Developments Toward an Intelligent Electric Arc Furnace at - Tenova
Developments Toward an Intelligent Electric Arc Furnace at - Tenova
Developments Toward an Intelligent Electric Arc Furnace at - Tenova
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
MODEL INPUTS AND OUTPUTS<br />
Initial work towards a dynamic control system for the EAF has focused on building a m<strong>at</strong>hem<strong>at</strong>ical model for calcul<strong>at</strong>ing a dynamic<br />
mass <strong>an</strong>d energy bal<strong>an</strong>ce applied to the gas-phase of the EAF. The idea of calcul<strong>at</strong>ing a mass/energy bal<strong>an</strong>ce of the gas-phase of the<br />
EAF is not new. Wh<strong>at</strong> differenti<strong>at</strong>es the current approach from th<strong>at</strong> of others is th<strong>at</strong> here the bal<strong>an</strong>ce is calcul<strong>at</strong>ed dynamically using<br />
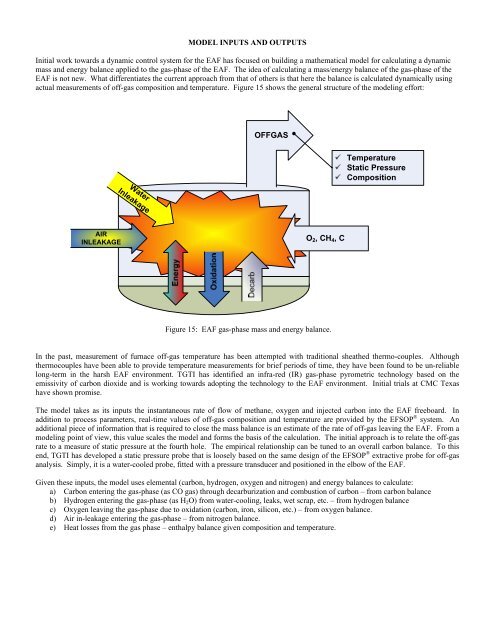
actual measurements of off-gas composition <strong>an</strong>d temper<strong>at</strong>ure. Figure 15 shows the general structure of the modeling effort:<br />
AIR<br />
INLEAKAGE<br />
W<strong>at</strong>er<br />
Inleakage<br />
OFFGAS<br />
O2, CH4, C<br />
Figure 15: EAF gas-phase mass <strong>an</strong>d energy bal<strong>an</strong>ce.<br />
Temper<strong>at</strong>ure<br />
St<strong>at</strong>ic Pressure<br />
Composition<br />
In the past, measurement of furnace off-gas temper<strong>at</strong>ure has been <strong>at</strong>tempted with traditional she<strong>at</strong>hed thermo-couples. Although<br />
thermocouples have been able to provide temper<strong>at</strong>ure measurements for brief periods of time, they have been found to be un-reliable<br />
long-term in the harsh EAF environment. TGTI has identified <strong>an</strong> infra-red (IR) gas-phase pyrometric technology based on the<br />
emissivity of carbon dioxide <strong>an</strong>d is working towards adopting the technology to the EAF environment. Initial trials <strong>at</strong> CMC Texas<br />
have shown promise.<br />
The model takes as its inputs the inst<strong>an</strong>t<strong>an</strong>eous r<strong>at</strong>e of flow of meth<strong>an</strong>e, oxygen <strong>an</strong>d injected carbon into the EAF freeboard. In<br />
addition to process parameters, real-time values of off-gas composition <strong>an</strong>d temper<strong>at</strong>ure are provided by the EFSOP ® system. An<br />
additional piece of inform<strong>at</strong>ion th<strong>at</strong> is required to close the mass bal<strong>an</strong>ce is <strong>an</strong> estim<strong>at</strong>e of the r<strong>at</strong>e of off-gas leaving the EAF. From a<br />
modeling point of view, this value scales the model <strong>an</strong>d forms the basis of the calcul<strong>at</strong>ion. The initial approach is to rel<strong>at</strong>e the off-gas<br />
r<strong>at</strong>e to a measure of st<strong>at</strong>ic pressure <strong>at</strong> the fourth hole. The empirical rel<strong>at</strong>ionship c<strong>an</strong> be tuned to <strong>an</strong> overall carbon bal<strong>an</strong>ce. To this<br />
end, TGTI has developed a st<strong>at</strong>ic pressure probe th<strong>at</strong> is loosely based on the same design of the EFSOP ® extractive probe for off-gas<br />
<strong>an</strong>alysis. Simply, it is a w<strong>at</strong>er-cooled probe, fitted with a pressure tr<strong>an</strong>sducer <strong>an</strong>d positioned in the elbow of the EAF.<br />
Given these inputs, the model uses elemental (carbon, hydrogen, oxygen <strong>an</strong>d nitrogen) <strong>an</strong>d energy bal<strong>an</strong>ces to calcul<strong>at</strong>e:<br />
a) Carbon entering the gas-phase (as CO gas) through decarburiz<strong>at</strong>ion <strong>an</strong>d combustion of carbon – from carbon bal<strong>an</strong>ce<br />
b) Hydrogen entering the gas-phase (as H2O) from w<strong>at</strong>er-cooling, leaks, wet scrap, etc. – from hydrogen bal<strong>an</strong>ce<br />
c) Oxygen leaving the gas-phase due to oxid<strong>at</strong>ion (carbon, iron, silicon, etc.) – from oxygen bal<strong>an</strong>ce.<br />
d) Air in-leakage entering the gas-phase – from nitrogen bal<strong>an</strong>ce.<br />
e) He<strong>at</strong> losses from the gas phase – enthalpy bal<strong>an</strong>ce given composition <strong>an</strong>d temper<strong>at</strong>ure.