PHY ICS - The Institute of Physics in Ireland
PHY ICS - The Institute of Physics in Ireland
PHY ICS - The Institute of Physics in Ireland
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>The</strong> blue spiral flame<br />
Austria<br />
An <strong>in</strong>troduction to ignition temperature/activation energy<br />
Follow these steps<br />
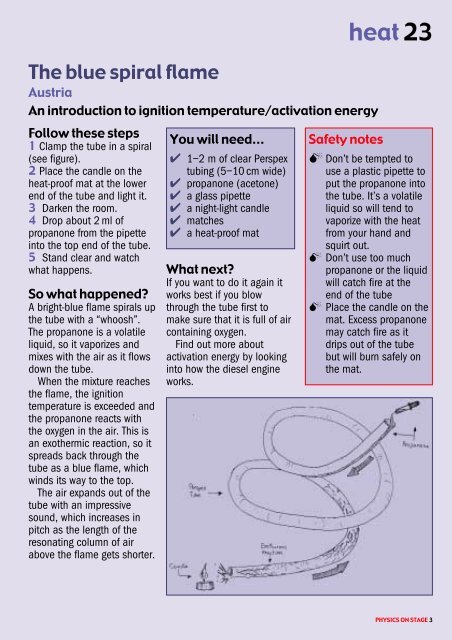
1 Clamp the tube <strong>in</strong> a spiral<br />
(see figure).<br />
2 Place the candle on the<br />
heat-pro<strong>of</strong> mat at the lower<br />
end <strong>of</strong> the tube and light it.<br />
3 Darken the room.<br />
4 Drop about 2 ml <strong>of</strong><br />
propanone from the pipette<br />
<strong>in</strong>to the top end <strong>of</strong> the tube.<br />
5 Stand clear and watch<br />
what happens.<br />
So what happened?<br />
A bright-blue flame spirals up<br />
the tube with a “whoosh”.<br />
<strong>The</strong> propanone is a volatile<br />
liquid, so it vaporizes and<br />
mixes with the air as it flows<br />
down the tube.<br />
When the mixture reaches<br />
the flame, the ignition<br />
temperature is exceeded and<br />
the propanone reacts with<br />
the oxygen <strong>in</strong> the air. This is<br />
an exothermic reaction, so it<br />
spreads back through the<br />
tube as a blue flame, which<br />
w<strong>in</strong>ds its way to the top.<br />
<strong>The</strong> air expands out <strong>of</strong> the<br />
tube with an impressive<br />
sound, which <strong>in</strong>creases <strong>in</strong><br />
pitch as the length <strong>of</strong> the<br />
resonat<strong>in</strong>g column <strong>of</strong> air<br />
above the flame gets shorter.<br />
You will need...<br />
✔ 1–2 m <strong>of</strong> clear Perspex<br />
tub<strong>in</strong>g (5–10 cm wide)<br />
✔ propanone (acetone)<br />
✔ a glass pipette<br />
✔ a night-light candle<br />
✔ matches<br />
✔ a heat-pro<strong>of</strong> mat<br />
What next?<br />
If you want to do it aga<strong>in</strong> it<br />
works best if you blow<br />
through the tube first to<br />
make sure that it is full <strong>of</strong> air<br />
conta<strong>in</strong><strong>in</strong>g oxygen.<br />
F<strong>in</strong>d out more about<br />
activation energy by look<strong>in</strong>g<br />
<strong>in</strong>to how the diesel eng<strong>in</strong>e<br />
works.<br />
heat 23<br />
Safety notes<br />
Don’t be tempted to<br />
use a plastic pipette to<br />
put the propanone <strong>in</strong>to<br />
the tube. It’s a volatile<br />
liquid so will tend to<br />
vaporize with the heat<br />
from your hand and<br />
squirt out.<br />
Don’t use too much<br />
propanone or the liquid<br />
will catch fire at the<br />
end <strong>of</strong> the tube<br />
Place the candle on the<br />
mat. Excess propanone<br />
may catch fire as it<br />
drips out <strong>of</strong> the tube<br />
but will burn safely on<br />
the mat.<br />
<strong>PHY</strong>S<strong>ICS</strong> ON STAGE 3