <strong>the</strong> diagonal line connecting pH, pC values ð0; 14Þ<br />
with ð14; 0Þ represents <strong>the</strong> hydroxide ion concentration, according to <strong>the</strong><br />
expression<br />
½H þ Š½OH Š¼KW ¼ 10 14<br />
ð2:14Þ